Gene Polymorphism of Biotransformation Enzymes and Ciprofloxacin Pharmacokinetics in Pediatric Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
- Maximum concentration (Cmax) for a period of 7.5 h after dosing.
- Time to reach maximum concentration (Tmax) over a period of 7.5 h and 12 h after dosing.
- The area under the concentration–time curve after taking the drug until the last measurement is above the limit of quantification for the observation period of 7.5 h and 12 h (AUC0-t).
- (1)
- Clinical and demographic
- -
- Age,
- -
- Sex,
- -
- Anthropometric data,
- -
- Fecal elastase concentration,
- -
- Chronic infection with P. aeruginosa,
- -
- Presence of meconium ileus,
- -
- Presence of genotype F508del/F508del,
- -
- Liver damage (estimated with liver enzymes elevation and ultrasound diagnostics),
- -
- Spirometry (FEV1, FVC).
- (2)
- Pharmacokinetic parameters.
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Pharmacokinetic Parameters of CPF in Children with CF
3.3. Study of the Effect of the Genotype of Xenobiotic Biotransformation Enzymes of the 1st and 2nd Phases on the Pharmacokinetic Parameters of CPF in Children and Adolescents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Kiseleva, A.; Klimushina, M.; Sotnikova, E.; Skirko, O.; Divashuk, M.; Kurilova, O.; Ershova, A.; Khlebus, E.; Zharikova, A.; Efimova, I.; et al. Cystic Fibrosis Polymorphic Variants in a Russian Population. Pharm. Pers. Med. 2020, 13, 679–686. [Google Scholar] [CrossRef]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ogden, H.; Kim, H.; Wikenheiser-Brokamp, K.; Naren, A.; Mun, K. Cystic Fibrosis Human Organs-on-a-Chip. Micromachines 2021, 12, 747. [Google Scholar] [CrossRef]
- De Lisle, R.C.; Borowitz, D. The Cystic Fibrosis Intestine. Cold Spring Harb. Perspect. Med. 2013, 3, a009753. [Google Scholar] [CrossRef]
- Sabharwal, S. Gastrointestinal Manifestations of Cystic Fibrosis. Gastroenterol. Hepatol. 2016, 12, 43–47. [Google Scholar]
- Dahlgren, D.; Lennernäs, H. Intestinal Permeability and Drug Absorption: Predictive Experimental, Computational and In Vivo Approaches. pharmaceuticals 2019, 11, 411. [Google Scholar] [CrossRef]
- Lusman, S.; Sullivan, J. Nutrition and Growth in Cystic Fibrosis. Pediatr. Clin. N. Am. 2016, 63, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Haupt, M.E.; Geller, D.E.; Hall, J.A.; Diez, P.M.Q. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017, 23, 7059–7076. [Google Scholar] [CrossRef] [PubMed]
- Mun, K.S.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D.; et al. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat. Commun. 2019, 10, 3124. [Google Scholar] [CrossRef]
- Kayani, K.; Mohammed, R.; Mohiaddin, H. Cystic Fibrosis-Related Diabetes. Front. Endocrinol. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.; Surana, P.; Koh, C. Liver disease in patients with cystic fibrosis. Curr. Opin. Gastroenterol. 2018, 34, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Drzymała-Czyż, S.; Dziedzic, K.; Szwengiel, A.; Krzyżanowska-Jankowska, P.; Nowak, J.K.; Nowicka, A.; Aringazina, R.; Drzymała, S.; Kashirskaya, N.; Walkowiak, J. Serum bile acids in cystic fibrosis patients–glycodeoxycholic acid as a potential marker of liver disease. Dig. Liver Dis. 2021, 54, 111–117. [Google Scholar] [CrossRef]
- Li, R.; Barton, H.; Maurer, T. A Mechanistic Pharmacokinetic Model for Liver Transporter Substrates Under Liver Cirrhosis Conditions. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 338–349. [Google Scholar] [CrossRef]
- Szalek, E.; Kamińska, A.; Gozdzik-Spychalska, J.; Grzeskowiak, E.; Batura-Gabryel, H. The PK/PD index (CMAX/MIC) for ciprofloxacin in patients with cystic fibrosis. Acta Pol. Pharm. 2011, 68, 777–783. [Google Scholar] [PubMed]
- Yahiaoui, Y.; Jablonski, M.; Hubert, D.; Mosnier-Pudar, H.; Noël, L.-H.; Stern, M.; Grenet, D.; Grünfeld, J.-P.; Chauveau, D.; Fakhouri, F. Renal Involvement in Cystic Fibrosis: Diseases Spectrum and Clinical Relevance. Clin. J. Am. Soc. Nephrol. 2009, 4, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Postorino, A.; Lucanto, C.; Costa, S.; Cristadoro, S.; Pellegrino, S.; Conti, G.; Buemi, M.; Magazzù, G.; Bellinghieri, G. Cystic Fibrosis: A Risk Condition for Renal Disease. J. Ren. Nutr. 2017, 27, 470–473. [Google Scholar] [CrossRef]
- Lai, S.; Mazzaferro, S.; Mitterhofer, A.P.; Bonci, E.; Marotta, P.G.; Pelligra, F.; Murciano, M.; Celani, C.; Troiani, P.; Cimino, G.; et al. Renal involvement and metabolic alterations in adults patients affected by cystic fibrosis. J. Transl. Med. 2019, 17, 388. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- Register of Patients with Cystic Fibrosis in Russian Federation. 2019. Available online: https://mukoviscidoz.org/doc/registr/site_Registre_2019.pdf (accessed on 20 February 2022).
- Caverly, L.J.; Zimbric, M.; Azar, M.; Opron, K.; LiPuma, J.J. Cystic fibrosis airway microbiota associated with outcomes of nontuberculous mycobacterial infection. ERJ Open Res. 2021, 7, 00578-2020. [Google Scholar] [CrossRef]
- Odoul, F.; Le Guellec, C.; Giraut, C.; de Gialluly, C.; Marchand, S.; Paintaud, G.; Saux, M.C.; Rolland, J.C.; Autret-Leca, E. Ciprofloxacin pharmacokinetics in young cystic fibrosis patients after repeated oral doses. Therapie 2001, 56, 519–524. [Google Scholar]
- Rubio, T.T.; Miles, M.V.; Lettieri, J.T.; Kuhn, R.J.; Echols, R.M.; Church, D.A. Pharmacokinetic disposition of sequential intravenous/oral ciprofloxacin in pediatric cystic fibrosis patients with acute pulmonary exacerbation. Pediatr. Infect. Dis. J. 1997, 16, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.G.; Stass, H.; Wedgwood, J.; Hampel, B.; Fischer, C.; Kuhlmann, J.; Schaad, U.B. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob. Agents Chemother. 1996, 40, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Payen, S.; Serreau, R.; Munck, A.; Aujard, Y.; Aigrain, Y.; Bressolle, F.; Jacqz-Aigrain, E. Population Pharmacokinetics of Ciprofloxacin in Pediatric and Adolescent Patients with Acute Infections. Antimicrob. Agents Chemother. 2003, 47, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, P.; Gastonguay, M.R. Population pharmacokinetics of ciprofloxacin in pediatric patients. J. Clinic. Pharmacol. 2003, 43, 698–710. [Google Scholar] [CrossRef]
- CFTR2. Available online: https://cftr2.org/ (accessed on 10 January 2022).
- Guidance for Industry. General Considerations for Pediatric Pharmacokinetic Studies for Drugs and Biological Products; FDA: Rockville, MD, USA, 1998.
- Paediatric Formulary Committee. BNF for Children 2011–2012. Available online: https://www.sbp.com.br/fileadmin/user_upload/pdfs/british_national_formulary_for_children_2011-2012.pdf (accessed on 10 January 2022).
- De Sutter, P.-J.; Gasthuys, E.; Van Braeckel, E.; Schelstraete, P.; Van Biervliet, S.; Van Bocxlaer, J.; Vermeulen, A. Pharmacokinetics in Patients with Cystic Fibrosis: A Systematic Review of Data Published Between 1999 and 2019. Clin. Pharmacokinet. 2020, 59, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, O.; Petrova, N.; Kondratyeva, E.; Krasovskii, S.; Voronkova, A.; Sherman, V.; Melyanovskaya, Y.; Zinchenko, R. Influence of gene polymorphism of the 1st phase of xenobiotics metabolism on antibacterial therapy efficacy in patients with cystic fibrosis homozygous for F508DEL mutation of CFTR gene. Pediatria 2018, 97, 638–646. [Google Scholar] [CrossRef]
- McCarthy, C.; O’Carroll, O.; Franciosi, A.N.; McElvaney, A.N.F. and N.G. Factors Affecting Prognosis and Prediction of Outcome in Cystic Fibrosis Lung Disease. Cyst. Fibros. Light New Res. 2015, 40, 627–632. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Gai, X.-Y.; Bo, S.-N.; Shen, N.; Zhou, Q.-T.; Yin, A.-Y.; Lu, W. Pharmacokinetic-pharmacodynamic analysis of ciprofloxacin in elderly Chinese patients with lower respiratory tract infections caused by Gram-negative bacteria. Chin. Med. J. 2019, 132, 638–646. [Google Scholar] [CrossRef]
- Madaras-Kelly, K.J.; Ostergaard, B.E.; Hovde, L.B.; Rotschafer, J.C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa and an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 1996, 40, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.N. Levofloxacin: Update and perspectives on one of the original ‘respiratory quinolone’. Expert Rev. Anti-Infect. Ther. 2003, 1, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Granfors, M.T.; Backman, J.T.; Neuvonen, M.; Neuvonen, P.J. Ciprofloxacin greatly increases concentrations and hypotensive effect of tizanidine by inhibiting its cytochrome P450 1A2-mediated presystemic metabolism. Clin. Pharmacol. Ther. 2004, 76, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Intersection of the Roles of Cytochrome P450 Enzymes with Xenobiotic and Endogenous Substrates: Relevance to Toxicity and Drug Interactions. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Stevens, J.C.; Marsh, S.A.; Zaya, M.J.; Regina, K.J.; Divakaran, K.; Le, M.; Hines, R.N. Developmental Changes in Human Liver CYP2D6 Expression. Drug Metab. Dispos. 2008, 36, 1587–1593. [Google Scholar] [CrossRef]
- Trzcinski, R.; Skrętkowicz, J.; Dziki, A.; Rychlik-Sych, M.; Baranska, M. Genetic Polymorphisms of CYP2D6 Oxidation in Patients with Inflammatory Bowel Disease. Am. J. Dig. Dis. 2009, 55, 1037–1043. [Google Scholar] [CrossRef]
- Terlizzi, V.; Tosco, A.; Tomaiuolo, R.; Sepe, A.; Amato, N.; Casale, A.; Mercogliano, C.; De Gregorio, F.; Improta, F.; Elce, A.; et al. Prediction of acute pancreatitis risk based on PIP score in children with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 579–584. [Google Scholar] [CrossRef][Green Version]
- Ooi, C.Y.; Dorfman, R.; Cipolli, M.; Gonska, T.; Castellani, C.; Keenan, K.; Freedman, S.D.; Zielenski, J.; Berthiaume, Y.; Corey, M.; et al. Type of CFTR Mutation Determines Risk of Pancreatitis in Patients With Cystic Fibrosis. Gastroenterology 2011, 140, 153–161. [Google Scholar] [CrossRef]
- McKone, E.F.; Emerson, S.S.; Edwards, K.L.; Aitken, M.L. Effect of genotype on phenotype and mortality in cystic fibrosis: A retrospective cohort study. Lancet 2003, 361, 1671–1676. [Google Scholar] [CrossRef]
- Petrova, N.V.; Kashirskaya, N.Y.; Vasileva, T.A.; Voronkova, A.Y.; Kondrateva, E.I.; Sherman, V.D.; Novoselova, O.G.; Krasovskiy, S.A.; CHernyak, A.V.; Amelina, E.L.; et al. Phenotypic features in patients with cystic fibrosis with L138ins (p.Leu138dup) mutation. Pediatrics 2017, 96, 64–71. [Google Scholar] [CrossRef]
- Hatziagorou, E.; Orenti, A.; Drevinek, P.; Kashirskaya, N.; Mei-Zahav, M.; De Boeck, K.; Pfleger, A.; Sciensano, M.T.; Lammertyn, E.; Macek, M., Jr. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis–data from the European cystic fibrosis society patient registry. J. Cyst. Fibros. 2020, 19, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K. Mechanism of fluoroquinolone action. Curr. Opin. Microbiol. 1999, 2, 504–508. [Google Scholar] [CrossRef]
- Sharma, D.; Patel, R.P.; Zaidi, S.T.R.; Sarker, M.R.; Lean, Q.Y.; Ming, L.C. Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries. Front. Pharmacol. 2017, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.F.; Shao, J.; Frangolias, D.D.; Keener, C.L.; Shephard, C.A.; Farin, F.M.; Tonelli, M.R.; Pare, P.D.; Sandford, A.J.; Aitken, M.L.; et al. Variants in the Glutamate-Cysteine-Ligase Gene Are Associated with Cystic Fibrosis Lung Disease. Am. J. Respir. Crit. Care Med. 2006, 174, 415–419. [Google Scholar] [CrossRef]
- Walsh, A.C.; Feulner, J.A.; Reilly, A. Evidence for functionally significant polymorphism of human glutamate cysteine ligase catalytic subunit: Association with glutathione levels and drug resistance in the National Cancer Institute tumor cell line panel. Toxicol. Sci. 2001, 61, 218–223. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Lazarus, P.; Richie, J.P. A GAG trinucleotide-repeat polymorphism in the gene for glutathione biosynthetic enzyme, GCLC, affects gene expression through translation. FASEB J. 2011, 25, 2180–2187. [Google Scholar] [CrossRef]
| Characteristics | Age Groups | ||||
|---|---|---|---|---|---|
| 2–6 Years | 6–12 Years | 12–18 Years | Total | ||
| Age, years | N | 8 | 14 | 11 | 33 |
| Mean (±SD) | 3.37 (0.91) | 8.14 (8.00) | 14.27 (1.61) | 9.03 (4.48) | |
| Median | 3.00 | 8.00 | 14.00 | 9.00 | |
| Weight, kg | Mean (±SD) | 15.37 (3.19) | 26.82 (8.75) | 46.95 (9.69) | 30.75 (14.79) |
| Median | 15.60 | 25.25 | 50.00 | 26.00 | |
| Height, cm | Mean (±SD) | 99.12 (10.06) | 129.07 (10.76) | 156.15 (12.56) | 130.8 (24.32) |
| Median | 100.00 | 128.00 | 157.70 | 130.00 | |
| BMI, kg/m2 | Mean (±SD) | 15.52 (1.31) | 15.74 (2.81) | 19.02 (1.67) | 16.78 (2.65) |
| Median | 15.25 | 14.80 | 18.50 | 16.40 | |
| BMI percentile | Mean (±SD) | 50.83 (29.11) | 38.95 (35.42) | 49.07 (26.23) | 45.08 (30.69) |
| Median | 46.00 | 30.10 | 18.50 | 36.90 | |
| Sex | Male, n (%) | 3 (37.5%) | 7 (50%) | 2 (18.2%) | 12 (36.4%) |
| Parameter | 2–6 Years (n = 8) | 6–12 Years (n = 14) | 12–18 Years (n = 11) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| Dose, mg/kg | 21.40 ± 3.38 | ------- | 21.97 ± 3.65 | --------- | 21.12 ± 2.21 | --------- |
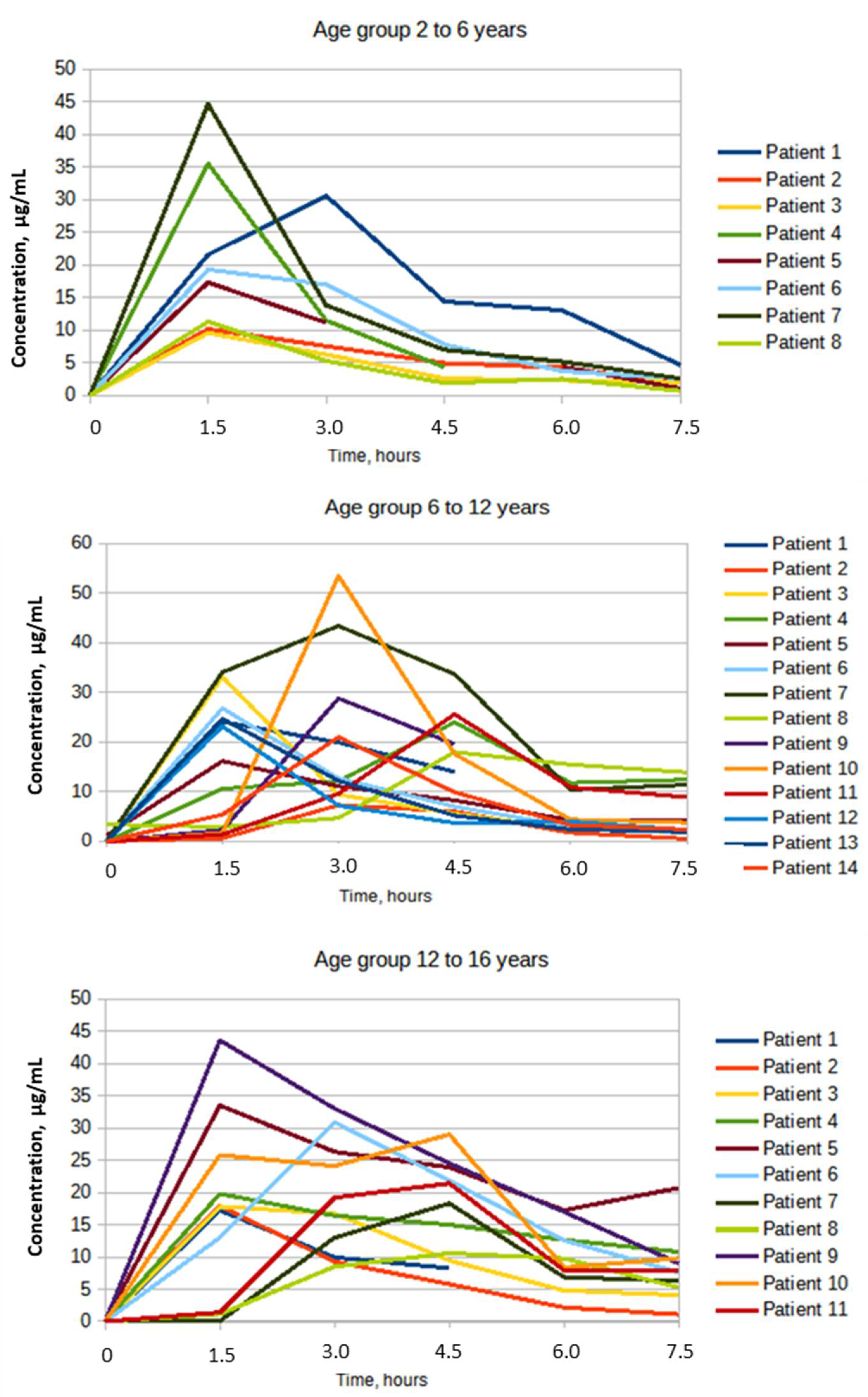
| Cmax, µg/mL | 22.32 ± 13.15 | 58.9 | 26.4 ± 11.33 | 42.89 | 23.63 ± 9.51 | 40.24 |
| Tmax, hours | 1.68 ± 0.53 | 31.42 | 2.67 ± 1.2 | 44.89 | 2.72 ± 1.47 | 53.99 |
| AUC0-t µg·h/mL | 69.84 ± 35.29 | 50.54 | 85.44 ± 38.55 | 45.13 | 100.53 ± 46.6 | 46.31 |
| AUC0-t_norm (µg·h/mL)/(mg/kg) | 3.33 ± 1.98 | 59.54 | 3.880 ± 1.46 | 37.71 | 4.77 ± 2.20 | 46.18 |
| Cmax_norm (µg/mL)/(mg/kg) | 1.03 ± 0.545 | 52.98 | 1.1886 ± 0.40 | 33.47 | 1.12 ± 0.47 | 41.70 |
| Genotypes | n | Age, Years Mean (±SD) | Male, n | ||
|---|---|---|---|---|---|
| Monooxygenases of the CYP450 family | CYP2C9*3 (c.1075A > C) | AA | 25 | 8.24 (4.22) | 9 |
| AC | 6 | 9.88 (5.38) | 5 | ||
| CC | 1 | 12 | 0 | ||
| CYP2C9*2 (c.430C > T) | CC | 27 | 9.1 (4.6) | 10 | |
| CT | 5 | 6.8 (3.6) | 4 | ||
| CYP2C19*2 (c.681G > A) | GG | 22 | 8.4 (4.5) | 8 | |
| GA | 9 | 9.4 (4.8) | 5 | ||
| AA | 1 | 10.0 | 1 | ||
| CYP2D6*4 (1846G > A) | GG | 19 | 8.9 (4.3) | 6 | |
| GA | 12 | 8.64 (4.9) | 7 | ||
| AA | 1 | 6.0 | 1 | ||
| Glutathione-S-transferase | GSTT1 | N/N | 26 | 9.4 (4.5) | 9 |
| D/D | 6 | 6.7 (2.6) | 4 | ||
| GSTMI | Without deletion | 14 | 9.5 (4.8) | 9 | |
| Genotype Zero | 18 | 8.5 (4.1) | 4 | ||
| GSTP1 | AA | 15 | 8.2 (4.9) | 5 | |
| AG | 14 | 9 (4.3) | 8 | ||
| GG | 2 | 9 (5.7) | 1 | ||
| N-acetyltransferase | NAT2 (282C > T) | CC | 18 | 8.16 (4.29) | 8 |
| CT | 13 | 9.47 (4.72) | 6 | ||
| NAT2 (590G > A) | GG | 21 | 9 (4.44) | 9 | |
| GA | 11 | 8.31 (4.64) | 5 | ||
| NAT2 (341T > C) | TT | 7 | 9.0 (3.40) | 2 | |
| TC | 14 | 10.13 (4.78) | 7 | ||
| CC | 11 | 6.83 (3.66) | 5 | ||
| NAT2 481C > T | CC | 8 | 9.4 (4.70) | 3 | |
| CT | 14 | 9.79 (4.39) | 6 | ||
| TT | 10 | 6.6 (3.98) | 5 | ||
| NAT2 803A> G | AA | 7 | 8.67 (4.85) | 5 | |
| AG | 13 | 10.38 (4.29) | 5 | ||
| GG | 11 | 6.82 (4.14) | 4 | ||
| Parameter | Statistical Indicators | Genotype | ||
|---|---|---|---|---|
| AA | CA | CC | ||
| Dose, mg/kg | N | 25 | 7 | 1 |
| Mean (±SD) | 21.51 (3.46) | 19.14 (8.76) | 20 | |
| Median (75%Q3–25%Q1) | 20 (24–19.5) | 21.7 (24.5–18.8) | 20 | |
| CV | 16.1 | 45.76 | - | |
| Concentration at point 1, µg/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 18.72 (13.17) | 16.19 (10.31) | 1.4 | |
| Median (75%Q3–25%Q1) | 17.89 (25.8–9.58) | 15.16 (19.79–10.69) | 1.4 | |
| CV | 70.33 | 63.69 | - | |
| Concentration at point 2, µg/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 17.37 (12.19) | 16.93 (9.89) | 19.2 | |
| Median (75%Q3–25%Q1) | 12.7 (21–9.43) | 14.26 (26.3–11.17) | 19.2 | |
| CV | 70.20 | 58.40 | - | |
| Concentration at point 3, µg/mL | N | 25 | 5 | 1 |
| Mean (±SD) | 11.78 (8.83) | 20.55 (3.94) | 21.4 | |
| Median (75%Q3–25%Q1) | 8.26 (17.6–5.56) | 21.85 (23.89–18.0) | 21.4 | |
| CV | 75.04 | 19.17 | - | |
| Concentration at point 4, µg/mL | N | 14 | 4 | 1 |
| Mean (±SD) | 5.16 (4.14) | 11.27 (4.78) | 7.92 | |
| Median (75%Q3–25%Q1) | 4.0 (5.16–2.6) | 12.6 (14.06–8.48) | 7.92 | |
| CV | 80.12 | 42.39 | - | |
| Concentration at point 5, µg/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 4.18 (3.14) | 11.08 (6.55) | 7.8 | |
| Median (75%Q3–25%Q1) | 2.6 (5.96–1.84) | 11.6 (13.9–7.62) | 7.8 | |
| CV | 75.24 | 59.14 | - | |
| AUC0-t, µg·h/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 84.13 (42.61) | 104.59 (37.47) | 80.73 | |
| Median (75%Q3–25%Q1) | 75.62 (107.94–61.17) | 100.51 (123.20–74.60) | 80.73 | |
| CV | 50.64 | 35.83 | - | |
| Cmax, µg/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 25.31 (11.94) | 23.92 (6.88) | 21.4 | |
| Median (75%Q3–25%Q1) | 24.09 (30.61–17.81) | 21.90 (30.9–18) | 21.4 | |
| CV | 47.17 | 28.75 | - | |
| Tmax, h | N | 25 | 6 | 1 |
| Mean (±SD) | 2.22 (1.07) | 2.75 (1.48) | 4.5 | |
| Median (75%Q3–25%Q1) | 1.5 (3.0–1.5) | 2.25 (4.5–1.5) | 4.5 | |
| CV | 48.25 | 53.63 | - | |
| AUC0-t_norm, µg·h/mL | N | 25 | 6 | one |
| Mean (±SD) | 3.95 (2.02) | 4.69 (1.49) | 4.04 | |
| Median (75%Q3–25%Q1) | 3.31 (4.56–2.89) | 4.59 (5.68–3.97) | 4.04 | |
| CV | 51.15 | 31.78 | - | |
| Cmax_norn, µg/mL | N | 25 | 6 | 1 |
| Mean (±SD) | 1.17 (0.48) | 1.08 (0.30) | 1.07 | |
| Median (75%Q3–25%Q1) | 1.05 (1.45–0.83) | 1.08 (1.37–0.82) | 1.07 | |
| CV | 41.51 | 27.71 | - | |
| Quantitative Characteristic Name | Mean Value in the Group/Number of Observations | The Level of Statistical Significance, p ≤ 0.05 | |||
|---|---|---|---|---|---|
| Kruskal-Wallis Test | Van der Waerden Test | AA | CA | CC | |
| CPF concentration at point 5 | 4.18/25 | 11.08/6 | 7.80/1 | p = 0.05 | p = 0.04 |
| n = 30 | The Result of the Analysis of the Discriminant Function Step 2, N Variables in Model l: 2, Grouping: CYP2C9*3 I359L(c.1075A > C) (2 Groups) Wilks’ Lambda: 0.42029 Approximation. F(2.27) = 18.621, p < 0.0000 | |||||
|---|---|---|---|---|---|---|
| Wilks’ Lambda | Partial Lambda | F-Remove (1.27) | p-Value | Toler. | 1-Toler. (R-Sqr.) | |
| CPF concentration at point 5 | 0.857958 | 0.489875 | 28.11609 | 0.000014 | 0.380098 | 0.619902 |
| CPF concentration at point 3 | 0.500211 | 0.840231 | 5.13404 | 0.031690 | 0.380098 | 0.619902 |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi2 | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| Free member 1 (CA genotype) | 3.3573 | 5.7513 | 0.0165 | 1.4000 | |
| Cmax, µg/mL | 0.4371 | 2.6625 | 4.6479 | 0.0311 | 0.2027 |
| AUC, µg·h/mL | −0.0900 | −2.0260 | 4.1340 | 0.0420 | 0.0442 |
| Total dose of CPF, mg | −0.0050 | −0.7412 | 4.2320 | 0.0397 | 0.0024 |
| Chronic staphylococcal infection | 3.9051 | 0.9151 | 6.3188 | 0.0119 | 1.5535 |
| Indicator | Meaning | GG | GA | AA |
|---|---|---|---|---|
| Dose, mg/kg | n | 19 | 13 | 1 |
| Mean (±SD) | 21.32 (3.13) | 20.01 (6.81) | 26.7 | |
| Median | 20.0 (24.5–19.4) | 21.7 (22.8–19.6) | 26.7 | |
| (75%Q3–25%Q1) | ||||
| CV | 14.67 | 34.05 | - | |
| Concentration at point 1, µg/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 15.25 (12.51) | 20.84 (13.03) | 26.8 | |
| Median (75%Q3–25%Q1) | 17.31 (24.09–2.30) | 18,51 (33.60–10.96) | 26.8 | |
| CV | 82.03 | 62.52 | - | |
| Concentration at point 2, µg/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 17.16 (11.07) | 18.01 (12.89) | 12.7 | |
| Median (75%Q3–25%Q1) | 13.8 (21.0–9.6) | 11.81 (30.76–8.37) | 12.7 | |
| CV | 64.51 | 71.55 | - | |
| Concentration at point 3, µg/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 13.86 (7.98) | 13.51 (10.43) | 7.00 | |
| Median (75%Q3–25%Q1) | 11.94 (19.6–7.0) | 11.34 (22.93–4.95) | 7.00 | |
| CV | 57.56 | 77.14 | - | |
| Concentration at point 4, µg/mL | n | 13 | 5 | 1 |
| Mean (±SD) | 5.83 (3.74) | 9.31(6.77) | 3.0 | |
| Median | 4.40 (6.88–3.72) | 12.56 (12.64–2.60) | 3.0 | |
| (75%Q3–25%Q1) | ||||
| CV | 64.26 | 72.72 | - | |
| Concentration at point 5, µg/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 5.71 (5.03) | 5.71 (4.44) | 1.7 | |
| Median (75%Q3–25%Q1) | 4.09 (8.55–2.30) | 5.32 (9.89–1.69) | 1.7 | |
| CV | 88.06 | 77.78 | - | |
| AUC0-t, µg·h/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 83.89 (31.87) | 95.18 (55.02) | 75.53 | |
| Median (75%Q3–25%Q1) | 74.59 (107.94–61.79) | 89.96 (125.33–50.15) | 75.53 | |
| CV | 37.88 | 57.81 | - | |
| Cmax, µg/mL | n | 19 | 12 | 1 |
| Mean (±SD) | 24.45 (10.32) | 25.53 (12.62) | 26.8 | |
| Median (75%Q3–25%Q1) | 21.4 (28.8–17.89) | 27.31 (34.33–14.26) | 26.8 | |
| CV | 42.20 | 49.42 | - | |
| Tmax, hours | n | 19 | 12 | 1 |
| Mean (±SD) | 2.53 (1.33) | 2.25 (1.01) | 1.5 | |
| Median | 1.5 (4.5–1.5) | 1.5 (3.0–1.5) | 1.5 | |
| (75%Q3–25%Q1) | ||||
| CV | 52.56 | 44.94 | - | |
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | n | 19 | 12 | 1 |
| Mean (±SD) | 3.96 (1.42) | 4.41 (2.56) | 2.83 | |
| Median (75%Q3–25%Q1) | 3.68 (4.56–3.06) | 3.86 (6.15–2.48) | 2.83 | |
| CV | 35.89 | 58.16 | - | |
| Cmax_norm, (µg/mL)/(mg/kg) | n | 19 | 12 | 1 |
| Mean (±SD) | 1.13 (0.37) | 1.18 (0.57) | 1.00 | |
| Median | 1.05 (1.38–0.94) | 1.31 (1.48–0.68) | 1.00 | |
| (75%Q3–25%Q1) | ||||
| CV | 32.47 | 48.49 | - |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi2 | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| Free member 0 (GG genotype) | −1495.1 | 860.1 | 3.0219 | 0.0821 | NA |
| Free member 1 (GA genotype) | −1428.2 | 821.7 | 3.0212 | 0.0822 | NA |
| Age, years | 25.6330 | 15.2937 | 2.8091 | 0.0937 | 59.9328 |
| Weight, kg | 25.2035 | 14.7289 | 2.9280 | 0.0871 | 187.1 |
| Height, cm | 6.5305 | 3.7882 | 2.9719 | 0.0847 | 83.1126 |
| BMI, kg/m2 | 19.7351 | 11.4131 | 2.9900 | 0.0838 | 27.7247 |
| Dose of CPF, mg/kg | 35.1704 | 20.2638 | 3.0124 | 0.0826 | 63.7317 |
| AUC, µg·h/mL | 1.8107 | 1.0713 | 2.8565 | 0.0910 | 40.7737 |
| Total dose of CPF, mg | −2.2508 | 1.3090 | 2.9566 | 0.0855 | −333.5 |
| Cmax_norm (µg/mL)/(mg/kg) | −90.7806 | 53.5454 | 2.8744 | 0.0900 | −22.4050 |
| Anamnesis of meconium ileus | 133.0 | 80.4820 | 2.7325 | 0.0983 | 24.9956 |
| Liver damage without cirrhosis (elevation of liver enzymes plus ultrasound diagnostics) | −71.2019 | 42.1590 | 2.8524 | 0.0912 | −37.3079 |
| Parameter | Statistical Indicators | AA | AG | GG |
|---|---|---|---|---|
| Dose, mg/kg | n | 15 | 15 | 2 |
| Mean (±SD) | 21.03 (3.37) | 21.03 (3.37) | 24.35 (3.75) | |
| Median (75%Q3–25%Q1) | 20.0 (22.8–19.4) | 20.0 (22.8–19.4) | 24.35 (27.0–21.70) | |
| CV | 16.01 | 16.01 | 15.39 | |
| Concentration at point 1, µg/mL | n | 15 | 15 | 2 |
| Mean (±SD) | 14.91 (10.66) | 14.91 (10.66) | 28.86 (22.40) | |
| Median (75%Q3–25%Q1) | 16.18 (21.57–5.40) | 16.18 (21.57–5.40) | 28.86 (44.7–13.02) | |
| CV | 71.52 | 71.52 | 77.63 | |
| Concentration at point 2, µg/mL | n | 15 | 15 | 2 |
| Mean (±SD) | 19.46 (14.30) | 19.46 (14.30) | 22.35 (12.09) | |
| Median (75%Q3–25%Q1) | 12.98 (28.80–9.43) | 12.98 (28.80–9.43) | 22.35 (30.90–13.80) | |
| CV | 73.50 | 73.50 | 54.10 | |
| Concentration at point 3, µg/mL | n | 14 | 14 | 2 |
| Mean (±SD) | 13.11 (9.60) | 13.11 (9.60) | 14.42 (10.50) | |
| Median (75%Q3–25%Q1) | 9.67 (8.30–5.56) | 9.67 (8.30–5.56) | 14.42 (21.85–7.0) | |
| CV | 73.24 | 73.24 | 72.78 | |
| Concentration at point 4, µg/mL | n | 7 | 7 | 2 |
| Mean (±SD) | 4.38 (1.34) | 4.38 (1.34) | 8.86 (5.23) | |
| Median (75%Q3–25%Q1) | 4.4 (4.8–3.28) | 4.4 (4.8–3.28) | 8.86 (12.56–5.16) | |
| CV | 30.67 | 30.67 | 59.06 | |
| Concentration at point 5, µg/mL | n | 15 | 15 | 2 |
| Mean (±SD) | 4.31 (3.08) | 4.31 (3.08) | 5.11 (3.55) | |
| Median (75%Q3–25%Q1) | 4.09 (5.96–1.84) | 4.09 (5.96–1.84) | 5.11 (7.62–2.60) | |
| CV | 71.33 | 71.33 | 69.47 | |
| AUC0-t, µg·h/mL | n | 15 | 15 | 2 |
| Mean(±SD) | 83.25 (44.05) | 83.25 (44.05) | 115.57 (10.79) | |
| Median (75%Q3–25%Q1) | 68.25 (118.65–61.17) | 68.25 (118.65–61.17) | 115.56 (123.20–107.94) | |
| CV | 52.92 | 52.92 | 9.34 | |
| Cmax, µg/mL | n | 15 | 15 | 2 |
| Mean(±SD) | 23.82 (12.57) | 23.82 (12.57) | 37.8 (9.76) | |
| Median (75%Q3–25%Q1) | 18.30 (30.6–16.18) | 18.30 (30.6–16.18) | 37.80 (44.70–30.90) | |
| CV | 52.78 | 52.78 | 25.82 | |
| Tmax, hours | n | 15 | 15 | 2 |
| Mean(±SD) | 2.4 (1.11) | 2.4 (1.11) | 2.25 (1.06) | |
| Median (75%Q3–25%Q1) | 1.5 (3.0–1.5) | 1.5 (3.0–1.5) | 2.25 (3.0–1.5) | |
| CV | 46.05 | 46.05 | 47.14 | |
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | n | 15 | 15 | 2 |
| Mean (±SD) | 3.93 (1.89) | 3.93 (1.89) | 4.84 (1.19) | |
| Median (75%Q3–25%Q1) | 3.27 (4.88–2.47) | 3.27 (4.88–2.47) | 4.84 (5.68–3.99) | |
| CV | 48.17 | 48.17 | 24.55 | |
| Cmax_norm, (µg/mL)/(mg/kg) | n | 15 | 15 | 2 |
| Mean (±SD) | 1.11 (0.49) | 1.11 (0.49) | 1.54 (0.16) | |
| Median (75%Q3–25%Q1) | 0.99 (1.45–0.69) | 0.99 (1.45–0.69) | 1.54 (1.66–1.42) | |
| CV | 43.87 | 43.87 | 10.64 |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi2 | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| Free member 0 (genotype AA) | 550.9 | 250.7 | 4.8289 | 0.0280 | ---------- |
| Free member 1 (GA genotype) | 634.3 | 286.4 | 4.9053 | 0.0268 | ---------- |
| Weight, kg | 1.4388 | 0.7370 | 3.8118 | 0.0509 | 10.3566 |
| Dose of ciprofloxacin, mg/kg | −28.7268 | 12.9844 | 4.8947 | 0.0269 | −52.7091 |
| Cmax, µg/mL | −15.2313 | 7.9661 | 3.6559 | 0.0559 | −94.2176 |
| Tmax, hours | 39.6197 | 17.8781 | 4.9111 | 0.0267 | 23.9541 |
| AUC, µg·h/mL | 8.7552 | 4.0921 | 4.5777 | 0.0324 | 200.5 |
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | −247.3 | 114.5 | 4.6616 | 0.0308 | −257.6 |
| Cmax_norm, (µg/mL)/(mg/kg) | 516.6 | 253 I | 4.1410 | 0.0419 | 129.6 |
| Use of azithromycin | −127.6 | 58.0950 | 4.8273 | 0.0280 | −31.6519 |
| Chronic persistent Pseudomonas aeruginosa infection | 102.0 | 46.7224 | 4.7666 | 0.0290 | 21.3175 |
| Chronic intermittent Pseudomonas aeruginosa infection | −87.2033 | 39.6337 | 4.8410 | 0.0278 | −20.6822 |
| Liver damage | 40.9851 | 18.8952 | 4.7049 | 0.0301 | 21.7897 |
| Pancreatic elastase | −108.1 | 49.2495 | 4.8144 | 0.0282 | −20.5988 |
| Homozygotes for F508del | 148.0 | 66.7351 | 4.9194 | 0.0266 | 36.7042 |
| Predictor | Modules of Standardized Regression Coefficients |
|---|---|
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | |257.6| |
| AUC, µg·h/mL | |200.5| |
| Cmax_norm, (µg/mL)/(mg/kg) | |129.6| |
| Cmax, µg/mL | |94.22| |
| CPF dose, mg/kg | |52.71| |
| Homozygotes for delF508 | |36.70| |
| Use of azithromycin | |31.65| |
| Tmax, hours | |23.95| |
| Liver damage | |21.79| |
| Chronic persistent Pseudomonas aeruginosa infection | |21.32| |
| Chronic intermittent Pseudomonas aeruginosa infection | |20.68| |
| Pancreatic elastase | |20.61| |
| Weight, kg | |10.36| |
| GSTP1 Genotype | Predictors | |
|---|---|---|
| Pharmacokinetic Parameters | Clinical Signs | |
| AA | High values of Cmax_norm High AUC Longer Tmax | Fecal elastase level above 200 µg/g (in stool) Liver damage without cirrhosis Chronic Pseudomonas Infection Genotype F508del/F508del High weight and percentile BMI |
| GA | High AUC0-t_norm | Intermittent Pseudomonas Infection |
| Parameter | Statistical Indicators | GCLC Genotypes | |
|---|---|---|---|
| “Other” | “7/7” Genotype | ||
| Dose, mg/kg | n | 18 | 14 |
| Mean (±SD) | 20.04 (5.66) | 22.1 (3.93) | |
| Median (75%Q3–25%Q1) | 20.0 (22.8–19.5) | 20.85 (25.0–19.40) | |
| CV | 28.26 | 17.78 | |
| Concentration at point 1, µg/mL | n | 17 | 14 |
| Mean (±SD) | 17.92 (12.81) | 17.79 (13.45) | |
| Median (75%Q3–25%Q1) | 17.90 (25.80–9.58) | 17.56 (24.09–5.40) | |
| CV | 71.47 | 75.63 | |
| Concentration at point 2, µg/mL | n | 17 | 14 |
| Mean (±SD) | 15.35 (12.84) | 18.80 (9.37) | |
| Median (75%Q3–25%Q1) | 11.09 (17.0–7.31) | 16.57 (21.0–12.13) | |
| CV | 83.66 | 49.87 | |
| Concentration at point 3, µg/mL | n | 17 | 13 |
| Mean (±SD) | 10.11 (8.27) | 17.29 (8.03) | |
| Median (75%Q3–25%Q1) | 6.03 (17.6–4.96) | 15.0 (23.89–9.90) | |
| CV | 81.86 | 46.40 | |
| Concentration at point 4, µg/mL | n | 10 | 8 |
| Mean (±SD) | 5.68 (5.65) | 6.99 (3.31) | |
| Median (75%Q3–25%Q1) | 3.36 (4.40–2.40) | 6.02 (9.40–4.60) | |
| CV | 99.47 | 47.28 | |
| Concentration at point 5, µg/mL | n | 17 | 14 |
| Mean (±SD) | 3.73 (3.72) | 7.69 (5.13) | |
| Median (75%Q3–25%Q1) | 2.01 (4.03–1.70) | 7.04 (10.79–4.09) | |
| CV | 99.59 | 66.73 | |
| AUC0-t, µg·h/mL | n | 17 | 14 |
| Mean (±SD) | 76.22 (40.84) | 99.48 (39.75) | |
| Median (75%Q3–25%Q1) | 73.68 (82.75–53.48) | 88.95 (110.34–68.25) | |
| CV | 53.58 | 39.95 | |
| Cmax, µg/mL | n | 17 | 14 |
| Mean(±SD) | 24.0 (12.56) | 25.63 (9.20) | |
| Median (75%Q3–25%Q1) | 23.2 (29.0–16.18) | 22.7 (30.61–18.30) | |
| CV | 52.32 | 35.89 | |
| Tmax, hours | n | 17 | 14 |
| Mean (±SD) | 2.12 (1.07) | 2.68 (1.34) | |
| Median (75%Q3–25%Q1) | 1.5 (3.0–1.5) | 2.25 (4.5–1.5) | |
| CV | 50.45 | 49.98 | |
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | n | 17 | 14 |
| Mean (±SD) | 3.62 (2.03) | 4.55 (1.68) | |
| Median (75%Q3–25%Q1) | 3.31 | 4.14 | |
| CV | (3.97–2.25) | (6.30–3.15) | |
| Cmax_norm, (µg/mL)/(mg/kg) | n | 56.09 | 36.86 |
| Mean (±SD) | 17 | 14 | |
| Median (75%Q3–25%Q1) | 1.12 (0.53) | 1.16 (0.34) | |
| CV | 1.00 (1.44–0.75) | 1.06 (1.38–0.94) | |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi-Squared Test | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| “Other” genotypes | 6.3620 | NA | 5.4146 | 0.0200 | 2.7341 |
| CPF concentration at point 5 | −0.5254 | −1.3908 | 6.0159 | 0.0142 | 0.2142 |
| Chronic staphylococcal infection | −4.2264 | −0.9606 | 3.6440 | 0.0563 | 2.2140 |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi-Squared Test | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| Weight, kg | −0.4636 | 0.7370 | 3.8118 | 0.0509 | 10.3566 |
| Height, cm | 0.2174 | 0.0934 | 5.4219 | 0.0199 | 2.7521 |
| Dose of ciprofloxacin, mg/kg | −0.4228 | 0.2168 | 3.8386 | 0.0501 | −0.7830 |
| Tmax, hours | −1.3875 | 0.7270 | 3.6426 | 0.0563 | −0.8905 |
| Chronic staphylococcal infection | −2.5359 | 1.5194 | 2.7859 | 0.0951 | −0.6015 |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi-Squared Test | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| TT genotype | 22.2390 | NA | 4.3622 | 0.0367 | 10.6479 |
| TS genotype | 25.0517 | NA | 5.2690 | 0.0217 | 10.9137 |
| F508del/F508del genotype | 2.4789 | 0.5809 | 4.6230 | 0.0315 | 1.1529 |
| Concentration at point 2, µg/mL | −0.1596 | −1.0190 | 5.2864 | 0.0215 | 0.0694 |
| Concentration at point 3, µg/mL | 0.4694 | 2.2766 | 3.9464 | 0.0470 | 0.2363 |
| Cmax, µg/mL | 1.7517 | 10.6082 | 6.4388 | 0.0112 | 0.6903 |
| Tmax, hours | −1.9260 | −1.2793 | 3.6026 | 0.0577 | 1.0147 |
| AUC0-t, µg·h/mL | −0.3396 | −7.7922 | 4.9033 | 0.0268 | 0.1534 |
| AUC0-t_norm, (µg·h/mL)/(mg/kg) | 6.7110 | 7.0541 | 4.4481 | 0.0349 | 3.1820 |
| Cmax_norm, (µg/mL)/(mg/kg) | −38.2657 | −1.3516 | 5.9664 | 0.0146 | 15.6659 |
| Dose of CPF, mg/kg | −0.9643 | −1.7227 | 3.8817 | 0.0488 | 0.4894 |
| Predictor | Regression Coefficient | Standardized Regression Coefficient | Wald Chi-Squared Test | The Level of Statistical Significance, p | Standard Error |
|---|---|---|---|---|---|
| Other genotypes | 54.9688 | NA | 5.1170 | 0.0237 | 24.2957 |
| Chronic and persistent Pseudomonas aeruginosa infection | −4.3270 | −1.0495 | 3.8955 | 0.0484 | 2.1923 |
| Concentration at point 1, µg/mL | −0.3704 | −2.6617 | 3.4964 | 0.0615 | 0.1981 |
| Concentration at point 2, µg/mL | −0.9071 | −5.7526 | 4.9559 | 0.0260 | 0.4075 |
| Concentration at point 5, µg/mL | −1.1133 | −2.8468 | 3.6473 | 0.0562 | 0.5829 |
| Cmax, µg/mL | 2.4391 | 14.9399 | 6.0739 | 0.0137 | 0.9897 |
| AUC0-t, µg·h/mL | 0.3349 | 7.6692 | 4.4298 | 0.0353 | 0.1591 |
| Cmax_norm, (µg·h/mL)/(mg/kg) | −48.1829 | −11.9427 | 5.4855 | 0.0192 | 20.5723 |
| Dose of CPF, mg/kg | −2.6767 | −4.7333 | 5.2891 | 0.0215 | 1.1639 |
| Genotype | Predictors | |
|---|---|---|
| Pharmacokinetic Parameters | Clinical Manifestations | |
| CYP2C9*3 (I359L, c.1075A > C) | ||
| AA | High values of Cmax | - |
| CYP2D6*4 (1846G > A) | ||
| GG | High values of AUC | History of meconium ileus A greater number of older children were here, thus weight, height and BMI were correspondingly higher |
| GCLC | ||
| 7/7 genotype | Longer Tmax | Chronic staphylococcal infection Higher weights compared to “other” genotypes |
| GSTP1 | ||
| AA | High values of Cmax_norm High values of AUC Longer Tmax | Fecal elastase level above 200 µg/g Liver damage Chronic persistent Pseudomonas aeruginosa infection Genotype F508del/F508del High weight and percentile of BMI |
| GA | High values of AUC0-t_norm High values of CPF dose, mg/kg | Intermittent Pseudomonas Infection Use of azithromycin |
| NAT2 (341T > C) | ||
| TT | Cmax AUC0-t_norm | Genotype F508del/F508del |
| TC CC | High values of CPF concentration after 1.5 h, 3.0 h, 7.5 h Cmax_norm Longer Tmax | - |
| F508del/F508del | ||
| Present | High concentrations of CPF after 1.5 h, 3.0 h and 7.5 h of taking the drug High values of Cmax_norm | Chronic Pseudomonas aerugenosae infection |
| Absent | Cmax | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyryanov, S.K.; Ushkalova, E.A.; Kondratyeva, E.I.; Butranova, O.I.; Kondakova, Y.A. Gene Polymorphism of Biotransformation Enzymes and Ciprofloxacin Pharmacokinetics in Pediatric Patients with Cystic Fibrosis. Biomedicines 2022, 10, 1050. https://doi.org/10.3390/biomedicines10051050
Zyryanov SK, Ushkalova EA, Kondratyeva EI, Butranova OI, Kondakova YA. Gene Polymorphism of Biotransformation Enzymes and Ciprofloxacin Pharmacokinetics in Pediatric Patients with Cystic Fibrosis. Biomedicines. 2022; 10(5):1050. https://doi.org/10.3390/biomedicines10051050
Chicago/Turabian StyleZyryanov, Sergey K., Elena A. Ushkalova, Elena I. Kondratyeva, Olga I. Butranova, and Yulia A. Kondakova. 2022. "Gene Polymorphism of Biotransformation Enzymes and Ciprofloxacin Pharmacokinetics in Pediatric Patients with Cystic Fibrosis" Biomedicines 10, no. 5: 1050. https://doi.org/10.3390/biomedicines10051050
APA StyleZyryanov, S. K., Ushkalova, E. A., Kondratyeva, E. I., Butranova, O. I., & Kondakova, Y. A. (2022). Gene Polymorphism of Biotransformation Enzymes and Ciprofloxacin Pharmacokinetics in Pediatric Patients with Cystic Fibrosis. Biomedicines, 10(5), 1050. https://doi.org/10.3390/biomedicines10051050






