FIB-4 and APRI as Predictive Factors for Short- and Long-Term Survival in Patients with Transjugular Intrahepatic Portosystemic Stent Shunts
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Cohort Characteristics
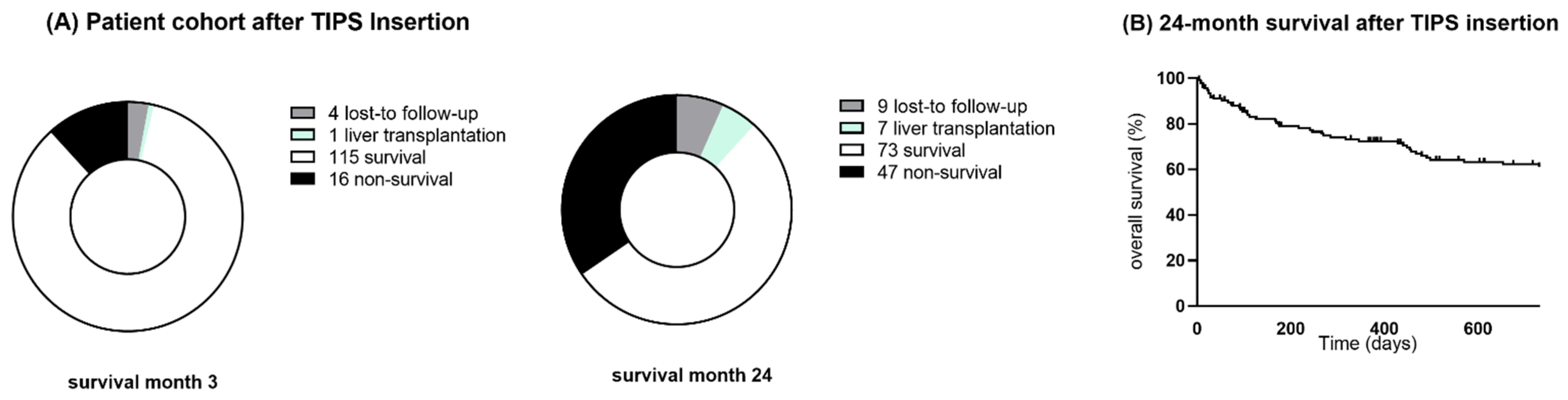
3.2. Cohort Follow-Up and Survival
3.3. Short-Term and Long-Term Survival Predictive Factors
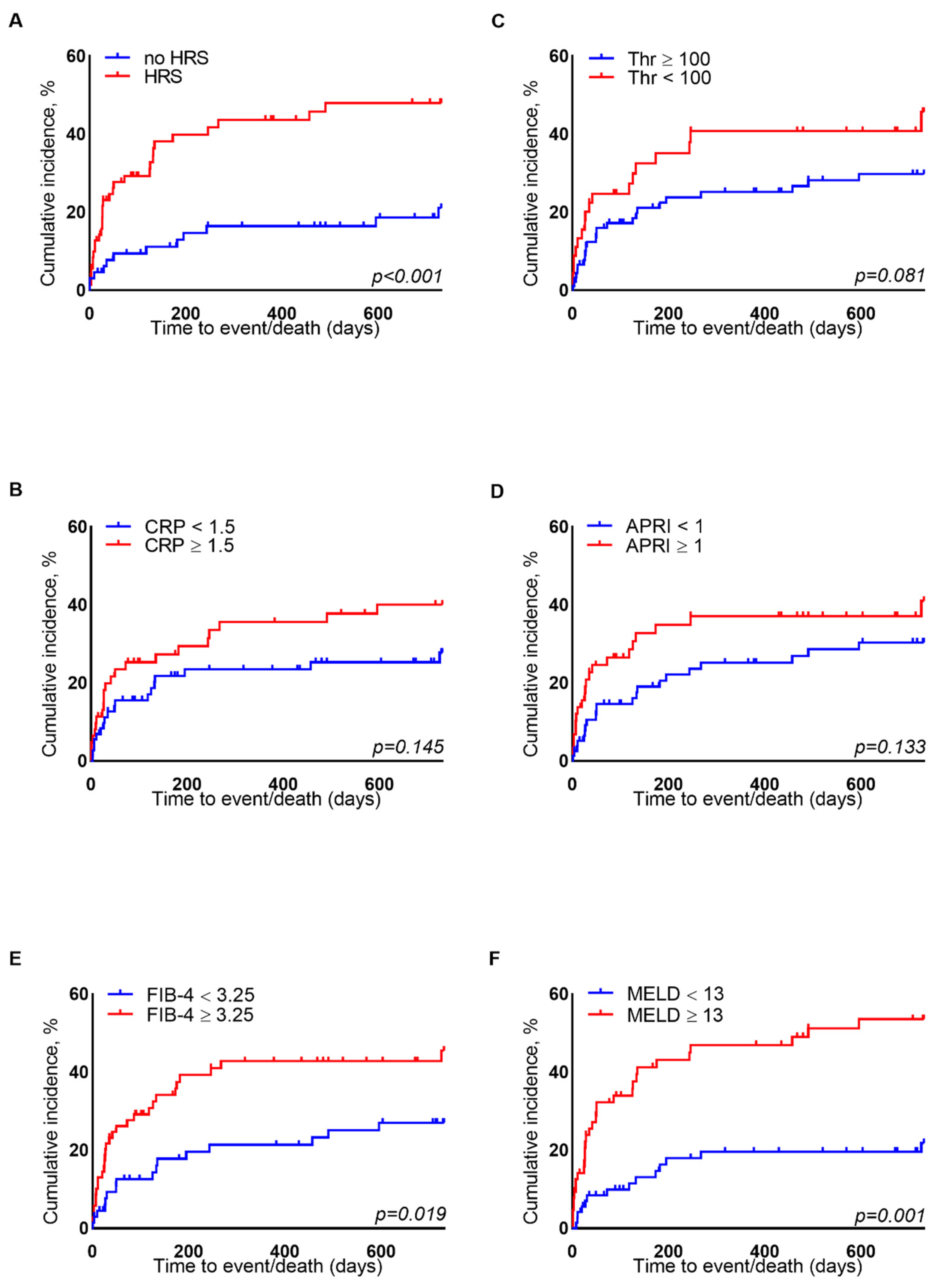
3.4. Liver-Related Event Incidence during Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trebicka, J. Predisposing Factors in Acute-on-Chronic Liver Failure. Semin. Liver Dis. 2016, 36, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Rössle, M.; Siegerstetter, V.; Euringer, W.; Olschewski, M.; Kromeier, J.; Kurz, K.; Langer, M. The use of a polytetrafluoroethylene-covered stent graft for transjugular intrahepatic portosystemic shunt (TIPS): Long-term follow-up of 100 patients. Acta Radiol. 2006, 47, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Banares, R.; Gonzalez, M.; Catalina, M.V.; Molinero, L.M. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J. Hepatol. 2005, 43, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Travis, S.; Armstrong, M.J.; A Tsochatzis, E.; A Rowe, I.; Roslund, N.; Ireland, H.; Lomax, M.; et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut 2020, 69, 1173–1192. [Google Scholar] [CrossRef] [PubMed]
- Bureau, C.; Thabut, D.; Oberti, F.; Dharancy, S.; Carbonell, N.; Bouvier, A.; Mathurin, P.; Otal, P.; Cabarrou, P.; Péron, J.M.; et al. Transjugular Intrahepatic Portosystemic Shunts with Covered Stents Increase Transplant-Free Survival of Patients with Cirrhosis and Recurrent Ascites. Gastroenterology 2017, 152, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllou, T.; Aggarwal, P.; Gupta, E.; Svetanoff, W.J.; Bhirud, D.P.; Singhal, S. Polytetrafluoroethylene-Covered Stent Graft Versus Bare Stent in Transjugular Intrahepatic Portosystemic Shunt: Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 867–879. [Google Scholar] [CrossRef]
- Berry, K.; Lerrigo, R.; Liou, I.W.; Ioannou, G.N. Association Between Transjugular Intrahepatic Portosystemic Shunt and Survival in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 118–123. [Google Scholar] [CrossRef]
- Sarwar, A.; Zhou, L.; Novack, V.; Tapper, E.B.; Curry, M.; Malik, R.; Ahmed, M. Hospital volume and mortality after transjugular intrahepatic portosystemic shunt creation in the United States. Hepatology 2018, 67, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Fagiuoli, S.; Bruno, R.; Venon, W.D.; Schepis, F.; Vizzutti, F.; Toniutto, P.; Senzolo, M.; Caraceni, P.; Salerno, F.; Angeli, P.; et al. Consensus conference on TIPS management: Techniques, indications, contraindications. Dig. Liver Dis. 2017, 49, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Bureau, C.; Métivier, S.; D’Amico, M.; Péron, J.M.; Otal, P.; Garcia-Pagan, J.C.; Chabbert, V.; Chagneau-Derrode, C.; Procopet, B.; Rousseau, H.; et al. Serum bilirubin and platelet count: A simple predictive model for survival in patients with refractory ascites treated by TIPS. J. Hepatol. 2011, 54, 901–907. [Google Scholar] [CrossRef]
- Khabbaz, R.C.; Lokken, R.P.; Chen, Y.-F.; Lipnik, A.J.; Bui, J.T.; Ray, C.E.; Gaba, R.C. Albumin-Bilirubin and Platelet-Albumin-Bilirubin Grades Do Not Predict Survival After Transjugular Intrahepatic Portosystemic Shunt Creation. Cardiovasc. Intervent. Radiol. 2018, 41, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Schepke, M.; Roth, F.; Fimmers, R.; Brensing, K.A.; Sudhop, T.; Schild, H.H.; Sauerbruch, T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am. J. Gastroenterol. 2003, 98, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Snyder, N.; Gajula, L.; Xiao, S.-Y.; Grady, J.; Luxon, B.; Lau, D.T.-Y.; Soloway, R.; Petersen, J. APRI: An easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J. Clin. Gastroenterol. 2006, 40, 535–542. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score, and APRI for NAFLD-related events: A systematic review. Liver Int. 2020, 41, 261–270. [Google Scholar] [CrossRef]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.K.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Bhangui, P.; Laurent, A.; Amathieu, R.; Azoulay, D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J. Hepatol. 2012, 57, 874–884. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Yang, Z.; Liu, L.; Li, K.; He, C.; Wang, Z.; Bai, W.; Guo, W.; Yu, T.; Yuan, X.; et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: A randomised controlled trial. Lancet Gastroenterol. Hepatol. 2019, 4, 587–598. [Google Scholar] [CrossRef]
- Bucsics, T.; Hoffman, S.; Grünberger, J.; Schoder, M.; Matzek, W.; Stadlmann, A.; Mandorfer, M.; Schwabl, P.; Ferlitsch, A.; Peck-Radosavljevic, M.; et al. ePTFE-TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018, 38, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casadaban, L.C.; Parvinian, A.; Zivin, S.P.; Lakhoo, J.; Minocha, J.; Knuttinen, M.G.; Ray, C.E.; Bui, J.T.; Gaba, R.C. MELD score for prediction of survival after emergent TIPS for acute variceal hemorrhage: Derivation and validation in a 101-patient cohort. Ann. Hepatol. 2015, 14, 380–388. [Google Scholar] [CrossRef]
- Angermayr, B.; Cejna, M.; Karnel, F.; Gschwantler, M.; Koenig, F.; Pidlich, J.; Mendel, H.; Pichler, L.; Wichlas, M.; Kreil, A.; et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut 2003, 52, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Gaba, R.C.; Couture, P.M.; Bui, J.T.; Knuttinen, M.G.; Walzer, N.M.; Kallwitz, E.R.; Berkes, J.L.; Cotler, S.J. Prognostic capability of different liver disease scoring systems for prediction of early mortality after transjugular intrahepatic portosystemic shunt creation. J. Vasc. Interv. Radiol. 2013, 24, 411–420.e4. [Google Scholar] [CrossRef]
- Schepis, F.; Vizzutti, F.; Garcia-Tsao, G.; Marzocchi, G.; Rega, L.; De Maria, N.; Di-Maira, T.; Gitto, S.; Caporali, C.; Colopi, S.; et al. Under-dilated TIPS Associate With Efficacy and Reduced Encephalopathy in a Prospective, Non-randomized Study of Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2018, 16, 1153–1162.e7. [Google Scholar] [CrossRef] [Green Version]
- Vozzo, C.F.; Singh, T.; Bullen, J.; Sarvepalli, S.; McCullough, A.; Kapoor, B. Hospital readmission following transjugular intrahepatic portosystemic shunt: A 14-year single-center experience. Gastroenterol. Rep. 2020, 8, 98–103. [Google Scholar] [CrossRef]
- Bosch, J. Small diameter shunts should lead to safe expansion of the use of TIPS. J. Hepatol. 2020, 74, 230–234. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Chalasani, N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018, 68, 305–315. [Google Scholar] [CrossRef]
- Vasconcelos, M.P.A.; DallÁcqua, D.V.; Wedemeyer, H.; Witkin, S.S.; Mendes-Corrêa, M.C.; Villalobos-Salcedo, J.M. Noninvasive Models for Predicting Liver Fibrosis in Individuals with Hepatitis D Virus/Hepatitis B Virus Coinfection in the Brazilian Amazon Region. Am. J. Trop. Med. Hyg. 2020, 103, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Mueller, S.; Szabo, G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J. Hepatol. 2019, 70, 273–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, C. Markers of immunity and bacterial translocation in cirrhosis. Dan. Med. J. 2015, 62, B5121. [Google Scholar] [PubMed]
- Pieri, G.; Agarwal, B.; Burroughs, A.K. C-reactive protein and bacterial infection in cirrhosis. Ann. Gastroenterol. 2014, 27, 113–120. [Google Scholar]
- Alexopoulou, A.; Agiasotelli, D.; Vasilieva, L.E.; Dourakis, S.P. Bacterial translocation markers in liver cirrhosis. Ann. Gastroenterol. 2017, 30, 486–497. [Google Scholar] [CrossRef]

| All Patients | Refractory Ascites | Variceal Bleeding | p-Value | |
|---|---|---|---|---|
| Patients, n (%) | 136 (100) | 117 (86) | 19 (14) | |
| Age, y | 57 (49–64) | 57 (50–64) | 54 (37–63) | 0.244 |
| Male/female, n (%) | 84/52 (62/38) | 74/43 (63/37) | 10/9 (53/47) | 0.377 |
| Etiology of cirrhosis | ||||
| Alcohol/viral/autoimmune/NASH/other, n | 102/5/9/12/8 | 93/3/6/9/6 | 9/2/3/3/2 | 0.025 |
| Child–Pugh A/B/C, n | 19/102/15 | 6/97/14 | 13/5/1 | 0.001 |
| MELD | 12 (10–15) | 13 (10–16) | 11 (8–12) | 0.049 |
| CLIF-C-AD Score | 47 (42–53) | 48 (43–53) | 43 (38–48) | 0.007 |
| CCI | 5 (4–7) | 5 (4–7) | 4 (3–7) | 0.414 |
| BMI, kg/m2 | 25 (22–29) | 25 (22–29) | 26 (22–33) | 0.355 |
| Esophageal varices | ||||
| No varices/Grad I-II / III-IV | 38/76/22 | 38/62/17 | 0/14/5 | 0.012 |
| GI bleeding, n (%) | 52 (38) | 33 (28) | 19 (100) | <0.0001 |
| HRS yes/no, n (%) | 71/65 (52/48) | 66/51 (56/44) | 5/14 (26/74) | 0.015 |
| Previous SPB yes/no, n (%) | 44/92 (32/68) | 41/76 (35/65) | 3/16 (16/84) | 0.096 |
| HE, n (%) | 29 (21) | 23 (20) | 6 (32) | 0.239 |
| Elective/urgent, n | 134/2 | 117/0 | 17/2 | 0.001 |
| TIPS procedural characteristics | ||||
| Pre-TIPS PSG, mmHg | 22 (18–25) | 22 (18–25) | 22 (18–25) | 0.934 |
| Post-TIPS PSG, mmHg | 9 (7–10) | 8 (7–10) | 9 (7–11) | 0.290 |
| Reduction PSG, mmHg | 13 (9–17) | 13 (9–17) | 13 (8–17) | 0.719 |
| Stent diameter, mm | 8 (6–8) | 8 (6–8) | 8 (6–8) | 0.999 |
| Coil embolization, n (%) | 19 (14) | 10 (9) | 9 (47) | <0.0001 |
| Laboratory characteristics | ||||
| Leukocytes, /nL | 5.9 (4.1–7.8) | 6.1 (4.8–8.5) | 3.9 (3.3–5.8) | 0.008 |
| Hemoglobin, g/dL | 10 (8.6–12) | 10 (8.8–12) | 9 (8.3–11) | 0.336 |
| Platelets, /nL | 130 (91–188) | 141 (97–219) | 90 (62–114) | 0.001 |
| INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.565 |
| Fibrinogen, mg/dL | 277 (201–377) | 279 (199–389) | 295 (222–311) | 0.635 |
| Sodium, mmol/L | 137 (134–139) | 137 (134–139) | 138 (136–141) | 0.074 |
| Creatinine, mg/dL | 1.2 (1.0–1.6) | 1.2 (1.0–1.7) | 1.0 (0.9–1.1) | 0.002 |
| Bilirubin, mg/dL | 1.2 (0.7–1.7) | 1.2 (0.7–1.7) | 1.3 (0.8–1.7) | 0.789 |
| AST, U/L | 36 (29–47) | 35 (28–42) | 46 (34–59) | 0.004 |
| ALT, U/L | 20 (14–28) | 19 (14–25) | 30 (18–53) | 0.001 |
| Protein, g/dL | 6.4 (5.5–7.1) | 6.2 (5.5–6.9) | 7.1 (6.1–8.0) | 0.005 |
| Albumin, g/dL | 3.2 (2.8–3.6) | 3.1 (2.8–3.5) | 3.6 (2.9–4.0) | 0.028 |
| CRP, mg/dL | 1.6 (0.6–3.1) | 1.6 (0.7–3.4) | 0.6 (0.5–1.8) | 0.029 |
| All Patients Month 3 | LTF Survival | LTF Non-Survival | p-Value | All Patients Month 24 | LTF Survival | LTF Non-Survival | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Patients, n (%) | 131 (100) | 115 (88) | 16 (12) | 120 (100) | 73 (61) | 47 (39) | ||
| Age, y | 58 (50–64) | 58 (50–64) | 58 (50–63) | 0.712 | 58 (50–65) | 57 (47–64) | 60 (53–66) | 0.046 |
| Male/female, n (%) | 80/51 (61/39) | 69/46 (60/40) | 11/5 (69/31) | 0.592 | 72/48 (60/40) | 42/31 (58/42) | 31/16 (66/34) | 0.444 |
| MELD | 12 (10–15) | 12 (10–15) | 16 (12–23) | <0.001 | 12 (10–15) | 12 (9–15) | 14 (11–17) | 0.004 |
| HRS yes/no, n (%) | 68/63 (52/48)) | 56/59 (49/51) | 12/4 (75/25) | 0.048 | 34/39 (47/53) | 30/17 (64/36) | 0.064 | |
| Pre-TIPS PSG, mmHg | 22 (18–25) | 22 (19–26) | 18 (14–22) | 0.004 | 22 (18–25) | 22 (19–26) | 20 (16–23) | 0.005 |
| Post-TIPS PSG, mmHg | 9 (7–10) | 9 (7–10) | 7 (6–10) | 0.109 | 8 (7–10) | 9 (7–11) | 8 (6–10) | 0.159 |
| Reduction PSG, mmHg | 13 (9–17) | 14 (10–17) | 11 (7–13) | 0.044 | 13 (10–17) | 14 (11–17) | 13 (8–16) | 0.042 |
| Laboratory characteristics | ||||||||
| Leukocytes, /nL | 5.9 (4.0–7.8) | 5.8 (3.9–7.8) | 6.6 (4.5–8.9) | 0.273 | 5.8 (4.3–8.0) | 6.1 (3.9–8.9) | 5.6 (4.4–7.4) | 0.258 |
| Hemoglobin, g/dL | 10 (8.8–12) | 10 (8.8–12) | 10 (8.3–11) | 0.174 | 10 (8.6–12.0) | 10 (9.0–12.0) | 9.7 (8.0–12.0) | 0.123 |
| Platelets, /nL | 130 (91–189) | 138 (95–206) | 106 (62–150) | 0.134 | 130 (91–186) | 144 (98–234) | 107 (74–154) | 0.001 |
| INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.2–1.4) | 0.052 | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.298 |
| Fibrinogen, mg/dL | 279 (201–377) | 284 (204–389) | 234 (126–312) | 0.052 | 277 (201–377) | 298 (216–384) | 250 (186–344) | 0.229 |
| Sodium, mmol/L | 137 (134–139) | 137 (134–139) | 137 (131–138) | 0.235 | 137 (134–139) | 137 (135–139) | 137 (134–139) | 0.629 |
| Creatinine, mg/dL | 1.1 (1.0–1.6) | 1.1 (1.0–1.5) | 1.3 (1.1–2.2) | 0.008 | 1.2 (1.0–1.6) | 1.1 (1.0–1.4) | 1.3 (1.0–2.0) | 0.009 |
| Bilirubin, mg/dL | 1.2 (0.7–1.7) | 1.2 (0.7–1.7) | 1.4 (0.6–2.5) | 0.201 | 1.2 (0.7–1.7) | 1.1 (0.7–1.6) | 1.2 (0.7–1.8) | 0.723 |
| AST, U/L | 35 (29–47) | 35 (29–47) | 36 (31–48) | 0.037 | 35 (29–47) | 34 (27–41) | 37 (30–51) | 0.051 |
| ALT, U/L | 20 (15–28) | 20 (15–28) | 16 (13–35) | 0.051 | 20 (14–28) | 20 (15–26) | 19 (14–33) | 0.183 |
| Protein, g/dL | 6.4 (5.5–7.1) | 6.5 (5.7–7.1) | 6.0 (5.0–6.4) | 0.009 | 6.3 (5.5–7.0) | 6.5 (5.8–7.1) | 5.9 (5.3–6.6) | 0.002 |
| Albumin, g/dL | 3.2 (2.8–3.6) | 3.2 (2.8–3.6) | 3.1 (2.6–3.5) | 0.291 | 3.2 (2.8–3.6) | 3.2 (2.8–3.6) | 3.1 (2.9–3.5) | 0.535 |
| CRP, mg/dL | 1.3 (0.6–2.9) | 1.3 (0.6–2.8) | 2.1 (0.9–6.6) | 0.0002 | 1.4 (0.6–2.8) | 1.3 (0.7–2.7) | 1.6 (0.6–3.3) | 0.067 |
| APRI | 0.8 (0.5–1.4) | 0.7 (0.5–1.4) | 1.0 (0.6–2.4) | 0.034 | 0.8 (0.5–1.4) | 0.7 (0.5–1.1) | 1.0 (0.6–1.7) | 0.008 |
| FIB-4 | 3.3 (2.2–5.5) | 3.2 (2.1–4.9) | 4.7 (3.0–8.2) | 0.011 | 3.3 (2.3–5.5) | 2.9 (2.0–4.0) | 4.7 (3.3–6.5) | <0.0001 |
| Risk Factor for Mortality | Multivariate Analysis 3 Months | Multivariate Analysis 24 Months | ||||
|---|---|---|---|---|---|---|
| HR | SE | p | HR | SE | p | |
| MELD | 1.228 | 0.089 | 0.022 | 1.094 | 0.059 | 0.129 |
| Pre-TIPS PSG | 0.839 | 0.074 | 0.19 | 0.889 | 0.045 | 0.009 |
| Protein | 0.673 | 0.388 | 0.307 | 0.623 | 0.247 | 0.060 |
| CRP | 1.240 | 0.100 | 0.031 | |||
| FIB-4 | 1.225 | 0.188 | 0.282 | 1.389 | 0.096 | 0.001 |
| No Event | Event | p-Value | |
|---|---|---|---|
| Patients, n (%) | 77 (57) | 59 (43) | |
| Age, y | 54 (47–63) | 60 (53–66) | 0.001 |
| Male/female, n (%) | 45/32 (58/42) | 39/20 (66/34) | 0.002 |
| Etiology of cirrhosis | |||
| Alcohol/viral/autoimmune/NASH/other, n | 60/3/3/6/5 | 41/2/8/6/2 | 0.283 |
| Child–Pugh A/B/C, n | 14/56/7 | 5/46/8 | 0.225 |
| MELD | 12 (10–14) | 13 (10–16) | 0.009 |
| CLIF C AD Score | 47 (42–53) | 48 (42–52) | 0.772 |
| CCI (points) | 4 (3–6) | 6 (5–8) | 0.001 |
| BMI, kg/m2 | 24 (22–29) | 25 (22–30) | 0.253 |
| Esophageal varices | |||
| No varices/Grade I-II/III-IV | 27/36/14 | 11/40/8 | 0.045 |
| GI bleeding, n (%) | 27 (35) | 25 (42) | 0.385 |
| HRS yes/no, n (%) | 33/44 (43/57) | 38/21 (78/22) | 0.013 |
| Previous SPB yes/no, n (%) | 20/57 (26/74) | 38/21 (64/36) | 0.069 |
| Elective /urgent, n | 77/0 | 57/2 | 0.104 |
| TIPS procedural characteristics | |||
| Pre-TIPS PSG, mmHg | 22 (19–26) | 21 (17–23) | 0.004 |
| Post-TIPS PSG, mmHg | 9 (7–11) | 8 (6–10) | 0.061 |
| Reduction PSG, mmHg | 14 (10–17) | 13 (9–16) | 0.055 |
| Stent diameter, mm | 8 (6–8) | 7 (6–8) | 0.065 |
| Coil embolization, n (%) | 14 (18) | 5 (9) | 0.106 |
| Laboratory characteristics | |||
| Leukocytes, /nL | 6.0 (4.1–8.8) | 5.7 (4.0–7.5) | 0.329 |
| Hemoglobin, g/dL | 10 (8.9–12) | 10 (8.3–12.0) | 0.119 |
| Platelets, /nL | 150 (104–227) | 111 (85–164) | 0.009 |
| INR | 1.2 (1.1–1.3) | 1.2 (1.1–1.3) | 0.828 |
| Fibrinogen, mg/dL | 289 (203–391) | 262 (190–344) | 0.275 |
| Sodium, mmol/L | 137 (134–140) | 137 (134–139) | 0.411 |
| Creatinine, mg/dL | 1.1 (1.0–1.4) | 1.3 (1.1–1.8) | 0.004 |
| Bilirubin, mg/dL | 1.2 (0.8–1.7) | 1.2 (0.7–1.6) | 0.372 |
| AST, U/L | 36 (30–42) | 35 (27–50) | 0.319 |
| ALT, U/L | 20 (15–27) | 18 (14–29) | 0.493 |
| Protein, g/dL | 6.6 (5.9–7.3) | 6.0 (5.3–6.6) | 0.0001 |
| Albumin, g/dL | 3.2 (2.8–3.6) | 3.1 (2.7–3.5) | 0.378 |
| CRP, mg/dL | 1.4 (0.7–3.0) | 1.4 (0.6–3.0) | 0.225 |
| APRI | 0.7 (0.5–1.1) | 1.0 (0.5–1.6) | 0.292 |
| FIB-4 | 2.9 (2.0–3.9) | 4.4 (3.0–6.3) | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keimburg, S.A.; Theysohn, J.; Buechter, M.; Rashidi-Alavijeh, J.; Willuweit, K.; Schneider, H.; Wetter, A.; Maasoumy, B.; Lange, C.; Wedemeyer, H.; et al. FIB-4 and APRI as Predictive Factors for Short- and Long-Term Survival in Patients with Transjugular Intrahepatic Portosystemic Stent Shunts. Biomedicines 2022, 10, 1018. https://doi.org/10.3390/biomedicines10051018
Keimburg SA, Theysohn J, Buechter M, Rashidi-Alavijeh J, Willuweit K, Schneider H, Wetter A, Maasoumy B, Lange C, Wedemeyer H, et al. FIB-4 and APRI as Predictive Factors for Short- and Long-Term Survival in Patients with Transjugular Intrahepatic Portosystemic Stent Shunts. Biomedicines. 2022; 10(5):1018. https://doi.org/10.3390/biomedicines10051018
Chicago/Turabian StyleKeimburg, Simone Anna, Jens Theysohn, Matthias Buechter, Jassin Rashidi-Alavijeh, Katharina Willuweit, Hannah Schneider, Axel Wetter, Benjamin Maasoumy, Christian Lange, Heiner Wedemeyer, and et al. 2022. "FIB-4 and APRI as Predictive Factors for Short- and Long-Term Survival in Patients with Transjugular Intrahepatic Portosystemic Stent Shunts" Biomedicines 10, no. 5: 1018. https://doi.org/10.3390/biomedicines10051018
APA StyleKeimburg, S. A., Theysohn, J., Buechter, M., Rashidi-Alavijeh, J., Willuweit, K., Schneider, H., Wetter, A., Maasoumy, B., Lange, C., Wedemeyer, H., & Markova, A. A. (2022). FIB-4 and APRI as Predictive Factors for Short- and Long-Term Survival in Patients with Transjugular Intrahepatic Portosystemic Stent Shunts. Biomedicines, 10(5), 1018. https://doi.org/10.3390/biomedicines10051018






