Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Effects of S-MenSCs and S-MenSCs* Administration on Cardiac Function
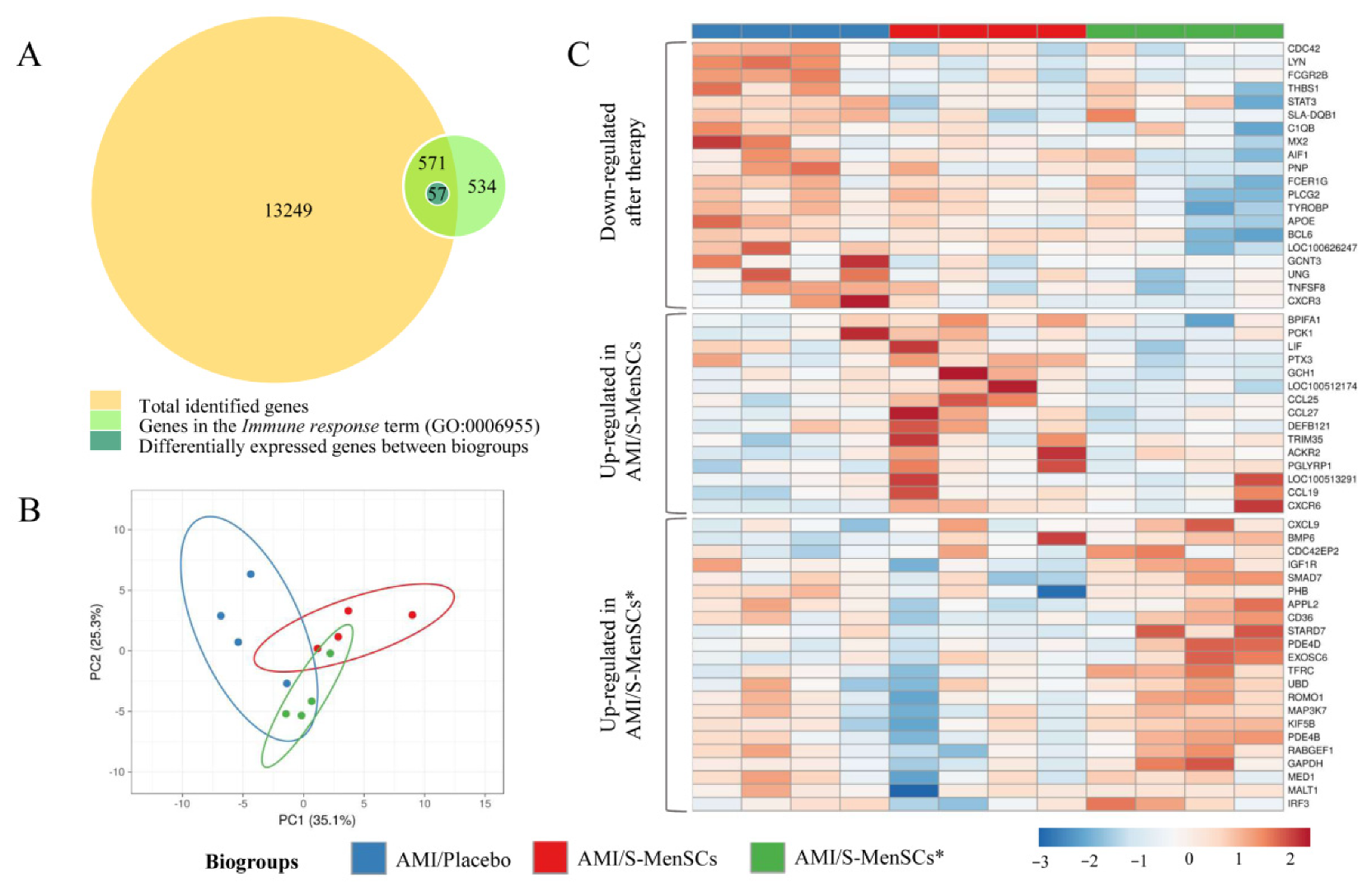
2.2. DEGs Identified and Related to the Immune System Process
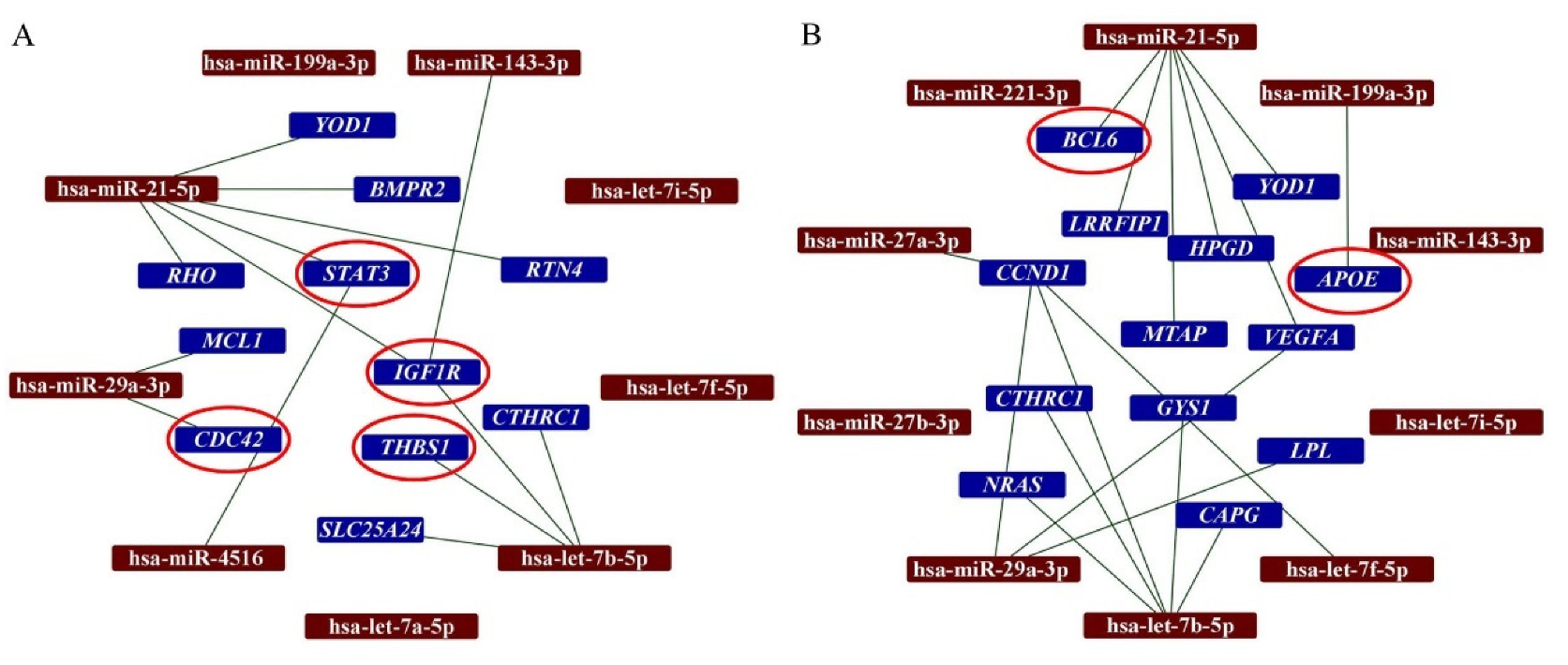
2.3. DEGs and Predicted Interactions with miRNAs in Secretomes
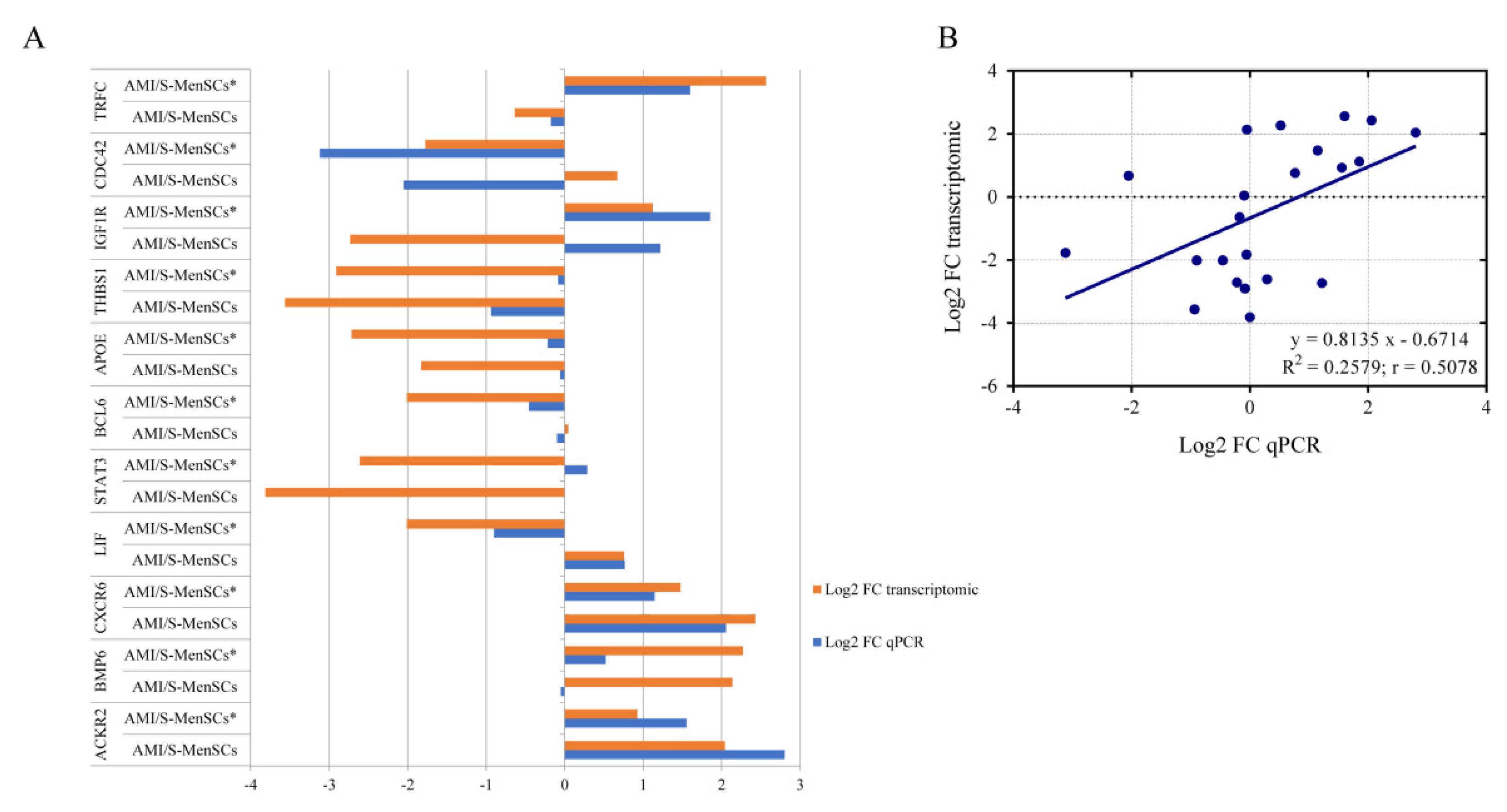
2.4. Validation of Pre-Selected Transcripts
3. Discussion
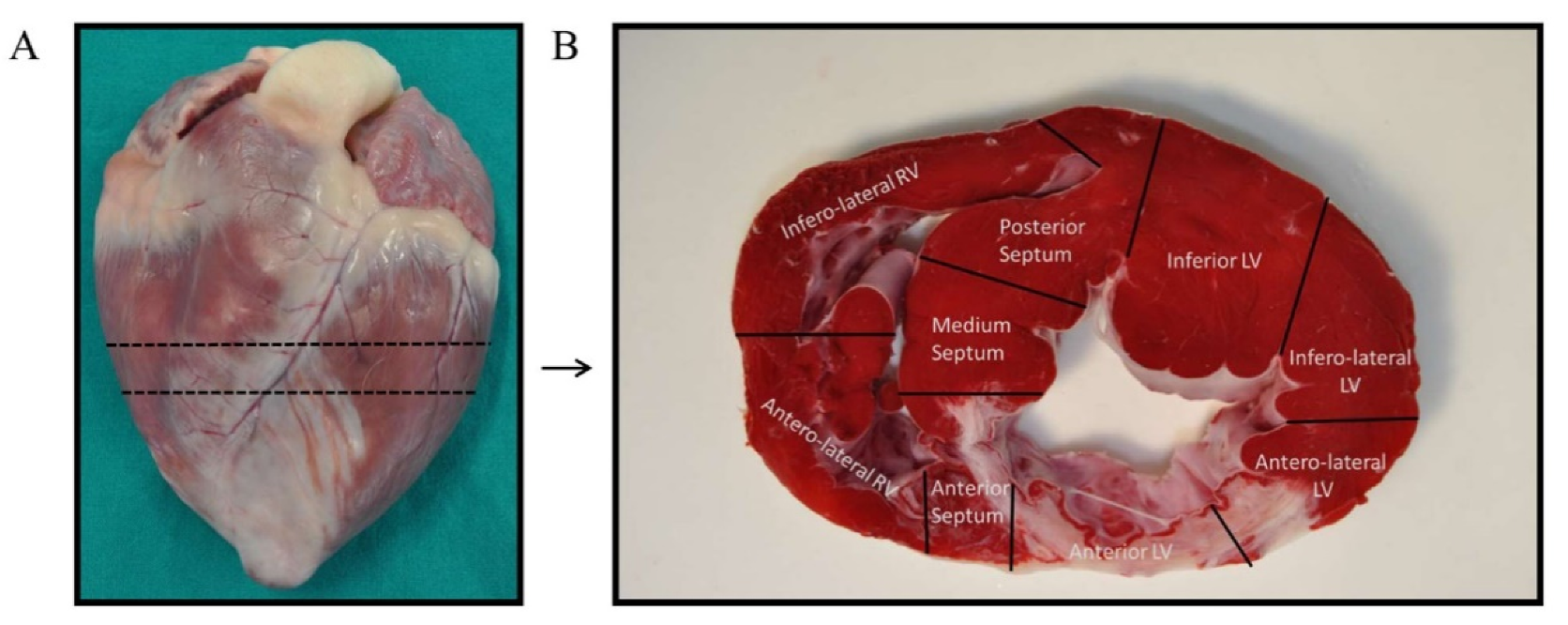
4. Materials and Methods
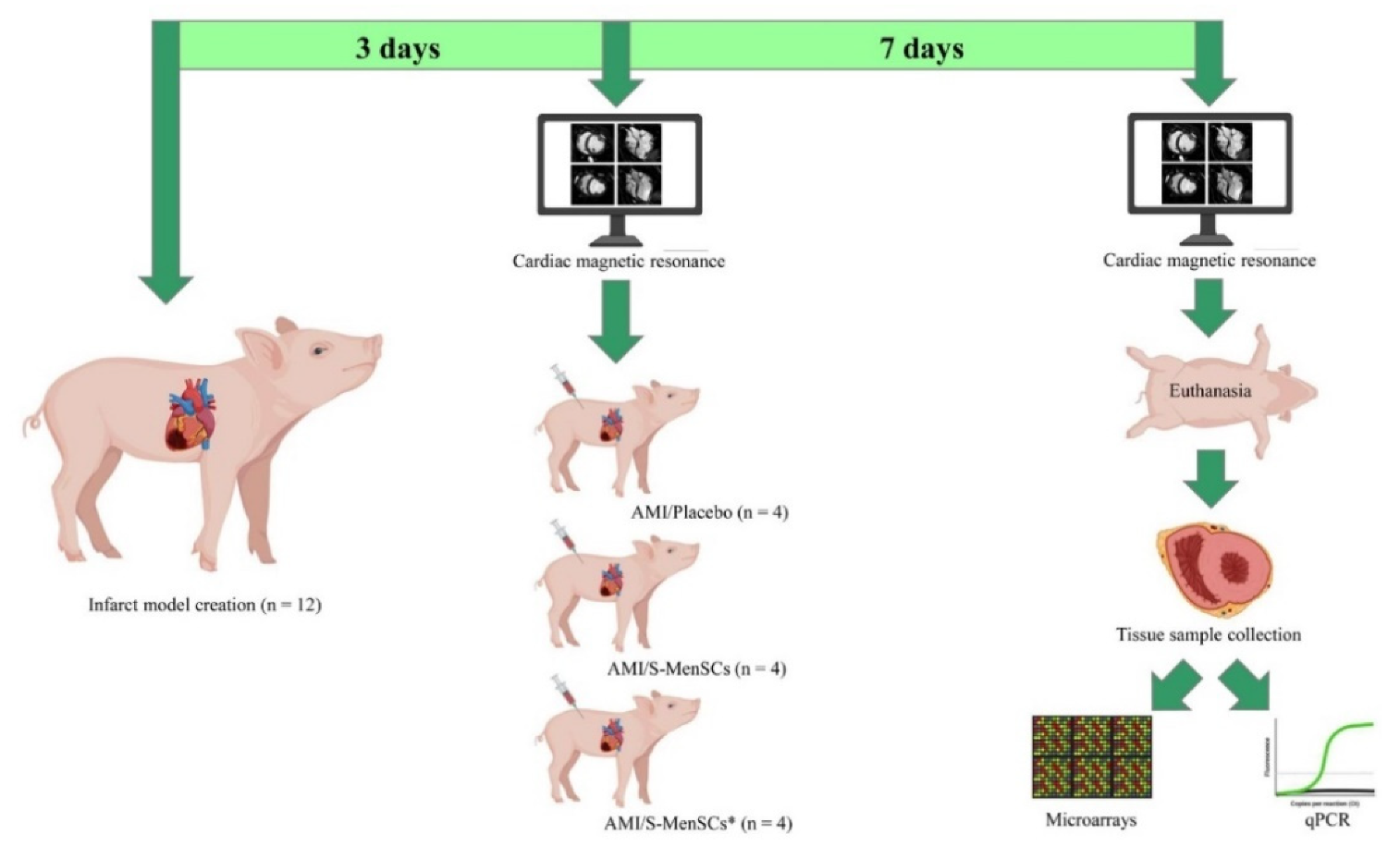
4.1. Study Design
4.2. Isolation, Preconditioning, and Characterization of S-MenSCs
4.3. AMI Model Creation and Monitoring
4.4. Intrapericardial Administration
4.5. Tissue Collection and RNA Isolation
4.6. Microarray Expression Analysis
4.7. Networks of miRNA and Gene Interactions
4.8. qPCR Analysis for Validation of Pre-Selected Transcripts
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hausenloy, D.J.; Yellon, D.M. Myocardial Ischemia-Reperfusion Injury: A Neglected Therapeutic Target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- van Zuylen, V.; den Haan, M.C.; Geutskens, S.B.; Roelofs, H.; Fibbe, W.E.; Schalij, M.J.; Atsma, D.E. Post-Myocardial Infarct Inflammation and the Potential Role of Cell Therapy. Cardiovasc. Drugs Ther. 2015, 29, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A Comparative Anatomic and Physiologic Overview of the Porcine Heart. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2014, 53, 432–438. [Google Scholar]
- Munz, M.R.; Faria, M.A.; Monteiro, J.R.; Aguas, A.P.; Amorim, M.J. Surgical Porcine Myocardial Infarction Model through Permanent Coronary Occlusion. Comp. Med. 2011, 61, 445–452. [Google Scholar] [PubMed]
- Dib, N.; Diethrich, E.B.; Campbell, A.; Gahremanpour, A.; McGarry, M.; Opie, S.R. A Percutaneous Swine Model of Myocardial Infarction. J. Pharmacol. Toxicol. Methods 2006, 53, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Crisóstomo, V.; Maestre, J.; Maynar, M.; Sun, F.; Báez-Díaz, C.; Usón, J.; Sánchez-Margallo, F.M. Development of a Closed Chest Model of Chronic Myocardial Infarction in Swine: Magnetic Resonance Imaging and Pathological Evaluation. ISRN Cardiol. 2013, 2013, 781762. [Google Scholar] [CrossRef][Green Version]
- Golubczyk, D.; Kalkowski, L.; Kwiatkowska, J.; Zawadzki, M.; Holak, P.; Glodek, J.; Milewska, K.; Pomianowski, A.; Janowski, M.; Adamiak, Z.; et al. Endovascular Model of Ischemic Stroke in Swine Guided by Real-Time MRI. Sci. Rep. 2020, 10, 17318. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Li, T.; Ma, L.; Fan, F.; Jin, Y.; Shen, J. The Whole Transcriptome and Proteome Changes in the Early Stage of Myocardial Infarction. Cell Death Discov. 2019, 5, 73. [Google Scholar] [CrossRef]
- Prat-Vidal, C.; Gálvez-Montón, C.; Nonell, L.; Puigdecanet, E.; Astier, L.; Solé, F.; Bayes-Genis, A. Identification of Temporal and Region-Specific Myocardial Gene Expression Patterns in Response to Infarction in Swine. PLoS ONE 2013, 8, e54785. [Google Scholar] [CrossRef]
- Binek, A.; Fernández-Jiménez, R.; Jorge, I.; Camafeita, E.; López, J.A.; Bagwan, N.; Galán-Arriola, C.; Pun, A.; Agüero, J.; Fuster, V.; et al. Proteomic Footprint of Myocardial Ischemia/Reperfusion Injury: Longitudinal Study of the at-Risk and Remote Regions in the Pig Model. Sci. Rep. 2017, 7, 12343. [Google Scholar] [CrossRef]
- Wu, R.; Gao, W.; Yao, K.; Ge, J. Roles of Exosomes Derived From Immune Cells in Cardiovascular Diseases. Front. Immunol. 2019, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- López, E.; Blázquez, R.; Marinaro, F.; Álvarez, V.; Blanco, V.; Báez, C.; González, I.; Abad, A.; Moreno, B.; Sánchez-Margallo, F.M.; et al. The Intrapericardial Delivery of Extracellular Vesicles from Cardiosphere-Derived Cells Stimulates M2 Polarization during the Acute Phase of Porcine Myocardial Infarction. Stem Cell Rev. Rep. 2020, 16, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel Cardiac Precursor-Like Cells from Human Menstrual Blood-Derived Mesenchymal Cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, Z.; Webster, K.A.; Chen, J.; Hu, H.; Zhou, Y.; Zhao, J.; Wang, L.; Wang, Y.; Zhong, Z.; et al. Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21: Superiority of Endometrium Mesenchymal Stem Cells. STEM CELLS Transl. Med. 2017, 6, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, V.; Sánchez-Margallo, F.M.; Macías-García, B.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; Blázquez, R.; Casado, J.G. The Immunomodulatory Activity of Extracellular Vesicles Derived from Endometrial Mesenchymal Stem Cells on CD4+ T Cells Is Partially Mediated by TGFbeta. J. Tissue Eng. Regen. Med. 2018, 12, 2088–2098. [Google Scholar] [CrossRef]
- Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Silla-Castro, J.C.; Vázquez, J.; Sánchez-Margallo, F.M.; Blázquez, R.; López, E.; Álvarez, V.; Casado, J.G. Unraveling the Molecular Signature of Extracellular Vesicles From Endometrial-Derived Mesenchymal Stem Cells: Potential Modulatory Effects and Therapeutic Applications. Front. Bioeng. Biotechnol. 2019, 7, 431. [Google Scholar] [CrossRef]
- de Pedro, M.Á.; Gómez-Serrano, M.; Marinaro, F.; López, E.; Pulido, M.; Preußer, C.; Pogge von Strandmann, E.; Sánchez-Margallo, F.M.; Álvarez, V.; Casado, J.G. IFN-Gamma and TNF-Alpha as a Priming Strategy to Enhance the Immunomodulatory Capacity of Secretomes from Menstrual Blood-Derived Stromal Cells. Int. J. Mol. Sci. 2021, 22, 12177. [Google Scholar] [CrossRef]
- Setubal, J.C.; Stadler, P.F. Gene Phylogenies and Orthologous Groups. In Comparative Genomics; Setubal, J.C., Stoye, J., Stadler, P.F., Eds.; Springer: New York, NY, USA, 2018; Volume 1704, pp. 1–28. ISBN 978-1-4939-7461-0. [Google Scholar]
- Biesbroek, P.S.; Amier, R.P.; Teunissen, P.F.A.; Hofman, M.B.M.; Robbers, L.F.H.J.; van de Ven, P.M.; Beek, A.M.; van Rossum, A.C.; van Royen, N.; Nijveldt, R. Changes in Remote Myocardial Tissue after Acute Myocardial Infarction and Its Relation to Cardiac Remodeling: A CMR T1 Mapping Study. PLoS ONE 2017, 12, e0180115. [Google Scholar] [CrossRef]
- Koudstaal, S.; Jansen of Lorkeers, S.; Gho, J.M.I.H.; van Hout, G.P.; Jansen, M.S.; Gründeman, P.F.; Pasterkamp, G.; Doevendans, P.A.; Hoefer, I.E.; Chamuleau, S.A.J. Myocardial Infarction and Functional Outcome Assessment in Pigs. J. Vis. Exp. 2014, 86, e51269. [Google Scholar] [CrossRef]
- Blázquez, R.; Álvarez, V.; Antequera-Barroso, J.A.; Báez-Díaz, C.; Blanco, V.; Maestre, J.; Moreno-Lobato, B.; López, E.; Marinaro, F.; Casado, J.G.; et al. Altered Hematological, Biochemical and Immunological Parameters as Predictive Biomarkers of Severity in Experimental Myocardial Infarction. Vet. Immunol. Immunopathol. 2018, 205, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; de Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes Secreted by Cardiosphere-Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2016, ehw240. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, L.; Wei, Y.; Krishnamurthy, P.; Walcott, G.P.; Menasché, P.; Zhang, J. Exosomes Secreted by HiPSC-Derived Cardiac Cells Improve Recovery from Myocardial Infarction in Swine. Sci. Transl. Med. 2020, 12, eaay1318. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell–Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Crisóstomo, V.; Báez, C.; Maestre, J.; García-Lindo, M.; Usón, A.; Álvarez, V.; Casado, J.G. Intrapericardial Administration of Mesenchymal Stem Cells in a Large Animal Model: A Bio-Distribution Analysis. PLoS ONE 2015, 10, e0122377. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, R.; Sánchez-Margallo, F.M.; Crisóstomo, V.; Báez, C.; Maestre, J.; Álvarez, V.; Casado, J.G. Intrapericardial Delivery of Cardiosphere-Derived Cells: An Immunological Study in a Clinically Relevant Large Animal Model. PLoS ONE 2016, 11, e0149001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally Invasive Delivery of Therapeutic Agents by Hydrogel Injection into the Pericardial Cavity for Cardiac Repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment–Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Lindsey, M.L.; Jin, Y.-F. Systems Analysis of Gene Ontology and Biological Pathways Involved in Post-Myocardial Infarction Responses. BMC Genom. 2015, 16, S18. [Google Scholar] [CrossRef]
- O’Sullivan, K.E.; Breen, E.P.; Gallagher, H.C.; Buggy, D.J.; Hurley, J.P. Understanding STAT3 Signaling in Cardiac Ischemia. Basic Res. Cardiol. 2016, 111, 27. [Google Scholar] [CrossRef]
- Harhous, Z.; Booz, G.W.; Ovize, M.; Bidaux, G.; Kurdi, M. An Update on the Multifaceted Roles of STAT3 in the Heart. Front. Cardiovasc. Med. 2019, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dézsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Jaafari, M.R.; Hatamipour, M.; Sahebkar, A. CD47: Role in the Immune System and Application to Cancer Therapy. Cell. Oncol. 2020, 43, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; Mallat, Z.; Booz, G.W.; Zouein, F.A. The CXCL10/CXCR3 Axis and Cardiac Inflammation: Implications for Immunotherapy to Treat Infectious and Noninfectious Diseases of the Heart. J. Immunol. Res. 2016, 2016, 4396368. [Google Scholar] [CrossRef]
- Mouton, A.J.; Ma, Y.; Rivera Gonzalez, O.J.; Daseke, M.J.; Flynn, E.R.; Freeman, T.C.; Garrett, M.R.; DeLeon-Pennell, K.Y.; Lindsey, M.L. Fibroblast Polarization over the Myocardial Infarction Time Continuum Shifts Roles from Inflammation to Angiogenesis. Basic Res. Cardiol. 2019, 114, 6. [Google Scholar] [CrossRef]
- Groover, M.K.; Richmond, J.M. Potential Therapeutic Manipulations of the CXCR3 Chemokine Axis for the Treatment of Inflammatory Fibrosing Diseases. F1000Research 2020, 9, 1197. [Google Scholar] [CrossRef]
- Chen, B.; Frangogiannis, N.G. Chemokines in Myocardial Infarction. J Cardiovasc. Transl. Res. 2021, 14, 35–52. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Wang, Z.; Sun, A.; Yang, X.; Qiu, Z.; Wu, C.; Zhang, W.; Li, H.; Zhang, Y.; et al. CXCR6 Deficiency Ameliorated Myocardial Ischemia/Reperfusion Injury by Inhibiting Infiltration of Monocytes and IFN-γ-Dependent Autophagy. Int. J. Cardiol. 2013, 168, 853–862. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, G.; Zhang, F.; Wu, J.; Xu, L.; Wang, Z.; Yin, P.; Wang, X.; You, J.; Yang, J.; et al. A Critical Role of CXCR6 in Murine Myocardial Ischemia-Reperfusion Injury through Activating IL-17a Producing INKT Cells. Int. J. Clin. Exp. Pathol. 2016, 9, 3513–3520. [Google Scholar]
- Woodcock, T.M.; Frugier, T.; Nguyen, T.T.; Semple, B.D.; Bye, N.; Massara, M.; Savino, B.; Besio, R.; Sobacchi, C.; Locati, M.; et al. The Scavenging Chemokine Receptor ACKR2 Has a Significant Impact on Acute Mortality Rate and Early Lesion Development after Traumatic Brain Injury. PLoS ONE 2017, 12, e0188305. [Google Scholar] [CrossRef]
- Cochain, C.; Auvynet, C.; Poupel, L.; Vilar, J.; Dumeau, E.; Richart, A.; Récalde, A.; Zouggari, Y.; Yin, K.Y.H.W.; Bruneval, P.; et al. The Chemokine Decoy Receptor D6 Prevents Excessive Inflammation and Adverse Ventricular Remodeling After Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, H.H.; Kiema, M.; Lappalainen, J.P.; Toropainen, A.; Beter, M.; Tirronen, A.; Holappa, L.; Niskanen, H.; Kaikkonen, M.U.; Ylä-Herttuala, S.; et al. BMP6/TAZ-Hippo Signaling Modulates Angiogenesis and Endothelial Cell Response to VEGF. Angiogenesis 2021, 24, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sibanda, V.L.; Zhang, H.-M.; Hu, H.; Liu, H.; Guo, A.-Y. MiRNA and TF Co-Regulatory Network Analysis for the Pathology and Recurrence of Myocardial Infarction. Sci. Rep. 2015, 5, 9653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.-F.; Morales, M.; Chou, J.; Chen, H.-L.; Chiang, Y.-H.; Lin, S.-Z.; Cadet, J.L.; Deng, X.; Wang, J.-Y.; et al. Bone Morphogenetic Protein-6 Reduces Ischemia-Induced Brain Damage in Rats. Stroke 2001, 32, 2170–2178. [Google Scholar] [CrossRef]
- Xu, W.; Barrientos, T.; Mao, L.; Rockman, H.A.; Sauve, A.A.; Andrews, N.C. Lethal Cardiomyopathy in Mice Lacking Transferrin Receptor in the Heart. Cell Rep. 2015, 13, 533–545. [Google Scholar] [CrossRef]
- Dai, W.; Kloner, R.A. Cardioprotection of Insulin-Like Growth Factor-1 During Reperfusion Therapy: What Is the Underlying Mechanism or Mechanisms? Circ. Cardiovasc. Interv. 2011, 4, 311–313. [Google Scholar] [CrossRef]
- O’Sullivan, J.F.; Leblond, A.-L.; Kelly, G.; Kumar, A.H.S.; Metharom, P.; Büneker, C.K.; Alizadeh-Vikali, N.; Hristova, I.; Hynes, B.G.; O’Connor, R.; et al. Potent Long-Term Cardioprotective Effects of Single Low-Dose Insulin-Like Growth Factor-1 Treatment Postmyocardial Infarction. Circ. Cardiovasc. Interv. 2011, 4, 327–335. [Google Scholar] [CrossRef]
- Haider, K.H.; Idris, N.M.; Kim, H.W.; Ahmed, R.P.H.; Shujia, J.; Ashraf, M. MicroRNA-21 Is a Key Determinant in IL-11/Stat3 Anti-Apoptotic Signalling Pathway in Preconditioning of Skeletal Myoblasts. Cardiovasc. Res. 2010, 88, 168–178. [Google Scholar] [CrossRef]
- Marinaro, F.; Macías-García, B.; Sánchez-Margallo, F.M.; Blázquez, R.; Álvarez, V.; Matilla, E.; Hernández, N.; Gómez-Serrano, M.; Jorge, I.; Vázquez, J.; et al. Extracellular Vesicles Derived from Endometrial Human Mesenchymal Stem Cells Enhance Embryo Yield and Quality in an Aged Murine Model†. Biol. Reprod. 2019, 100, 1180–1192. [Google Scholar] [CrossRef]
- Crisostomo, V.; Baez-Diaz, C.; Maestre, J.; Garcia-Lindo, M.; Sun, F.; Casado, J.G.; Blazquez, R.; Abad, J.L.; Palacios, I.; Rodriguez-Borlado, L.; et al. Delayed Administration of Allogeneic Cardiac Stem Cell Therapy for Acute Myocardial Infarction Could Ameliorate Adverse Remodeling: Experimental Study in Swine. J. Transl. Med. 2015, 13, 156. [Google Scholar] [CrossRef]
- Galati, G.; Leone, O.; Pasquale, F.; Olivotto, I.; Biagini, E.; Grigioni, F.; Pilato, E.; Lorenzini, M.; Corti, B.; Foà, A.; et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy: A Clinical-Pathological Study of 30 Explanted Hearts. Circ. Heart Fail. 2016, 9, e003090. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, R.A. Exploration, Normalization, and Summaries of High Density Oligonucleotide Array Probe Level Data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.S.; Irizarry, R.A. A Framework for Oligonucleotide Microarray Preprocessing. Bioinformatics 2010, 26, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- de Jonge, H.J.M.; Fehrmann, R.S.N.; de Bont, E.S.J.M.; Hofstra, R.M.W.; Gerbens, F.; Kamps, W.A.; de Vries, E.G.E.; van der Zee, A.G.J.; te Meerman, G.J.; ter Elst, A. Evidence Based Selection of Housekeeping Genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pedro, M.Á.; Pulido, M.; Marinaro, F.; Álvarez, V.; Báez-Díaz, C.; Blanco, V.; Silla-Castro, J.C.; Sanchez-Cabo, F.; Sánchez-Margallo, F.M.; Crisóstomo, V.; et al. Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction. Biomedicines 2022, 10, 1117. https://doi.org/10.3390/biomedicines10051117
de Pedro MÁ, Pulido M, Marinaro F, Álvarez V, Báez-Díaz C, Blanco V, Silla-Castro JC, Sanchez-Cabo F, Sánchez-Margallo FM, Crisóstomo V, et al. Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction. Biomedicines. 2022; 10(5):1117. https://doi.org/10.3390/biomedicines10051117
Chicago/Turabian Stylede Pedro, María Ángeles, María Pulido, Federica Marinaro, Verónica Álvarez, Claudia Báez-Díaz, Virginia Blanco, Juan Carlos Silla-Castro, Fátima Sanchez-Cabo, Francisco Miguel Sánchez-Margallo, Verónica Crisóstomo, and et al. 2022. "Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction" Biomedicines 10, no. 5: 1117. https://doi.org/10.3390/biomedicines10051117
APA Stylede Pedro, M. Á., Pulido, M., Marinaro, F., Álvarez, V., Báez-Díaz, C., Blanco, V., Silla-Castro, J. C., Sanchez-Cabo, F., Sánchez-Margallo, F. M., Crisóstomo, V., Casado, J. G., & López, E. (2022). Intrapericardial Administration of Secretomes from Menstrual Blood-Derived Mesenchymal Stromal Cells: Effects on Immune-Related Genes in a Porcine Model of Myocardial Infarction. Biomedicines, 10(5), 1117. https://doi.org/10.3390/biomedicines10051117







