Cathelicidin LL-37 in Health and Diseases of the Oral Cavity
Abstract
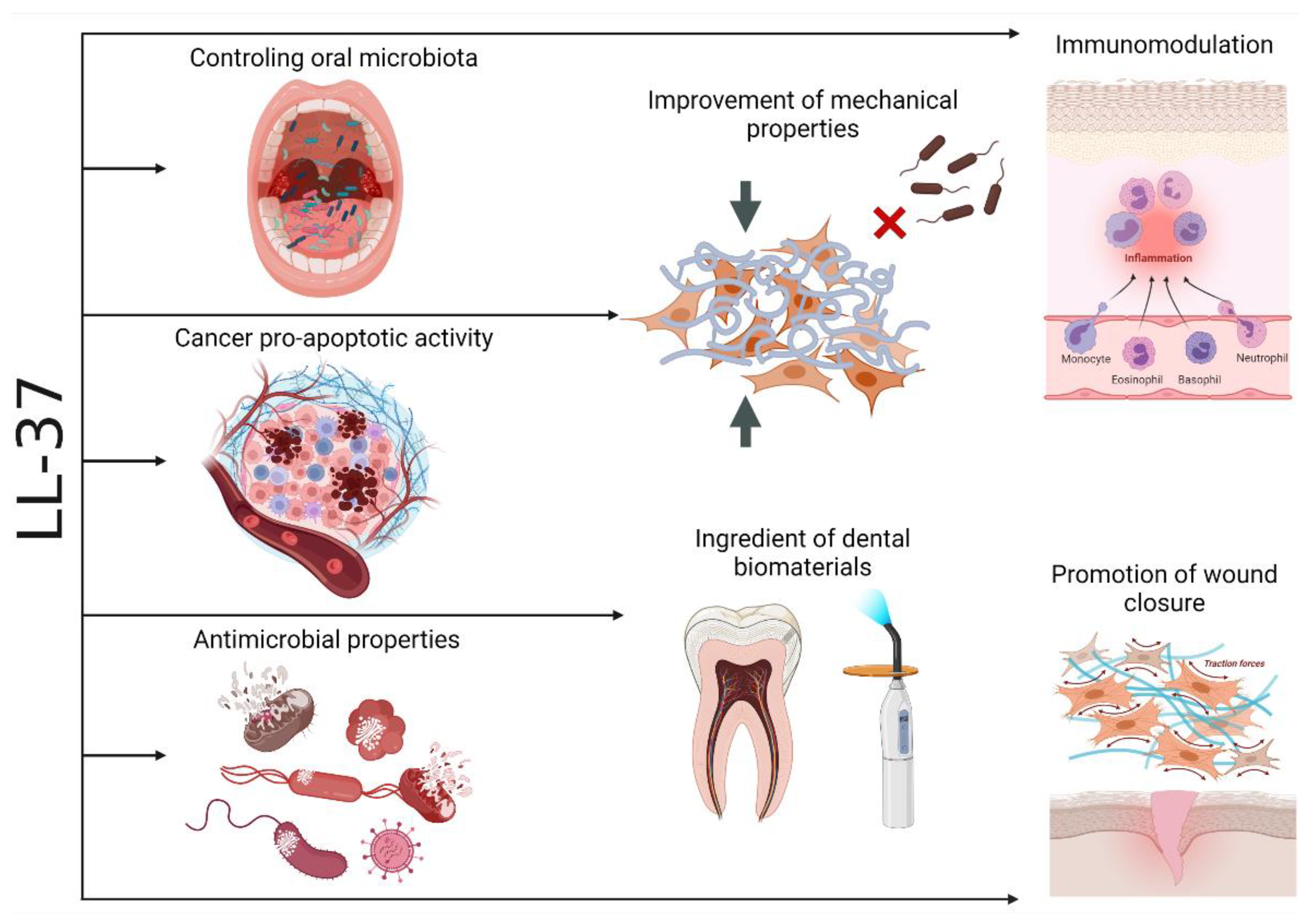
1. Introduction
2. Mechanism of LL-37 Expression in the Oral Cavity and Its Action at the Molecular Level
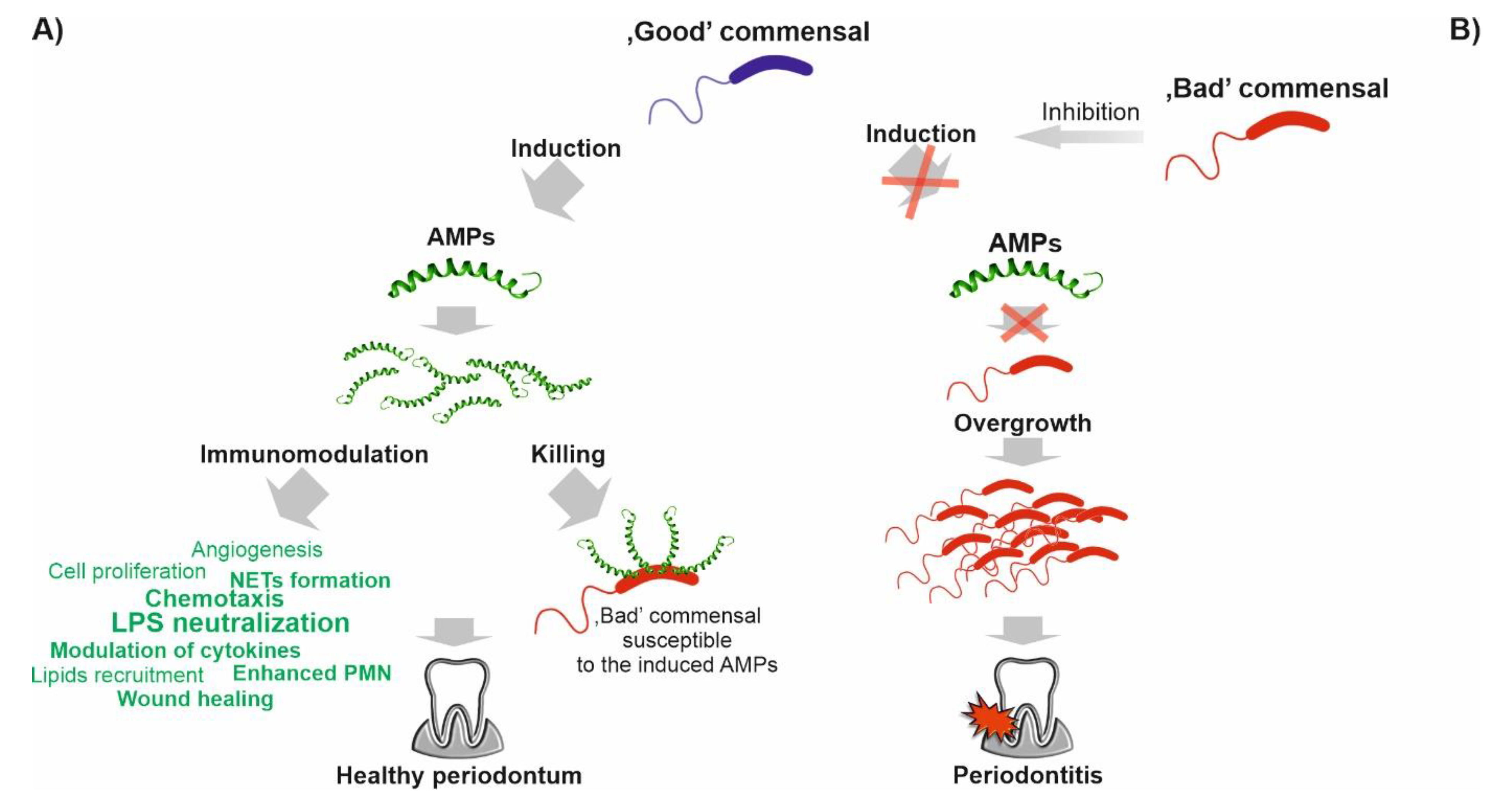
3. Involvement of the LL-37 Peptide in Maintaining Homeostasis of Oral Microbiota
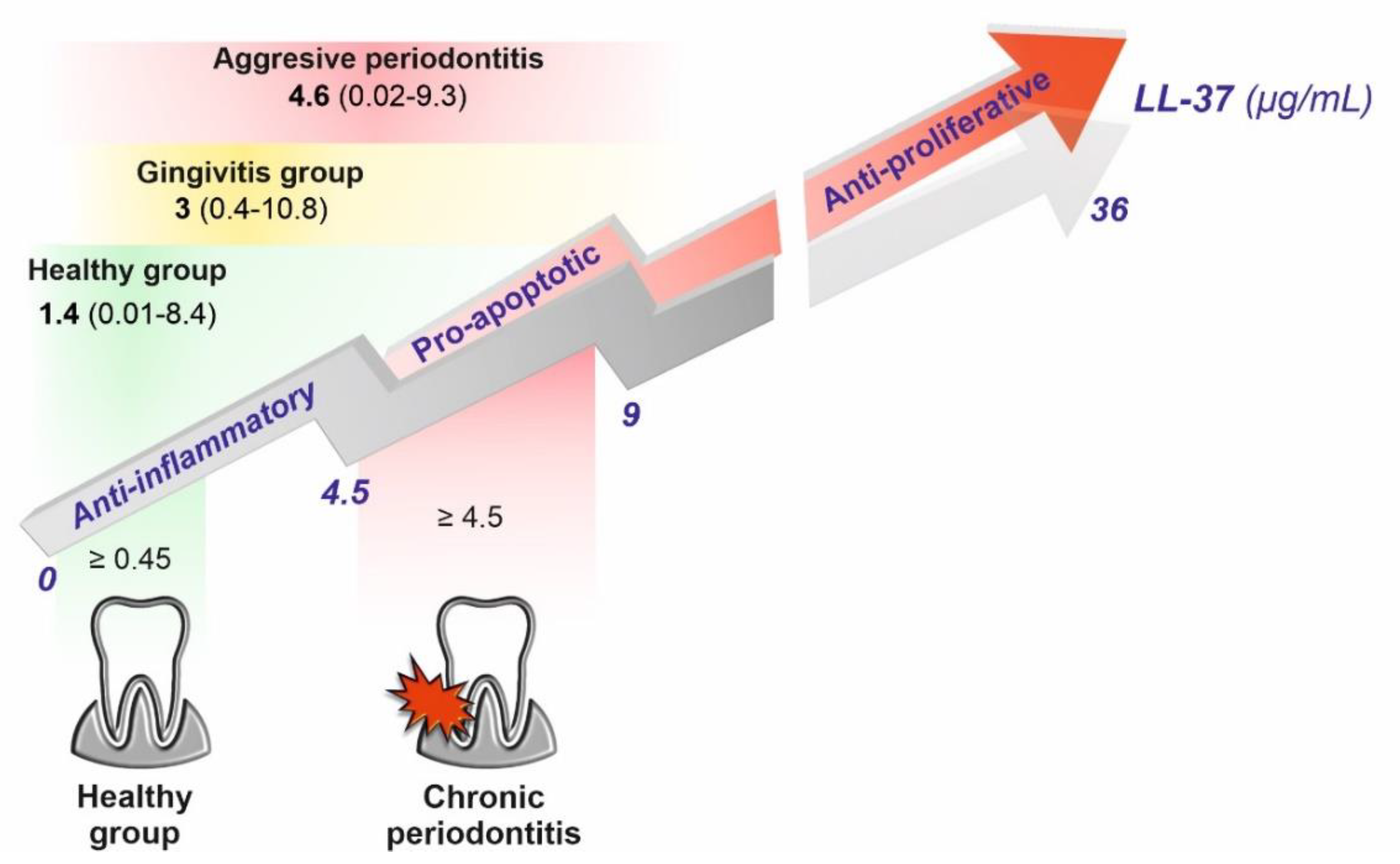
4. LL-37 Peptide in Dental Caries, Pulpitis, Refractory Apical Periodontitis, and Periodontal Diseases
5. LL-37 Peptide in Diseases of Oral Mucosa and Its Implication in Oral Cancer Development
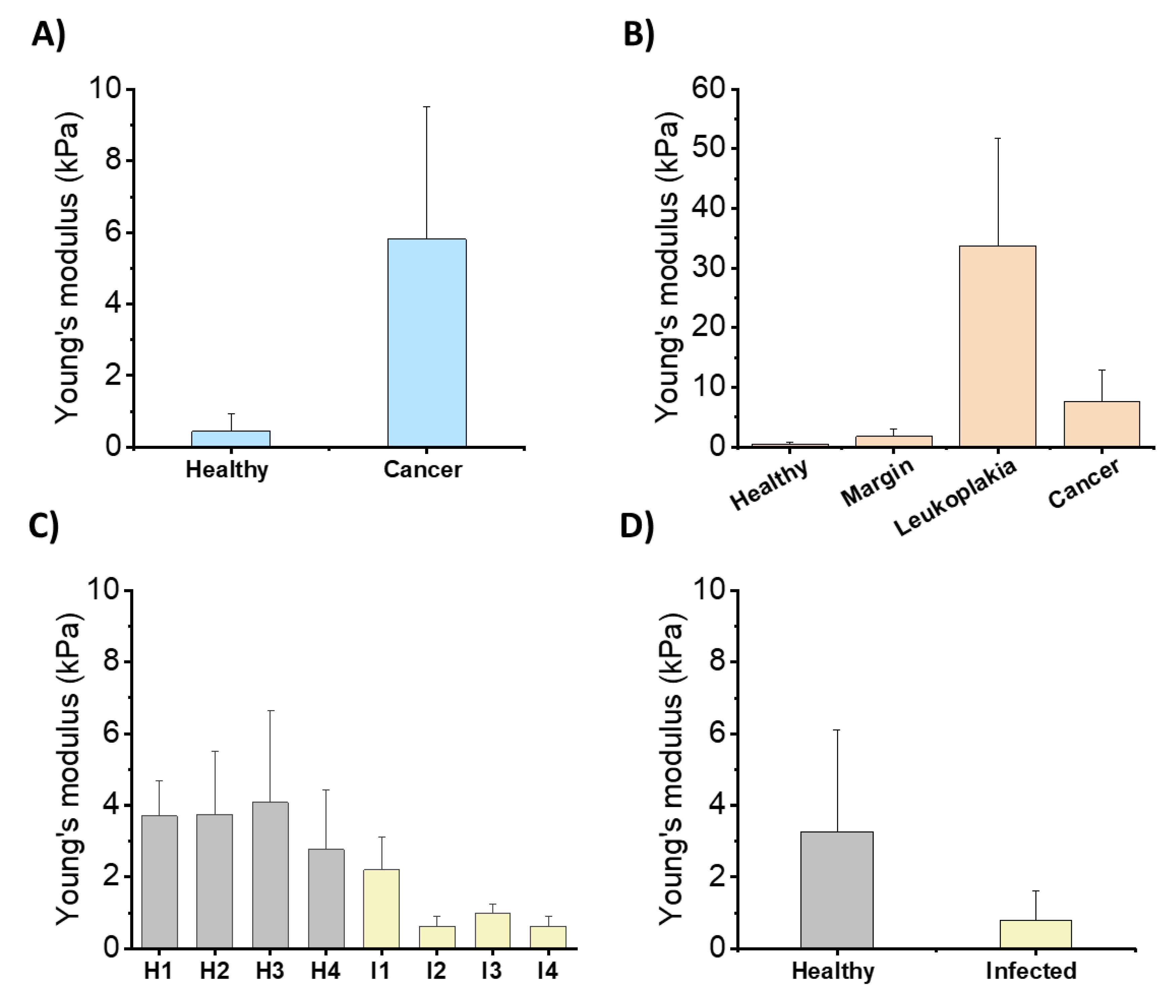
6. LL-37 Peptide as a Guardian of Oral Mucosa Mechanical Properties (LL-37 in Saliva and Saliva–Mucosal Surface Interference)
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Gorr, S.U. Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 2012, 15, 84–98. [Google Scholar] [CrossRef]
- Gorr, S.U.; Abdolhosseini, M. Antimicrobial peptides and periodontal disease. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 126–141. [Google Scholar] [CrossRef]
- Lin, B.; Li, R.; Handley, T.N.G.; Wade, J.D.; Li, W.; O’Brien-Simpson, N.M. Cationic Antimicrobial Peptides Are Leading the Way to Combat Oropathogenic Infections. ACS Infect. Dis. 2021, 7, 2959–2970. [Google Scholar] [CrossRef]
- Prasad, S.V.; Fiedoruk, K.; Daniluk, T.; Piktel, E.; Bucki, R. Expression and Function of Host Defense Peptides at Inflammation Sites. Int. J. Mol. Sci. 2019, 21, 104. [Google Scholar] [CrossRef]
- da Silva, B.R.; de Freitas, V.A.; Nascimento-Neto, L.G.; Carneiro, V.A.; Arruda, F.V.; de Aguiar, A.S.; Cavada, B.S.; Teixeira, E.H. Antimicrobial peptide control of pathogenic microorganisms of the oral cavity: A review of the literature. Peptides 2012, 36, 315–321. [Google Scholar] [CrossRef]
- Cole, A.M.; Liao, H.I.; Stuchlik, O.; Tilan, J.; Pohl, J.; Ganz, T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J. Immunol. 2002, 169, 6985–6991. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Chapple, I.L.; Working Group 3 of Seventh European Workshop on Periodontology. Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 114–118. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Uehara, A.; Fujimoto, Y.; Fukase, K.; Takada, H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 2007, 44, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Diana, J. The Dual Role of Antimicrobial Peptides in Autoimmunity. Front. Immunol. 2020, 11, 2077. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Feng, Z.; Fujioka, H.; Lux, R.; McCormick, T.S.; Weinberg, A. Conceptual Perspectives: Bacterial Antimicrobial Peptide Induction as a Novel Strategy for Symbiosis with the Human Host. Front. Microbiol. 2018, 9, 302. [Google Scholar] [CrossRef]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. Adv. Exp. Med. Biol. 2019, 1117, 215–240. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Sidorowicz, A.; Mikosinski, J.; Krzyżanowski, M.; Orleanski, J.; Twardowska-Saucha, K.; Nykaza, A.; Dyaczynski, M.; Belz-Lagoda, B.; Dziwiszek, G.; et al. Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial. Wound Repair Regen. 2021, 29, 938–950. [Google Scholar] [CrossRef]
- Peek, N.; Nell, M.J.; Brand, R.; Jansen-Werkhoven, T.; van Hoogdalem, E.J.; Verrijk, R.; Vonk, M.J.; Wafelman, A.R.; Valentijn, A.; Frijns, J.H.M.; et al. Ototopical drops containing a novel antibacterial synthetic peptide: Safety and efficacy in adults with chronic suppurative otitis media. PLoS ONE 2020, 15, e0231573. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R.; et al. Significance of LL-37 on Immunomodulation and Disease Outcome. Biomed. Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef] [PubMed]
- Samaras, P.; Schmidt, T.; Frejno, M.; Gessulat, S.; Reinecke, M.; Jarzab, A.; Zecha, J.; Mergner, J.; Giansanti, P.; Ehrlich, H.C.; et al. ProteomicsDB: A multi-omics and multi-organism resource for life science research. Nucleic Acids Res. 2020, 48, D1153–D1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Herrmann, C.J.; Simonovic, M.; Szklarczyk, D.; von Mering, C. Version 4.0 of PaxDb: Protein abundance data, integrated across model organisms, tissues, and cell-lines. Proteomics 2015, 15, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Schlegl, J.; Hahne, H.; Gholami, A.M.; Lieberenz, M.; Savitski, M.M.; Ziegler, E.; Butzmann, L.; Gessulat, S.; Marx, H.; et al. Mass-spectrometry-based draft of the human proteome. Nature 2014, 509, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Leszczyńska, K.; Namiot, A.; Sokołowski, W. Cathelicidin LL-37: A multitask antimicrobial peptide. Arch. Immunol. Exp. 2010, 58, 15–25. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The human cathelicidin LL-37--A pore-forming antibacterial peptide and host-cell modulator. Biochim. Biophys. Acta 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Henderson, B.; Martin, A.C. Protein moonlighting: A new factor in biology and medicine. Biochem. Soc. Trans. 2014, 42, 1671–1678. [Google Scholar] [CrossRef]
- Verjans, E.T.; Zels, S.; Luyten, W.; Landuyt, B.; Schoofs, L. Molecular mechanisms of LL-37-induced receptor activation: An overview. Peptides 2016, 85, 16–26. [Google Scholar] [CrossRef]
- Dang, X.; Wang, G. Spotlight on the Selected New Antimicrobial Innate Immune Peptides Discovered During 2015-2019. Curr. Top. Med. Chem. 2020, 20, 2984–2998. [Google Scholar] [CrossRef]
- Murakami, M.; Kameda, K.; Tsumoto, H.; Tsuda, T.; Masuda, K.; Utsunomiya, R.; Mori, H.; Miura, Y.; Sayama, K. TLN-58, an Additional hCAP18 Processing Form, Found in the Lesion Vesicle of Palmoplantar Pustulosis in the Skin. J. Investig. Derm. 2017, 137, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution Structures of Human LL-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, M.; Enciso, M.; Adda, C.G.; Smith, B.J.; Perugini, M.A.; Mechler, A. Membrane Core-Specific Antimicrobial Action of Cathelicidin LL-37 Peptide Switches Between Pore and Nanofibre Formation. Sci. Rep. 2016, 6, 38184. [Google Scholar] [CrossRef] [PubMed]
- Zeth, K.; Sancho-Vaello, E. Structural Plasticity of LL-37 Indicates Elaborate Functional Adaptation Mechanisms to Bacterial Target Structures. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Zamlynny, V.; Pieta, I.S.; Nowakowski, R.; Pieta, P. Interaction of LL-37 human cathelicidin peptide with a model microbial-like lipid membrane. Bioelectrochemistry 2021, 141, 107842. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, Y.; Landau, M. The Human LL-37(17-29) antimicrobial peptide reveals a functional supramolecular structure. Nat. Commun. 2020, 11, 3894. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.-J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The structure of the antimicrobial human cathelicidin LL-37 shows oligomerization and channel formation in the presence of membrane mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Vaello, E.; François, P.; Bonetti, E.J.; Lilie, H.; Finger, S.; Gil-Ortiz, F.; Gil-Carton, D.; Zeth, K. Structural remodeling and oligomerization of human cathelicidin on membranes suggest fibril-like structures as active species. Sci. Rep. 2017, 7, 15371. [Google Scholar] [CrossRef]
- Lee, E.Y.; Zhang, C.; Di Domizio, J.; Jin, F.; Connell, W.; Hung, M.; Malkoff, N.; Veksler, V.; Gilliet, M.; Ren, P.; et al. Helical antimicrobial peptides assemble into protofibril scaffolds that present ordered dsDNA to TLR9. Nat. Commun. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.A.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Neumann, A.; Völlger, L.; Berends, E.T.M.; Molhoek, E.M.; Stapels, D.A.C.; Midon, M.; Friães, A.; Pingoud, A.; Rooijakkers, S.H.M.; Gallo, R.L.; et al. Novel Role of the Antimicrobial Peptide LL-37 in the Protection of Neutrophil Extracellular Traps against Degradation by Bacterial Nucleases. J. Innate Immun. 2014, 6, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Bryzek, D.; Golda, A.; Budziaszek, J.; Kowalczyk, D.; Wong, A.; Bielecka, E.; Shakamuri, P.; Svoboda, P.; Pohl, J.; Potempa, J.; et al. Citrullination-Resistant LL-37 Is a Potent Antimicrobial Agent in the Inflammatory Environment High in Arginine Deiminase Activity. Int. J. Mol. Sci. 2020, 21, 9126. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Namiot, D.B.; Namiot, Z.; Savage, P.B.; Janmey, P.A. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J. Antimicrob. Chemother. 2008, 62, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Gutner, M.; Chaushu, S.; Balter, D.; Bachrach, G. Saliva enables the antimicrobial activity of LL-37 in the presence of proteases of Porphyromonas gingivalis. Infect. Immun. 2009, 77, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, O.; Emingil, G.; Eren, G.; Atmaca, H.; Kutukculer, N.; Atilla, G. Gingival crevicular fluid and serum hCAP18/LL-37 levels in generalized aggressive periodontitis. Clin. Oral Investig. 2017, 21, 763–769. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Huth, K.C.; Hickel, R.; Kollmuss, M. Inhibitory effect of LL-37 and human lactoferricin on growth and biofilm formation of anaerobes associated with oral diseases. Anaerobe 2021, 67, 102301. [Google Scholar] [CrossRef]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Horibe, K.; Takahashi, N.; Udagawa, N. Roles of cathelicidins in inflammation and bone loss. Odontology 2014, 102, 137–146. [Google Scholar] [CrossRef]
- Scott, A.; Weldon, S.; Buchanan, P.J.; Schock, B.; Ernst, R.K.; McAuley, D.F.; Tunney, M.M.; Irwin, C.R.; Elborn, J.S.; Taggart, C.C. Evaluation of the Ability of LL-37 to Neutralise LPS In Vitro and Ex Vivo. PLoS ONE 2011, 6, e26525. [Google Scholar] [CrossRef]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The Relationship between Vitamin D and Periodontal Pathology. Medicina 2018, 54, 45. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Facchetti, F. TREM-1 (triggering receptor expressed on myeloid cells): A new player in acute inflammatory responses. J. Infect. Dis. 2003, 187 (Suppl. 2), S397–S401. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y. Innate immune response to oral bacteria and the immune evasive characteristics of periodontal pathogens. J. Periodontal Implant Sci. 2013, 43, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Hyun, J.; Park, E.; Lee, B.L.; Kim, K.K.; Choi, Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodontal Res. 2007, 42, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Kim, Y.; Min, B.M.; Han, S.H.; Choi, Y. Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J. Periodontal Res. 2007, 42, 503–510. [Google Scholar] [CrossRef]
- Leszczynska, K.; Namiot, D.; Byfield, F.J.; Cruz, K.; Zendzian-Piotrowska, M.; Fein, D.E.; Savage, P.B.; Diamond, S.; McCulloch, C.A.; Janmey, P.A.; et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J. Antimicrob. Chemother. 2013, 68, 610–618. [Google Scholar] [CrossRef]
- Ouhara, K.; Komatsuzawa, H.; Yamada, S.; Shiba, H.; Fujiwara, T.; Ohara, M.; Sayama, K.; Hashimoto, K.; Kurihara, H.; Sugai, M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J. Antimicrob. Chemother. 2005, 55, 888–896. [Google Scholar] [CrossRef]
- Altman, H.; Steinberg, D.; Porat, Y.; Mor, A.; Fridman, D.; Friedman, M.; Bachrach, G. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 2006, 58, 198–201. [Google Scholar] [CrossRef]
- Sol, A.; Ginesin, O.; Chaushu, S.; Karra, L.; Coppenhagen-Glazer, S.; Ginsburg, I.; Bachrach, G. LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations. Infect. Immun. 2013, 81, 3577–3585. [Google Scholar] [CrossRef]
- Horie, T.; Inomata, M.; Into, T. OmpA-Like Proteins of Porphyromonas gingivalis Mediate Resistance to the Antimicrobial Peptide LL-37. J. Pathog. 2018, 2018, 2068435. [Google Scholar] [CrossRef] [PubMed]
- Pütsep, K.; Carlsson, G.; Boman, H.G.; Andersson, M. Deficiency of antibacterial peptides in patients with morbus Kostmann: An observation study. Lancet 2002, 360, 1144–1149. [Google Scholar] [CrossRef]
- Tanaka, D.; Miyasaki, K.T.; Lehrer, R.I. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: A cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 2000, 15, 226–231. [Google Scholar] [CrossRef]
- Rosenfeld, Y.; Papo, N.; Shai, Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J. Biol. Chem. 2006, 281, 1636–1643. [Google Scholar] [CrossRef]
- Sambri, V.; Marangoni, A.; Giacani, L.; Gennaro, R.; Murgia, R.; Cevenini, R.; Cinco, M. Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J. Antimicrob. Chemother. 2002, 50, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, L.; Pan, Y.; Feng, X.; Liu, J.; Guo, Y.; Pan, C.; Geng, F.; Tang, X. LL-37-Induced Autophagy Contributed to the Elimination of Live Porphyromonas gingivalis Internalized in Keratinocytes. Front. Cell. Infect. Microbiol. 2020, 10, 561761. [Google Scholar] [CrossRef]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef]
- Ahmad, S.M.; Bhattacharyya, P.; Jeffries, N.; Gisselbrecht, S.S.; Michelson, A.M. Two Forkhead transcription factors regulate cardiac progenitor specification by controlling the expression of receptors of the fibroblast growth factor and Wnt signaling pathways. Development 2016, 143, 306–317. [Google Scholar] [CrossRef]
- Darveau, R.P.; Belton, C.M.; Reife, R.A.; Lamont, R.J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 1998, 66, 1660–1665. [Google Scholar] [CrossRef]
- Bachrach, G.; Altman, H.; Kolenbrander, P.E.; Chalmers, N.I.; Gabai-Gutner, M.; Mor, A.; Friedman, M.; Steinberg, D. Resistance of Porphyromonas gingivalis ATCC 33277 to direct killing by antimicrobial peptides is protease independent. Antimicrob. Agents Chemother. 2008, 52, 638–642. [Google Scholar] [CrossRef]
- Laugisch, O.; Schacht, M.; Guentsch, A.; Kantyka, T.; Sroka, A.; Stennicke, H.R.; Pfister, W.; Sculean, A.; Potempa, J.; Eick, S. Periodontal pathogens affect the level of protease inhibitors in gingival crevicular fluid. Mol. Oral Microbiol. 2012, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Velarde, J.J.; Ashbaugh, M.; Wessels, M.R. The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor histidine kinase of group A Streptococcus, to activate expression of virulence factors. J. Biol. Chem. 2014, 289, 36315–36324. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, O.; Scheb-Wetzel, M.; Rohde, M.; Bravo, A. New Insights into the Antimicrobial Effect. Infect. Immun. 2014, 82, 4496. [Google Scholar]
- Hsu, C.-M.; Liao, Y.-L.; Chang, C.-K.; Lan, C.-Y. Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37. Int. J. Mol. Sci. 2021, 22, 10633. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G. Antimicrobial peptides (AMPs) with dual mechanisms: Membrane disruption and apoptosis. J. Microbiol. Biotechnol. 2015, 25, 759–764. [Google Scholar] [CrossRef]
- Nireeksha; Varma, S.R.; Damdoum, M.; Alsaegh, M.A.; Hegde, M.N.; Kumari, S.N.; Ramamurthy, S.; Narayanan, J.; Imran, E.; Shabbir, J.; et al. Immunomodulatory Expression of Cathelicidins Peptides in Pulp Inflammation and Regeneration: An Update. Curr. Issues Mol. Biol. 2021, 43, 116–126. [Google Scholar] [CrossRef]
- Dale, B.A.; Fredericks, L.P. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr. Issues Mol. Biol. 2005, 7, 119–134. [Google Scholar]
- Davidopoulou, S.; Diza, E.; Sakellari, D.; Menexes, G.; Kalfas, S. Salivary concentration of free LL-37 in edentulism, chronic periodontitis and healthy periodontium. Arch. Oral Biol. 2013, 58, 930–934. [Google Scholar] [CrossRef]
- Offenbacher, S. Periodontal diseases: Pathogenesis. Ann. Periodontol. 1996, 1, 821–878. [Google Scholar] [CrossRef]
- Li, Y.-H.; Huang, X.; Tian, X.-L. Recent advances in dental biofilm: Impacts of microbial interactions on the biofilm ecology and pathogenesis. Aims Bioeng. 2017, 4, 335–350. [Google Scholar] [CrossRef]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental plaque biofilms: Communities, conflict and control. Periodontology 2000 2011, 55, 16–35. [Google Scholar] [CrossRef]
- Guo, L.; He, X.; Shi, W. Intercellular communications in multispecies oral microbial communities. Front. Microbiol. 2014, 5, 328. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, S.; Diza, E.; Menexes, G.; Kalfas, S. Salivary concentration of the antimicrobial peptide LL-37 in children. Arch. Oral Biol. 2012, 57, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Thiruvanamalai Sivakumar, A.R.H.; Mednieks, M. Secretory proteins in the saliva of children. J. Oral Sci. 2009, 51, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.C.; Evans, C.A.; Crosby, T.R.; Mednieks, M.I. Expression of secretory proteins in oral fluid after orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2002, 121, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.; Sherriff, A.; Lappin, D.F.; Ramage, G.; Conway, D.I.; Macpherson, L.M.; Culshaw, S. Salivary antimicrobial proteins associate with age-related changes in streptococcal composition in dental plaque. Mol. Oral Microbiol. 2014, 29, 284–293. [Google Scholar] [CrossRef]
- Colombo, N.H.; Ribas, L.F.; Pereira, J.A.; Kreling, P.F.; Kressirer, C.A.; Tanner, A.C.; Duque, C. Antimicrobial peptides in saliva of children with severe early childhood caries. Arch. Oral Biol. 2016, 69, 40–46. [Google Scholar] [CrossRef]
- Phattarataratip, E.; Olson, B.; Broffitt, B.; Qian, F.; Brogden, K.A.; Drake, D.R.; Levy, S.M.; Banas, J.A. Streptococcus mutans strains recovered from caries-active or caries-free individuals differ in sensitivity to host antimicrobial peptides. Mol. Oral Microbiol. 2011, 26, 187–199. [Google Scholar] [CrossRef]
- Puklo, M.; Guentsch, A.; Hiemstra, P.S.; Eick, S.; Potempa, J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol. Immunol. 2008, 23, 328–335. [Google Scholar] [CrossRef]
- Koziel, J.; Karim, A.Y.; Przybyszewska, K.; Ksiazek, M.; Rapala-Kozik, M.; Nguyen, K.-A.; Potempa, J. Proteolytic inactivation of LL-37 by karilysin, a novel virulence mechanism of Tannerella forsythia. J. Innate Immun. 2010, 2, 288–293. [Google Scholar] [CrossRef]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.; Veerman, E.C. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; O’Donnell, K.; Irwin, C.R.; Lundy, F.T. Effects of LL-37 on gingival fibroblasts: A role in periodontal tissue remodeling? Vaccines 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, N.; Krunić, J.; Popović, B.; Stojičić, S.; Živković, S. Prevalence of Enterococcus faecalis and Porphyromonas gingivalis in infected root canals and their susceptibility to endodontic treatment procedures: A molecular study. Srp. Arh. Za Celok. Lek. 2014, 142, 535–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, D.; Davies, J.K.; Figdor, D. Starvation survival and recovery in serum of Candida albicans compared with Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 125–130. [Google Scholar] [CrossRef]
- Jönsson, D.; Nilsson, B.O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 330–335. [Google Scholar] [CrossRef]
- Harder, J.; Schröder, J.-M. Antimicrobial Peptides: Role in Human Health and Disease; Springer: Berlin/Heidelberg, Germany, 2016; p. 158. [Google Scholar]
- Yu, X.; Quan, J.; Long, W.; Chen, H.; Wang, R.; Guo, J.; Lin, X.; Mai, S. LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp. Cell Res. 2018, 372, 178–187. [Google Scholar] [CrossRef]
- 10Liu, Z.; Yuan, X.; Liu, M.; Fernandes, G.; Zhang, Y.; Yang, S.; Ionita, C.N. Antimicrobial Peptide Combined with BMP2-Modified Mesenchymal Stem Cells Promotes Calvarial Repair in an Osteolytic Model. Mol. Ther. 2018, 26, 199–207. [Google Scholar] [CrossRef]
- 10Cheng, Q.; Zeng, K.; Kang, Q.; Qian, W.; Zhang, W.; Gan, Q.; Xia, W. The antimicrobial peptide LL-37 promotes migration and odonto/osteogenic differentiation of stem cells from the apical papilla through the AKT/Wnt/β-catenin signaling pathway. J. Endod. 2020, 46, 964–972. [Google Scholar]
- Kajiya, M.; Shiba, H.; Komatsuzawa, H.; Ouhara, K.; Fujita, T.; Takeda, K.; Uchida, Y.; Mizuno, N.; Kawaguchi, H.; Kurihara, H. The antimicrobial peptide LL37 induces the migration of human pulp cells: A possible adjunct for regenerative endodontics. J. Endod. 2010, 36, 1009–1013. [Google Scholar] [CrossRef]
- Sarmiento, B.F.; Aminoshariae, A.; Bakkar, M.; Bonfield, T.; Ghosh, S.; Montagnese, T.A.; Mickel, A.K. The Expression of the Human Cathelicidin LL- 37 in the Human Dental Pulp: An In Vivo St. Int. J. Pharm. 2016, 1, 5. [Google Scholar] [CrossRef]
- Khung, R.; Shiba, H.; Kajiya, M.; Kittaka, M.; Ouhara, K.; Takeda, K.; Mizuno, N.; Fujita, T.; Komatsuzawa, H.; Kurihara, H. LL37 induces VEGF expression in dental pulp cells through ERK signalling. Int. Endod. J. 2015, 48, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Milhan, N.V.M.; de Barros, P.P.; de Lima Zutin, E.A.; de Oliveira, F.E.; Camargo, C.H.R.; Camargo, S.E.A. The Antimicrobial Peptide LL-37 as a Possible Adjunct for the Proliferation and Differentiation of Dental Pulp Stem Cells. J. Endod. 2017, 43, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Horibe, K.; Hosoya, A.; Hiraga, T.; NAkamura, H. Expression and localization of CRAMP in rat tooth germ and during reparative dentin formation. Clin. Oral Investig. 2018, 22, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, O.; Emingil, G.; Kütükçüler, N.; Atilla, G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J. Periodontol. 2009, 80, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, A.B.; Behm, C. A Review of Antimicrobial Activity of Dental Mesenchymal Stromal Cells: Is There Any Potential? Front. Oral Health 2021, 2, 832976. [Google Scholar] [CrossRef]
- Deng, H.; Liu, F.; Pan, Y.; Jin, X.; Wang, H.; Cao, J. BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor gene and periodontitis: A meta-analysis of 15 studies including 1338 cases and 1302 controls. J. Clin. Periodontol. 2011, 38, 199–207. [Google Scholar] [CrossRef]
- Wu, D.; Weng, Y.; Feng, Y.; Liang, B.; Wang, H.; Li, L.; Wang, Z. Trem1 Induces Periodontal Inflammation via Regulating M1 Polarization. J. Dent. Res. 2021, 101, 437–447. [Google Scholar] [CrossRef]
- Bostanci, N.; Abe, T.; Belibasakis, G.N.; Hajishengallis, G. TREM-1 Is Upregulated in Experimental Periodontitis, and Its Blockade Inhibits IL-17A and RANKL Expression and Suppresses Bone loss. J. Clin. Med. 2019, 8, 1579. [Google Scholar] [CrossRef]
- Inanc, N.; Mumcu, G.; Can, M.; Yay, M.; Silbereisen, A.; Manoil, D.; Direskeneli, H.; Bostanci, N. Elevated serum TREM-1 is associated with periodontitis and disease activity in rheumatoid arthritis. Sci. Rep. 2021, 11, 2888. [Google Scholar] [CrossRef]
- Willi, M.; Belibasakis, G.; Bostanci, N. Expression and regulation of TREM-1 in periodontal diseases. Clin. Exp. Immunol. 2014, 178, 190–200. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, M.L.; Velard, F.; Pierrard, L.; Sergheraert, J.; Gangloff, S.C.; Braux, J. Cationic antimicrobial peptides and periodontal physiopathology: A systematic review. J. Periodontal Res. 2019, 54, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.-O. What can we learn about functional importance of human antimicrobial peptide LL-37 in the oral environment from severe congenital neutropenia (Kostmann disease)? Peptides 2020, 128, 170311. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Puklo, M.; Adamowicz, K.; Kantyka, T.; Hiemstra, P.; Stennicke, H.; Guentsch, A.; Schacher, B.; Eickholz, P.; Potempa, J. Lack of cathelicidin processing in Papillon-Lefèvre syndrome patients reveals essential role of LL-37 in periodontal homeostasis. Orphanet J. Rare Dis. 2014, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.C.; Hart, P.S.; Michalec, M.D.; Zhang, Y.; Firatli, E.; Van Dyke, T.E.; Stabholz, A.; Zlorogorski, A.; Shapira, L.; Soskolne, W.A. Haim-Munk syndrome and Papillon-Lefèvre syndrome are allelic mutations in cathepsin C. J. Med. Genet. 2000, 37, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, O.; Azarsız, E.; Emingil, G.; Kütükçüler, N.; Atilla, G. Are Proteinase 3 and Cathepsin C Enzymes Related to Pathogenesis of Periodontitis? Biomed. Res. Int. 2014, 2014, 420830. [Google Scholar] [CrossRef]
- Katz, J.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community development between Porphyromonas gingivalis and Candida albicans mediated by InlJ and Als3. MBio 2018, 9, e00202–e00218. [Google Scholar] [CrossRef]
- Amer, A.; Galvin, S.; Healy, C.; Moran, G.P. The microbiome of oral leukoplakia shows enrichment in Fusobacteria and Rothia species. J. Oral Microbiol. 2017, 9, 1325253. [Google Scholar] [CrossRef][Green Version]
- Mumcu, G.; Cimilli, H.; Karacayli, U.; Inanc, N.; Ture-Ozdemir, F.; Eksioglu-Demiralp, E.; Ergun, T.; Direskeneli, H. Salivary levels of antimicrobial peptides Hnp 1-3, Ll-37 and S100 in Behcet’s disease. Arch. Oral Biol. 2012, 57, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sztukowska, M.; Ojo, A.; Scott, D.A.; Wang, H.; Lamont, R.J. FOXO responses to P orphyromonas gingivalis in epithelial cells. Cell. Microbiol. 2015, 17, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Wang, T.-H.; Shieh, T.-M.; Tseng, Y.-H. Oral submucous fibrosis: A review on etiopathogenesis, diagnosis, and therapy. Int. J. Mol. Sci. 2019, 20, 2940. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, M.; Gondhalekar, R.V.; Shashikanth, M.; George, J.; Thippeswamy, S.; Shukla, A. Pathogenesis of oral lichen planus–a review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef]
- Okumura, K. Cathelicidins—therapeutic antimicrobial and antitumor host defense peptides for oral diseases. Jpn. Dent. Sci. Rev. 2011, 47, 67–81. [Google Scholar] [CrossRef]
- Piktel, E.; Niemirowicz, K.; Wnorowska, U.; Wątek, M.; Wollny, T.; Głuszek, K.; Góźdź, S.; Levental, I.; Bucki, R. The Role of Cathelicidin LL-37 in Cancer Development. Arch. Immunol. Exp. 2016, 64, 33–46. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Yoneyama, H.; Katayama, M.; Isogai, H.; Okumura, K.; Isogai, E. Anti-proliferative effect of an analogue of the LL-37 peptide in the colon cancer derived cell line HCT116 p53+/+ and p53-/-. Oncol Rep. 2012, 28, 829–834. [Google Scholar] [CrossRef]
- Prevete, N.; Liotti, F.; Visciano, C.; Marone, G.; Melillo, R.M.; de Paulis, A. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 2014. [Google Scholar] [CrossRef]
- Wu, W.K.; Sung, J.J.; To, K.F.; Yu, L.; Li, H.T.; Li, Z.J.; Chu, K.M.; Yu, J.; Cho, C.H. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J. Cell Physiol. 2010, 223, 178–186. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Marini, F.C.; Watson, K.; Zwezdaryk, K.J.; Dembinski, J.L.; LaMarca, H.L.; Tomchuck, S.L.; Honer zu Bentrup, K.; Danka, E.S.; Henkle, S.L.; et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3806–3811. [Google Scholar] [CrossRef]
- von Haussen, J.; Koczulla, R.; Shaykhiev, R.; Herr, C.; Pinkenburg, O.; Reimer, D.; Wiewrodt, R.; Biesterfeld, S.; Aigner, A.; Czubayko, F.; et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer 2008, 59, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Jimenez, C.I.; Sandstedt, B.; Borregaard, N.; Tham, E.; Sørensen, O.E.; Weber, G.; Ståhle, M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J. Cancer 2005, 114, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qi, G.; Qin, M.; Zou, Y.; Zhong, K.; Tang, Y.; Guo, Y.; Jiang, X.; Liang, L.; Zou, X. DNA methylation directly downregulates human cathelicidin antimicrobial peptide gene (CAMP) promoter activity. Oncotarget 2017, 8, 27943–27952. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, S.; Si, J.; Zhang, X.; Wang, X.; Guo, Y.; Zou, X. Human cathelicidin antimicrobial peptide suppresses proliferation, migration and invasion of oral carcinoma HSC-3 cells via a novel mechanism involving caspase-3 mediated apoptosis. Mol. Med. Rep. 2020, 22, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Torz, K.; Gülses, A.; Wieker, H.; Gerle, M.; Purcz, N.; Will, O.M.; Eduard Meyer, J.; Wiltfang, J. An experimental study on antitumoral effects of KI-21-3, a synthetic fragment of antimicrobial peptide LL-37, on oral squamous cell carcinoma. J. Craniomaxillofac Surg 2018, 46, 1586–1592. [Google Scholar] [CrossRef]
- Okumura, K.; Itoh, A.; Isogai, E.; Hirose, K.; Hosokawa, Y.; Abiko, Y.; Shibata, T.; Hirata, M.; Isogai, H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004, 212, 185–194. [Google Scholar] [CrossRef]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: Current status and potential future applications. J. Nanobiotechnology 2020, 18, 3. [Google Scholar] [CrossRef]
- Vierthaler, M.; Rodrigues, P.C.; Sundquist, E.; Siponen, M.; Salo, T.; Risteli, M. Fluctuating role of antimicrobial peptide hCAP18/LL-37 in oral tongue dysplasia and carcinoma. Oncol Rep. 2020, 44, 325–338. [Google Scholar] [CrossRef]
- Brice, D.C.; Toth, Z.; Diamond, G. LL-37 disrupts the Kaposi’s sarcoma-associated herpesvirus envelope and inhibits infection in oral epithelial cells. Antivir. Res. 2018, 158, 25–33. [Google Scholar] [CrossRef]
- Sgambato, A.; Cittadini, A. Inflammation and cancer: A multifaceted link. Eur. Rev. Med. Pharm. Sci. 2010, 14, 263–268. [Google Scholar]
- Janmey, P.A.; Winer, J.P.; Murray, M.E.; Wen, Q. The hard life of soft cells. Cell Motil Cytoskelet. 2009, 66, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Jaczewska, J.; Wiltowska-Zuber, J.; Klymenko, O.; Zuber, K.; Fornal, M.; Lekka, M. Depth-sensing analysis of cytoskeleton organization based on AFM data. Eur. Biophys. J. 2012, 41, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Lekka, M.; Stylianopoulos, T. AFM assessing of nanomechanical fingerprints for cancer early diagnosis and classification: From single cell to tissue level. Nanoscale 2018, 10, 20930–20945. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Iwata, M. Stiffness of cancer cells measured with an AFM indentation method. J. Mech. Behav. Biomed. Mater. 2015, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, P.; Łysik, D.; Pogoda, K.; Cieśluk, M.; Namiot, A.; Mystkowska, J.; Król, G.; Głuszek, S.; Janmey, P.A.; Bucki, R. Tissue Rheology as a Possible Complementary Procedure to Advance Histological Diagnosis of Colon Cancer. Acs Biomater. Sci. Eng. 2020, 6, 5620–5631. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, P.; Suprewicz, Ł.; Daniluk, T.; Namiot, A.; Chmielewska, S.J.; Daniluk, U.; Lebensztejn, D.; Bucki, R. Nanomechanical Hallmarks of Helicobacter pylori Infection in Pediatric Patients. Int. J. Mol. Sci. 2021, 22, 5624. [Google Scholar] [CrossRef]
- Song, D.; Shivers, J.L.; MacKintosh, F.C.; Patteson, A.E.; Janmey, P.A. Cell-induced confinement effects in soft tissue mechanics. J. Appl. Phys. 2021, 129, 140901. [Google Scholar] [CrossRef]
- Stylianou, A.; Stylianopoulos, T. Atomic force microscopy probing of cancer cells and tumor microenvironment components. BioNanoScience 2016, 6, 33–46. [Google Scholar] [CrossRef]
- Pogoda, K.; Cieśluk, M.; Deptuła, P.; Tokajuk, G.; Piktel, E.; Król, G.; Reszeć, J.; Bucki, R. Inhomogeneity of stiffness and density of the extracellular matrix within the leukoplakia of human oral mucosa as potential physicochemical factors leading to carcinogenesis. Transl. Oncol. 2021, 14, 101105. [Google Scholar] [CrossRef]
- Ganesh, D.; Sreenivasan, P.; Öhman, J.; Wallström, M.; Braz-Silva, P.H.; Giglio, D.; Kjeller, G.; Hasseus, B. Potentially malignant oral disorders and cancer transformation. Anticancer Res. 2018, 38, 3223–3229. [Google Scholar] [CrossRef]
- Cieśluk, M.; Pogoda, K.; Deptuła, P.; Werel, P.; Kułakowska, A.; Kochanowicz, J.; Mariak, Z.; Łysoń, T.; Reszeć, J.; Bucki, R. Nanomechanics and Histopathology as Diagnostic Tools to Characterize Freshly Removed Human Brain Tumors. Int. J. Nanomed. 2020, 15, 7509. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.E.R.A.; Lourenço, A.G.; Motta, A.C.F.; Komesu, M.C. Salivary Expression of Antimicrobial Peptide LL37 and Its Correlation with Pro-inflammatory Cytokines in Patients with Different Periodontal Treatment Needs. Int. J. Pept. Res. Ther. 2020, 26, 2547–2553. [Google Scholar] [CrossRef]
- Grant, M.; Kilsgård, O.; Åkerman, S.; Klinge, B.; Demmer, R.T.; Malmström, J.; Jönsson, D. The human salivary antimicrobial peptide profile according to the oral microbiota in health, periodontitis and smoking. J. Innate Immun. 2019, 11, 432–444. [Google Scholar] [CrossRef]
- Schenkels, L.C.; Veerman, E.C.; Nieuw Amerongen, A.V. Biochemical composition of human saliva in relation to other mucosal fluids. Crit. Rev. Oral Biol. Med. 1995, 6, 161–175. [Google Scholar] [CrossRef]
- Łysik, D.; Mystkowska, J.; Markiewicz, G.; Deptuła, P.; Bucki, R. The Influence of Mucin-Based Artificial Saliva on Properties of Polycaprolactone and Polylactide. Polymers 2019, 11, 1880. [Google Scholar] [CrossRef]
- Byfield, F.J.; Kowalski, M.; Cruz, K.; Leszczyńska, K.; Namiot, A.; Savage, P.B.; Bucki, R.; Janmey, P.A. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J. Immunol. 2011, 187, 6402–6409. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
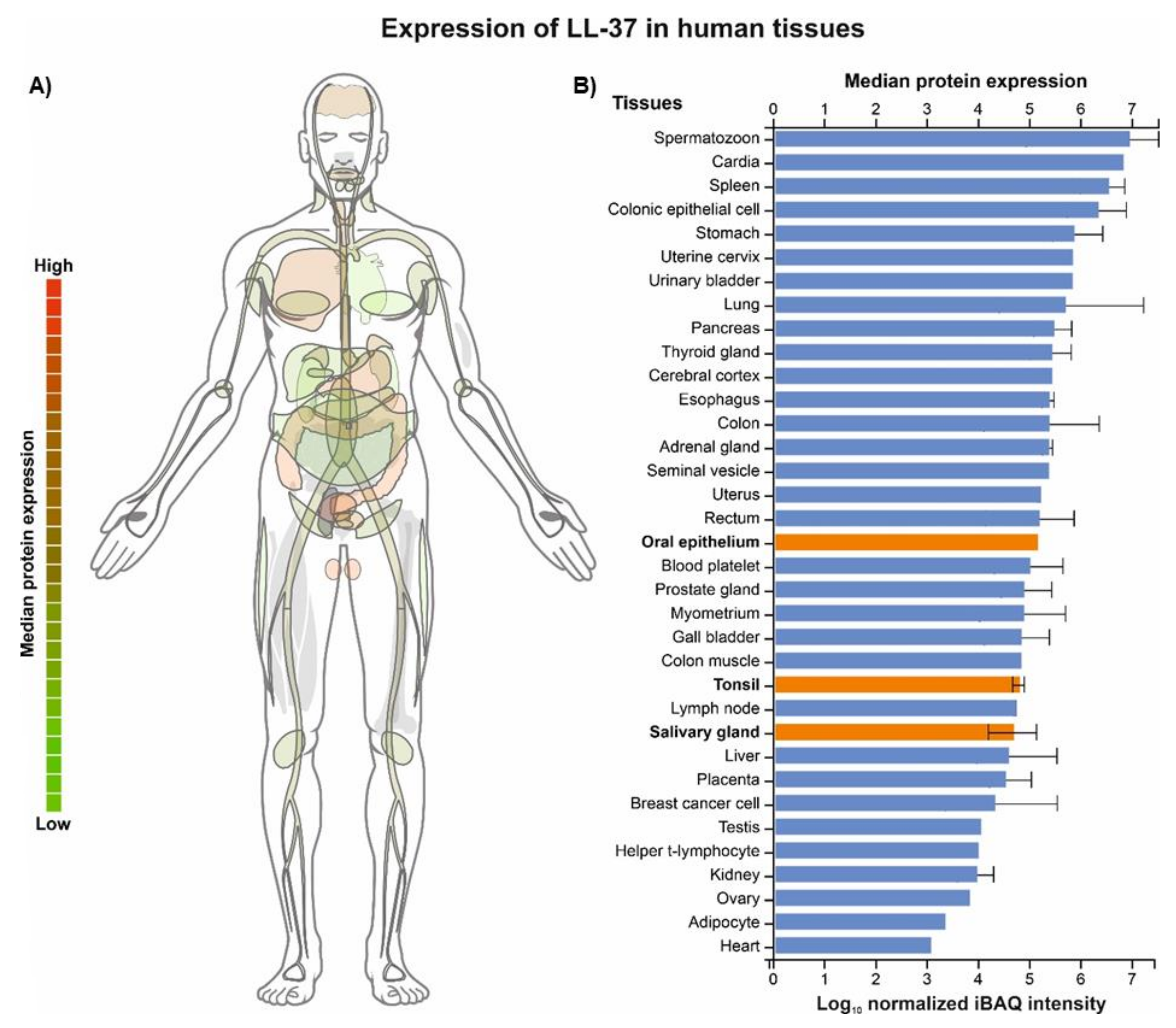



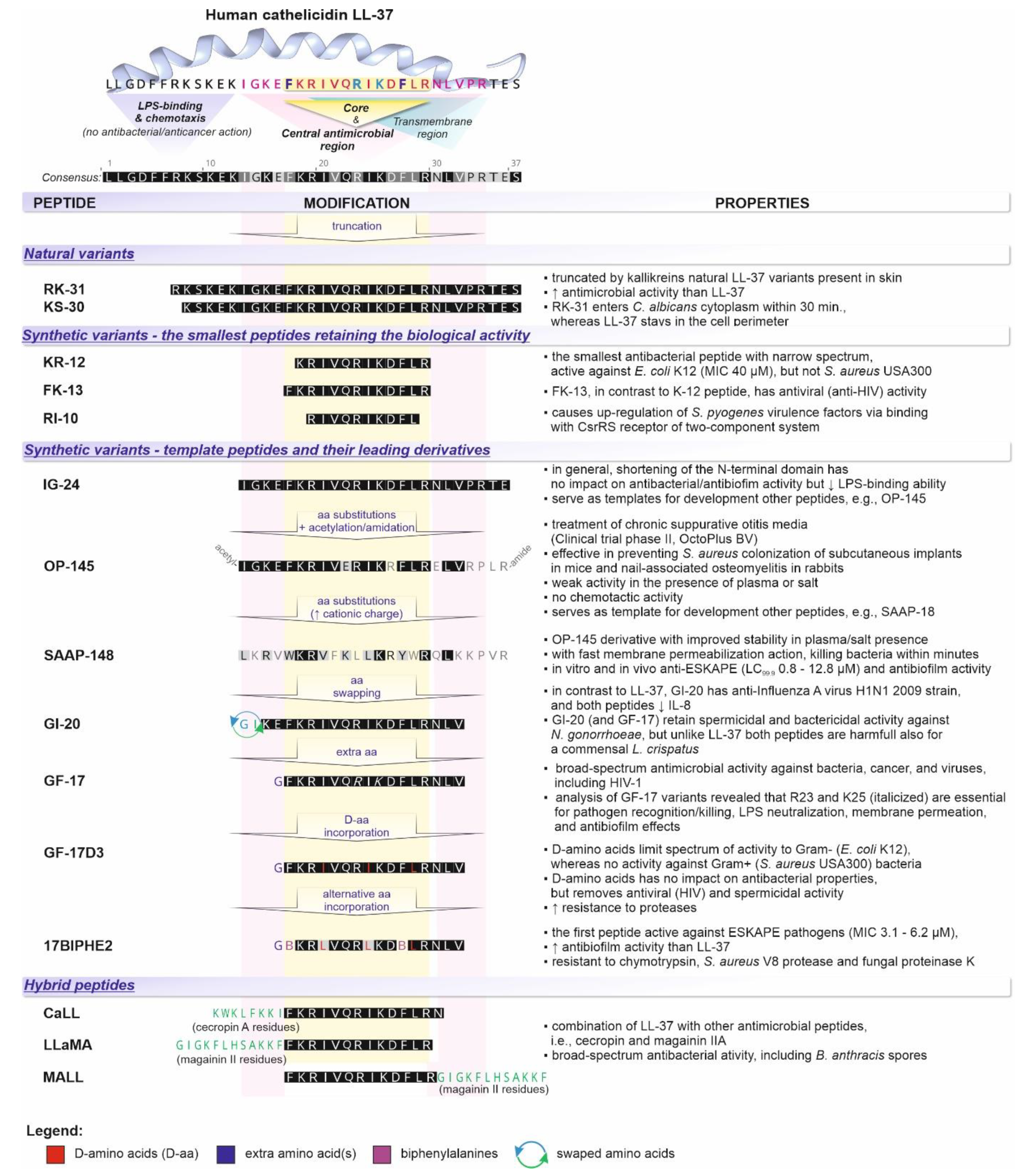
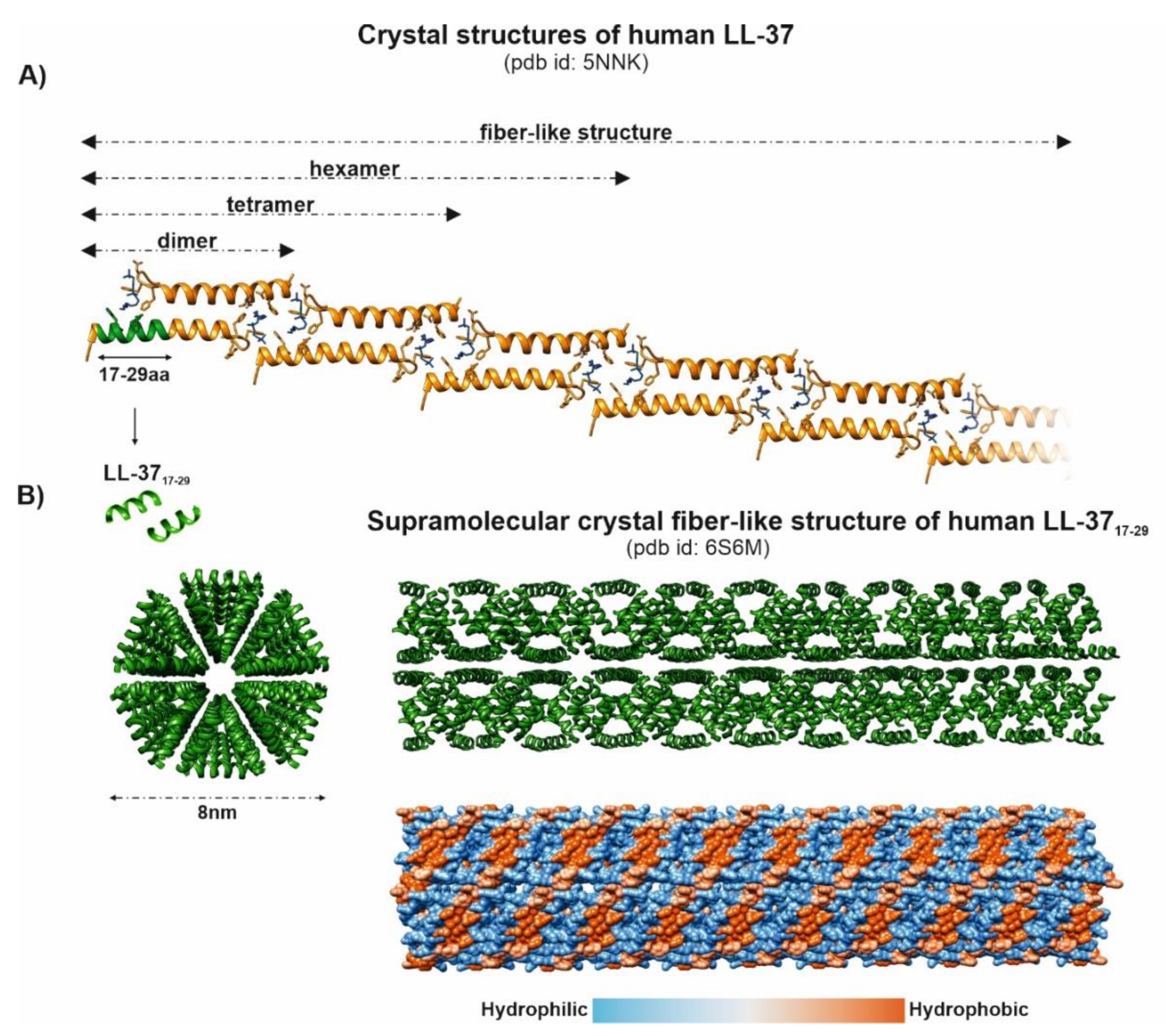
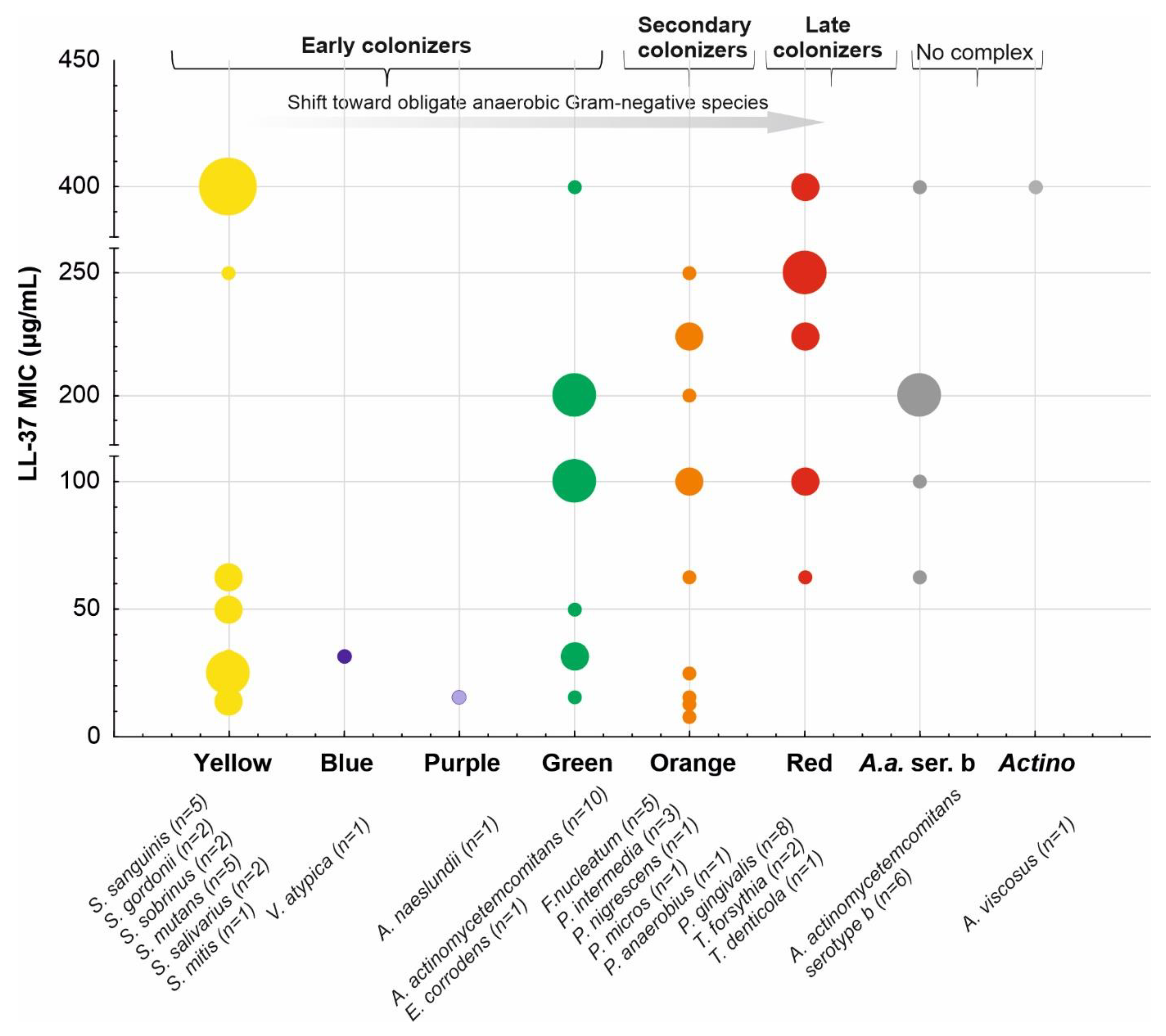
 , i.e., up- or downregulation) and their correlation with the periodontal status (
, i.e., up- or downregulation) and their correlation with the periodontal status ( , represented by probing depth, PD; bleeding on probing, BOP; clinical attachment loss, CAL; plaque index, PI; gingival index, GI; papillary bleeding index, PBI) and microbiological parameters (
, represented by probing depth, PD; bleeding on probing, BOP; clinical attachment loss, CAL; plaque index, PI; gingival index, GI; papillary bleeding index, PBI) and microbiological parameters ( , i.e., changes in occurrence of P. gingivalis, T. forsythia, and T. denticola) were obtained from the references [5,6,113].
, i.e., changes in occurrence of P. gingivalis, T. forsythia, and T. denticola) were obtained from the references [5,6,113].
 , i.e., up- or downregulation) and their correlation with the periodontal status (
, i.e., up- or downregulation) and their correlation with the periodontal status ( , represented by probing depth, PD; bleeding on probing, BOP; clinical attachment loss, CAL; plaque index, PI; gingival index, GI; papillary bleeding index, PBI) and microbiological parameters (
, represented by probing depth, PD; bleeding on probing, BOP; clinical attachment loss, CAL; plaque index, PI; gingival index, GI; papillary bleeding index, PBI) and microbiological parameters ( , i.e., changes in occurrence of P. gingivalis, T. forsythia, and T. denticola) were obtained from the references [5,6,113].
, i.e., changes in occurrence of P. gingivalis, T. forsythia, and T. denticola) were obtained from the references [5,6,113].
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokajuk, J.; Deptuła, P.; Piktel, E.; Daniluk, T.; Chmielewska, S.; Wollny, T.; Wolak, P.; Fiedoruk, K.; Bucki, R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines 2022, 10, 1086. https://doi.org/10.3390/biomedicines10051086
Tokajuk J, Deptuła P, Piktel E, Daniluk T, Chmielewska S, Wollny T, Wolak P, Fiedoruk K, Bucki R. Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines. 2022; 10(5):1086. https://doi.org/10.3390/biomedicines10051086
Chicago/Turabian StyleTokajuk, Joanna, Piotr Deptuła, Ewelina Piktel, Tamara Daniluk, Sylwia Chmielewska, Tomasz Wollny, Przemysław Wolak, Krzysztof Fiedoruk, and Robert Bucki. 2022. "Cathelicidin LL-37 in Health and Diseases of the Oral Cavity" Biomedicines 10, no. 5: 1086. https://doi.org/10.3390/biomedicines10051086
APA StyleTokajuk, J., Deptuła, P., Piktel, E., Daniluk, T., Chmielewska, S., Wollny, T., Wolak, P., Fiedoruk, K., & Bucki, R. (2022). Cathelicidin LL-37 in Health and Diseases of the Oral Cavity. Biomedicines, 10(5), 1086. https://doi.org/10.3390/biomedicines10051086







