Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment
Abstract
:1. Introduction
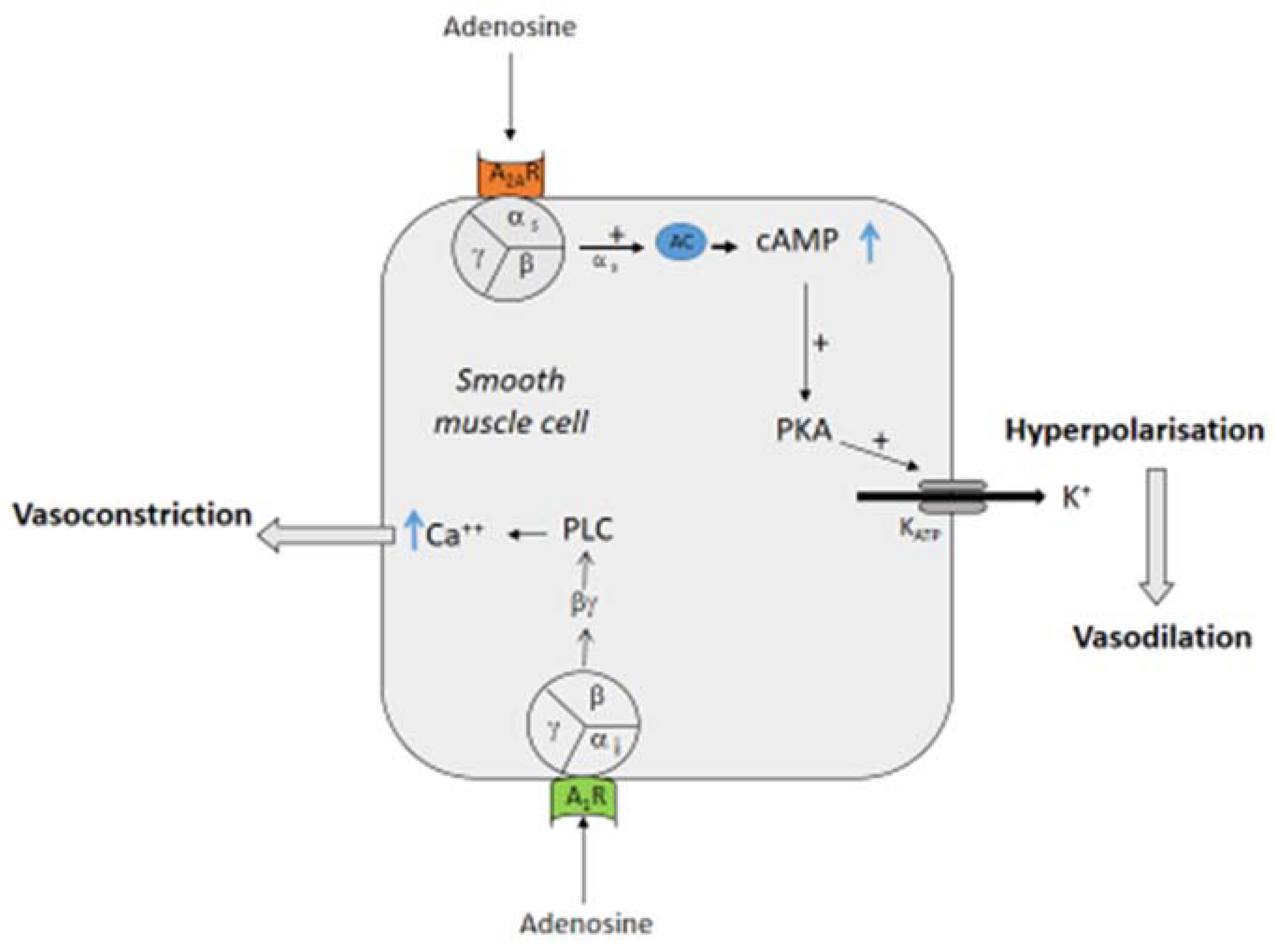
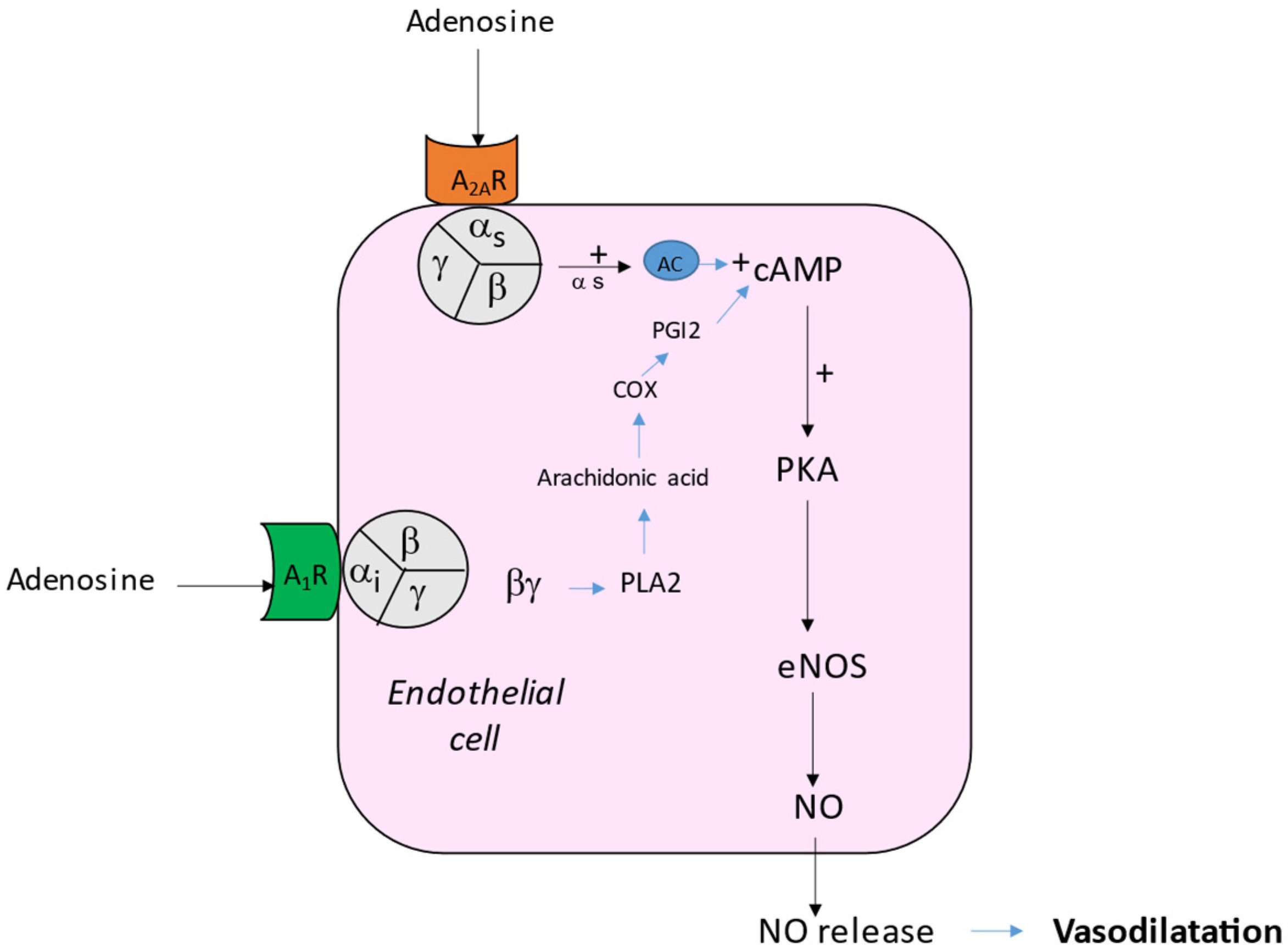
2. Effects of Adenosine on Vessels
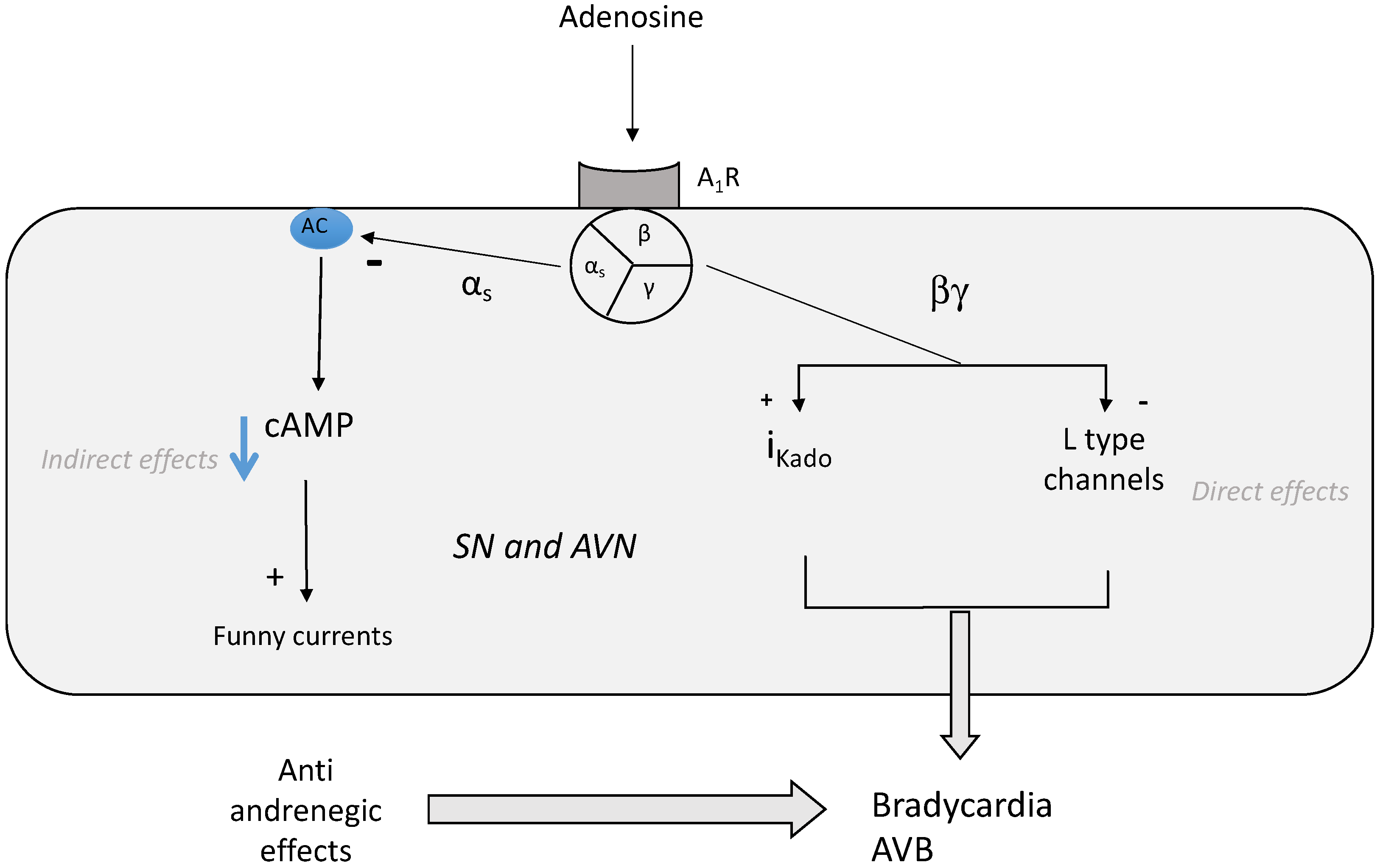
3. Effects of Adenosine on Heart Rhythm
4. Role of Adenosinergic System in NHS
5. Possible Consequences of Presence of Spare Receptors in NHS Forms
6. Possible Role of Adenosine Receptors in Central Nervous System
7. Effects of Adenosine Receptor Antagonists
8. Biased Ligands
9. Role of Nucleotidases
10. Conclusions
11. Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brignole, M.; Groppelli, A.; Brambilla, R.; Caldara, G.L.; Torresani, E.; Parati, G.; Solari, D.; Ungar, A.; Rafanelli, M.; Deharo, J.C.; et al. Plasma adenosine and neurally mediated syncope: Ready for clinical use. Europace 2020, 22, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Day, S.C.; Cook, E.F.; Funkenstein, H.; Goldman, L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am. J. Med. 1982, 73, 15–23. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Singer, D.; Mulley, A.G.; Thibault, G.E.; Barnett, G.O. Patients with syncope admitted to medical intensive care units. JAMA 1982, 248, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, T.; Umemura, S.; Toya, Y.; Uchibori, T.; Kogi, K.; Takagi, N.; Ishii, M. Identification of adenosine A2 receptor-cAMP system in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 1994, 199, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K.; Mullane, K.; Flameng, W. Role of adenosine in the heart and circulation. Cardiovasc. Res. 1996, 32, 797–813. [Google Scholar] [CrossRef]
- Ponnoth, D.S.; Sanjani, M.S.; Ledent, C.; Roush, K.; Krahn, T.; Mustafa, S.J. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1655–H1660. [Google Scholar] [CrossRef] [Green Version]
- Kusano, Y.; Echeverry, G.; Miekisiak, G.; Kulik, T.B.; Aronhime, S.N.; Chen, J.F.; Winn, H.R. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J. Cereb. Blood Flow Metab. 2010, 30, 808–815. [Google Scholar] [CrossRef]
- Arsyad, A.; Dobson, G.P. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC Pharmacol. Toxicol. 2016, 17, 23. [Google Scholar] [CrossRef] [Green Version]
- Kleppisch, T.; Nelson, M.T. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1995, 92, 12441–12445. [Google Scholar] [CrossRef] [Green Version]
- Hein, T.W.; Kuo, L. cAMP-independent dilation of coronary arterioles to adenosine: Role of nitric oxide, G proteins, and K(ATP) channels. Circ. Res. 1999, 85, 634–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, H.E.; Schnermann, J.; Oldenburg, P.J.; Mustafa, S.J. Role of A1 adenosine receptors in regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1411–H1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, A.B.; Grossfeld, D.; Kasselman, L.J.; Renna, H.A.; Vernice, N.A.; Drewes, W.; Konig, J.; Carsons, S.E.; DeLeon, J. Adenosine and the Cardiovascular System. Am. J. Cardiovasc. Drugs 2019, 19, 449–464. [Google Scholar] [CrossRef]
- Ray, C.J.; Marshall, J.M. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J. Physiol. 2006, 570 Pt 1, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Preti, D.; Borea, P.A.; Varani, K. Medicinal chemistry of A3 adenosine receptor modulators: Pharmacological activities and therapeutic implications. J. Med. Chem. 2012, 55, 5676–5703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Makaritsis, K.; Francis, C.E.; Gavras, H.; Ravid, K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: Studies in knock-out mice. Biochim. Biophys. Acta 2000, 1500, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Jammes, Y.; Joulia, F.; Steinberg, J.G.; Ravailhe, S.; Delpierre, S.; Condo, J.; Guieu, R.; Delliaux, S. Endogenous adenosine release is involved in the control of heart rate in rats. Can. J. Physiol. Pharmacol. 2015, 93, 667–675. [Google Scholar] [CrossRef]
- Pelleg, A.; Hurt, C.; Miyagawa, A.; Michelson, E.L.; Dreifus, L.S. Differential sensitivity of cardiac pacemakers to exogenous adenosine in vivo. Am. J. Physiol. 1990, 258, H1815–H1822. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Di Francesco, D.; Borer, J.S. The funny current: Cellular basis for the control of heart rate. Drugs 2007, 67 (Suppl. S2), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Fassina, G.; de Biasi, M.; Ragazzi, E.; Caparrotta, L. Adenosine: A natural modulator of L-type calcium channels in atrial myocardium? Pharmacol. Res. 1991, 23, 319–326. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H., 4th; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B adenosine receptor reduces left ventriculardysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 2014, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Cross, H.R.; Murphy, E.; Black, R.G.; Auchampach, J.; Steenbergen, C. Overexpression of A(3) adenosine receptors decreases heart rate, preserves energetics, and protects ischemic hearts. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1562–H1568. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.N.; Wang, Y.; Garcia-Roves, P.M.; Björnholm, M.; Fredholm, B.B. Adenosine A(3) receptors regulate heart rate, motor activity and body temperature. Acta Physiol. 2010, 199, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Saadjian, A.Y.; Lévy, S.; Franceschi, F.; Zouher, I.; Paganelli, F.; Guieu, R.P. Role of endogenous adenosine as a modulator of syncope induced during tilt testing. Circulation 2002, 106, 569–574. [Google Scholar] [CrossRef] [Green Version]
- Carrega, L.; Saadjian, A.Y.; Mercier, L.; Zouher, I.; Bergé-Lefranc, J.L.; Gerolami, V.; Giaime, P.; Sbragia, P.; Paganelli, F.; Fenouillet, E.; et al. Increased expression of adenosine A2A receptors in patients with spontaneous and head-up- tilt-induced syncope. Heart Rhythm 2007, 4, 870–876. [Google Scholar] [CrossRef]
- Saadjian, A.Y.; Gerolami, V.; Giorgi, R.; Mercier, L.; Berge-Lefranc, J.L.; Paganelli, F.; Ibrahim, Z.; By, Y.; Guéant, J.L.; Lévy, S.; et al. Head-up tilt induced syncope and adenosine A2A receptor gene polymorphism. Eur. Heart J. 2009, 30, 1510–1515. [Google Scholar] [CrossRef]
- Lo Mauro, R.; Sabella, F.P.; Enia, F. Sinus arrest associated with dypiridamole infusion. Chest 1994, 105, 604–605. [Google Scholar] [CrossRef]
- Buitrago, I.; Wolinski, D.; Asher, C.R. Syncope during a pharmacologic nuclear test. Clev. Clin. J. Med. 2014, 5, 279–280. [Google Scholar] [CrossRef]
- Del Rosso, A.; Bartoletti, A.; Brignole, M. The clinical utility and diagnosticvalue of the head-up tilt testing (HUT) protocol. J. Cardiovasc. Electrophysiol. 2004, 15, 615. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Fedorowski, A.; Olshansky, B.; van Dijk, J.; Abe, H.M.; de Lange, F.; Kenny, R.A.; Lim, P.B.; Moya, A.; Rosen, S.D.; et al. Tilt testing remains a valuable asset. Eur. Heart J. 2021, 42, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Mitro, P.; Mudrakova, K.; Valocik, G. Diagnostic yield of adenosine and nitroglycerine stimulated tilt test in patients with unexplained syncope. Bratisl. Lek. Listy 2007, 108, 259–264. [Google Scholar]
- Brignole, M.; Gaggioli, G.; Menozzi, C.; Gianfranchi, L.; Bartoletti, A.; Bottoni, N.; Lolli, G.; Oddone, D.; Del Rosso, A.; Pellinghelli, G. Adenosine-induced atrioventricular block in patients with unexplained syncope: The diagnosticvalue of ATP testing. Circulation 1997, 96, 3921–3927. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.C.; Brignole, M.; Guieu, R. Adenosine and neurohumoral syncope. Minerva Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Gaggioli, G.; Menozzi, C.; Del Rosso, A.; Costa, S.; Bartoletti, A.; Bottoni, N.; Lolli, G. Clinical features of adenosine sensitive syncope and tilt induced vasovagal syncope. Heart 2000, 83, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deharo, J.C.; Mechulan, A.; Giorgi, R.; Franceschi, F.; Prevot, S.; Peyrouse, E.; Condo, J.; By, Y.; Ruf, J.; Brignole, M.; et al. Adenosine plasma level and A2A adenosine receptor expression: Correlation with laboratory tests in patients with neutrally mediated syncope. Heart 2012, 98, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Vaugeois, J.M.; Schiffmann, S.N.; Pedrazzini, T.; El Yacoubi, M.; Vanderhaeghen, J.J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef]
- Jacquin, L.; Franceschi, F.; By, Y.; Durand-Gorde, J.M.; Condo, J.; Deharo, J.C.; Michelet, P.; Fenouillet, E.; Guieu, R.; Ruf, J. Search for adenosine A2A spare receptors onperipheral human lymphocytes. FEBS Open Bio. 2012, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, F.; By, Y.; Peyrouse, E.; Fromonot, J.; Gerolami, V.; Kipson, N.; Boussuges, A.; Brignole, M.; Fenouillet, E.; Deharo, J.C.; et al. A2A adenosine receptor function in patients with vasovagal syncope. Europace 2013, 15, 1328–1332. [Google Scholar] [CrossRef]
- Stephenson, R.P. A modification of receptor theory. 1956. Br. J. Pharmacol. 1997, 120 (Suppl. S4), 106–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenouillet, E.; Mottola, G.; Kipson, N.; Paganelli, F.; Guieu, R.; Ruf, J. Adenosine Receptor Profiling Reveals an Association between the Presence of Spare Receptors and Cardiovascular Disorders. Int. J. Mol. Sci. 2019, 20, 5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guieu, R.; Brignole, M.; Deharo, J.C.; Deharo, P.; Mottola, G.; Groppelli, A.; Paganelli, F.; Ruf, J. Adenosine Receptor Reserve and Long-Term Potentiation: Unconventional Adaptive Mechanisms in Cardiovascular Diseases? Int. J. Mol. Sci. 2021, 22, 7584. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, M.; Shryock, J.C.; Dennis, D.M.; Baker, S.P.; Belardinelli, L. Differential A1 adenosine receptor reserve for two actions of adenosine on guinea pig atrial myocytes. Mol. Pharmacol. 1997, 52, 683–691. [Google Scholar] [CrossRef] [Green Version]
- By, Y.; Durand-Gorde, J.M.; Condo, J.; Lejeune, P.J.; Mallet, B.; Carayon, P.; Guieu, R.; Ruf, J. Production of an agonist-like monoclonal antibody to the human A2A receptor of adenosine for clinical use. Mol. Immunol. 2009, 46, 400–405. [Google Scholar] [CrossRef]
- Cohen, F.R.; Lazareno, S.; Birdsall, N.J. The affinity of adenosine for the high- and low-affinity states of the human adenosine A1 receptor. Eur. J. Pharmacol. 1996, 309, 111–114. [Google Scholar] [CrossRef]
- Marlinge, M.; Vairo, D.; Marolda, V.; Bruzzese, L.; Adjriou, N.; Guiol, C.; Kipson, N.; Bonnardel, A.; Gastaldi, M.; Kerbaul, F.; et al. Rapid Measurement of Adenosine Concentration in Human Blood Using Fixed Potential Amperometry: Comparison with Mass Spectrometry and High-Performance Liquid Chromatography. J. Analytical. Bioanal. Tech. 2017, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; St Lambert, J.H.; Dashwood, M.R.; Spyer, K.M. Localization and action of adenosine A2a receptors in regions of the brainstem important in cardiovascular control. Neuroscience 2000, 95, 513–518. [Google Scholar] [CrossRef]
- Zyśko, D.; Szewczuk-Bogusławska, M.; Kaczmarek, M.; Agrawal, A.K.; Rudnicki, J.; Gajek, J.; Melander, O.; Sutton, R.; Fedorowski, A. Reflex syncope, anxiety level, and family history of cardiovascular disease in young women: Case-control study. Europace 2015, 17, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry. 2016, 80, 12–22. [Google Scholar] [CrossRef]
- El-Ani, D.; Jacobson, K.A.; Shainberg, A. Effects of theophylline and dibutyryl-cAMP on adenosine receptors and heart rate in cultured cardiocytes. J. Basic. Clin. Physiol. Pharmacol. 1996, 7, 347–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conlay, L.A.; Conant, J.A.; de Bros, F.; Wurtman, R. Caffeine alters plasma adenosine levels. Nature 1997, 389, 136. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Portaluppi, F.; Merighi, S.; Ongini, E.; Belardinelli, L.; Borea, P.A. Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation 1999, 99, 2499–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renda, G.; Zimarino, M.; Antonucci, I.; Tatasciore, A.; Ruggieri, B.; Bucciarelli, T.; Prontera, T.; Stuppia, L.; De Caterina, R. Genetic determinants of blood pressure responses to caffeine drinking. Am. J. Clin. Nutr. 2012, 95, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Committee on Military Nutrition Research. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academy Press: Washington, DC, USA, 2001; p. 4. [Google Scholar]
- Brignole, M.; Solari, D.; Iori, M.; Bottoni, N.; Guieu, R.; Deharo, J.C. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm 2016, 13, 1151–1154. [Google Scholar] [CrossRef]
- Brignole, M.; Iori, M.; Solari, D.; Bottoni, N.; Rivasi, G.; Ungar, A.; Deharo, J.C.; Guieu, R. Efficacy of theophylline in patients with syncope without prodromes with normal heart and normal ECG. Int. J. Cardiol. 2019, 289, 70–73. [Google Scholar] [CrossRef]
- Nurminen, M.L.; Niittynen, L.; Korpela, R.; Vapaatalo, H. Coffee, caffeine and blood pressure: A critical review. Eur. J. Clin. Nutr. 1999, 53, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Hutson, P.; Guieu, R.; Deharo, J.C.; Michelet, P.; Brignole, M.; Vander Ark, C.; Hamdan, M.H. Safety, Pharmacokinetic, and Pharmacodynamic Study of a Sublingual Formula for the Treatment of Vasovagal Syncope. Drugs R D 2022, 22, 61–70. [Google Scholar] [CrossRef]
- Berry, N.M.; Rickards, C.A.; Newman, D.G. The effect of caffeine on the cardiovascular responses to head-up tilt. Aviat. Space Environ. Med. 2003, 74, 725–730. [Google Scholar]
- Gibbon, J.R.; Frith, J. The effects of caffeine in adults with neurogenic orthostatic hypotension: A systematic review. Clin. Auton. Res. 2021, 31, 499–509. [Google Scholar] [CrossRef]
- Brignole, M.; Iori, M.; Strano, S.; Tomaino, M.; Rivasi, G.; Ungar, A.; Carretta, D.; Solari, D.; Napoli, P.; Deharo, J.C.; et al. Theophylline in patients with syncope without prodrome, normal heart, and normal electrocardiogram: A propensity-score matched study verified by implantable cardiac monitor. Europace 2021, euab300. [Google Scholar] [CrossRef] [PubMed]
- Jarpe, M.B.; Knall, C.; Mitchell, F.M.; Buhl, A.M.; Duzic, E.; Johnson, G.L. [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J. Biol. Chem. 1998, 273, 3097–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeill, S.M.; Baltos, J.A.; White, P.J.; May, L.T. Biased agonism at adenosine receptors. Cell Signal. 2021, 82, 109954. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.J. Evidence for biased agonists and antagonists at the endothelin receptors. Life Sci. 2016, 159, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelleg, A.; Schulman, E.S.; Barnes, P.J. Adenosine 5′-triphosphate’s role in bradycardia and syncope associated with pulmonary embolism. Respir. Res. 2018, 19, 142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guieu, R.; Degioanni, C.; Fromonot, J.; De Maria, L.; Ruf, J.; Deharo, J.C.; Brignole, M. Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment. Biomedicines 2022, 10, 1127. https://doi.org/10.3390/biomedicines10051127
Guieu R, Degioanni C, Fromonot J, De Maria L, Ruf J, Deharo JC, Brignole M. Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment. Biomedicines. 2022; 10(5):1127. https://doi.org/10.3390/biomedicines10051127
Chicago/Turabian StyleGuieu, Régis, Clara Degioanni, Julien Fromonot, Lucille De Maria, Jean Ruf, Jean Claude Deharo, and Michele Brignole. 2022. "Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment" Biomedicines 10, no. 5: 1127. https://doi.org/10.3390/biomedicines10051127
APA StyleGuieu, R., Degioanni, C., Fromonot, J., De Maria, L., Ruf, J., Deharo, J. C., & Brignole, M. (2022). Adenosine, Adenosine Receptors and Neurohumoral Syncope: From Molecular Basis to Personalized Treatment. Biomedicines, 10(5), 1127. https://doi.org/10.3390/biomedicines10051127






