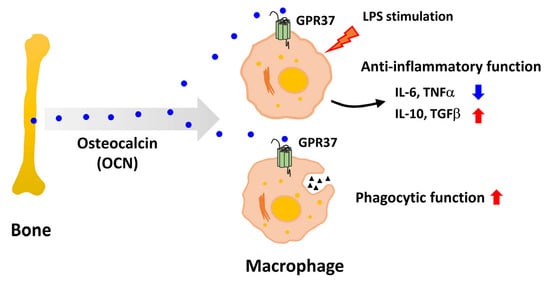
Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Serum Inflammatory Cytokines Analysis
2.3. Flow Cytometry
2.4. Peptides
2.5. Cell Culture and Treatment
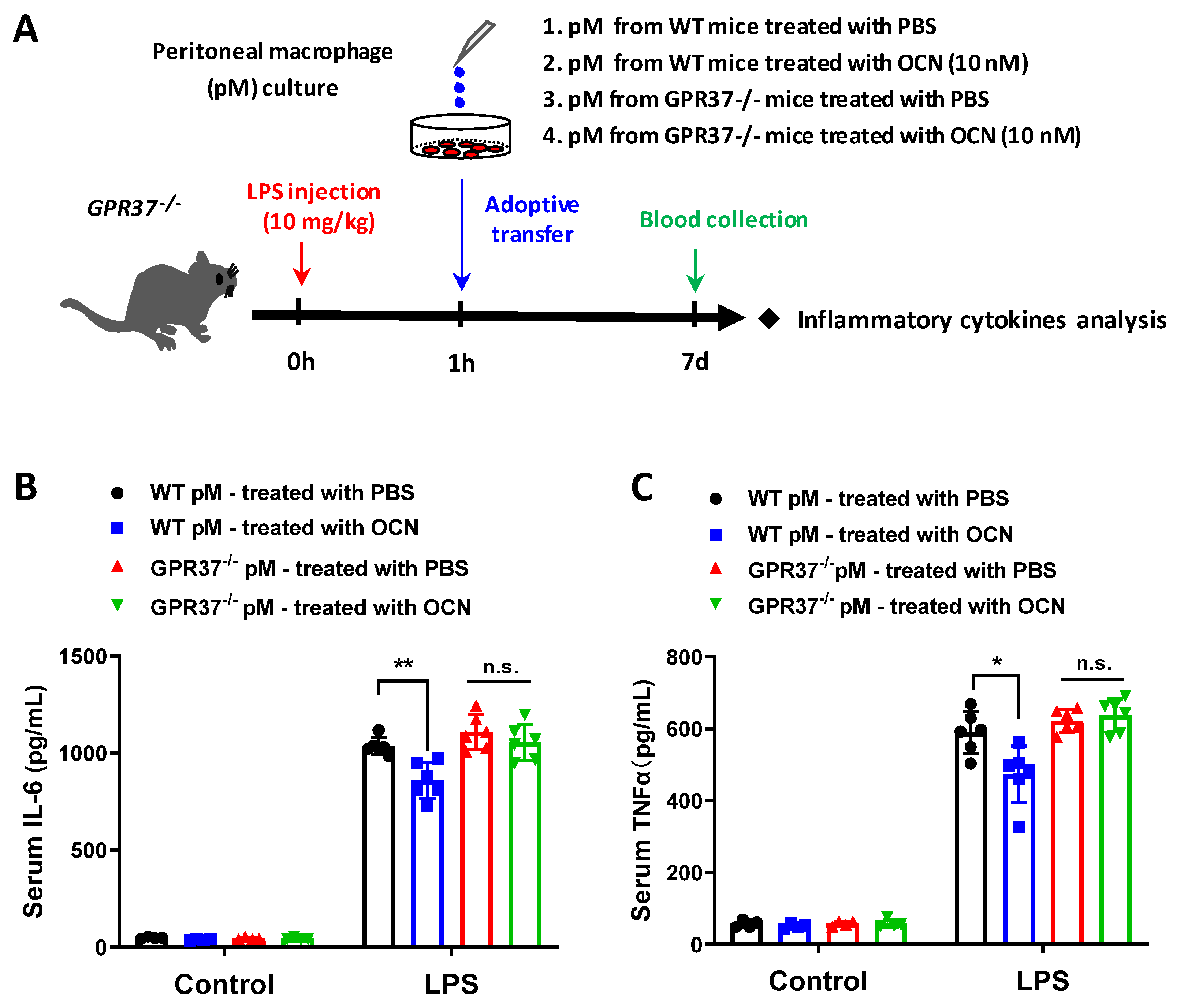
2.6. Adoptive Transfer of Macrophage
2.7. RNA Extraction and qRT-PCR
2.8. Western Blotting
2.9. cAMP Production Assays
2.10. Macrophage Phagocytosis Assay
2.11. Statistical Analysis
3. Results
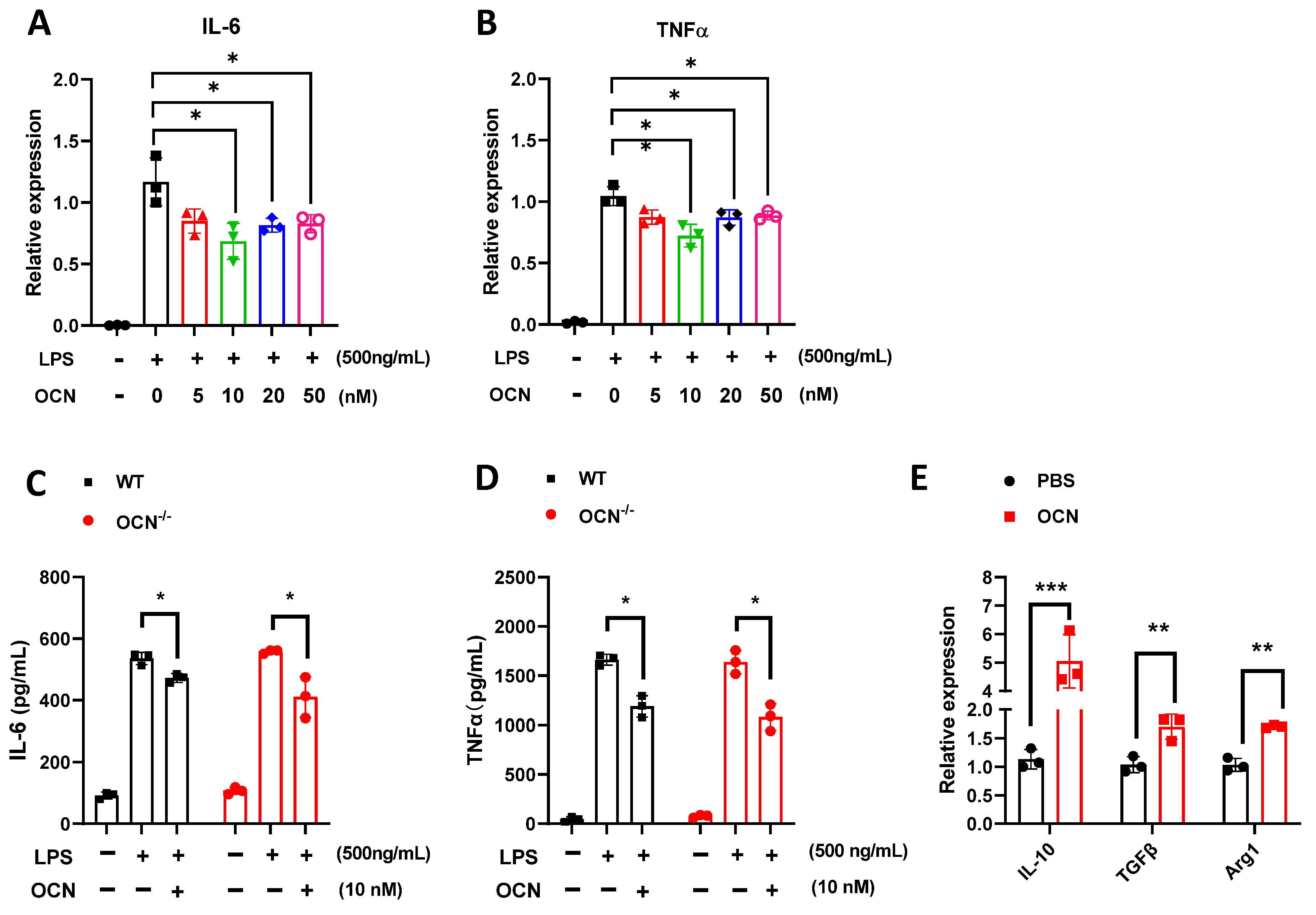
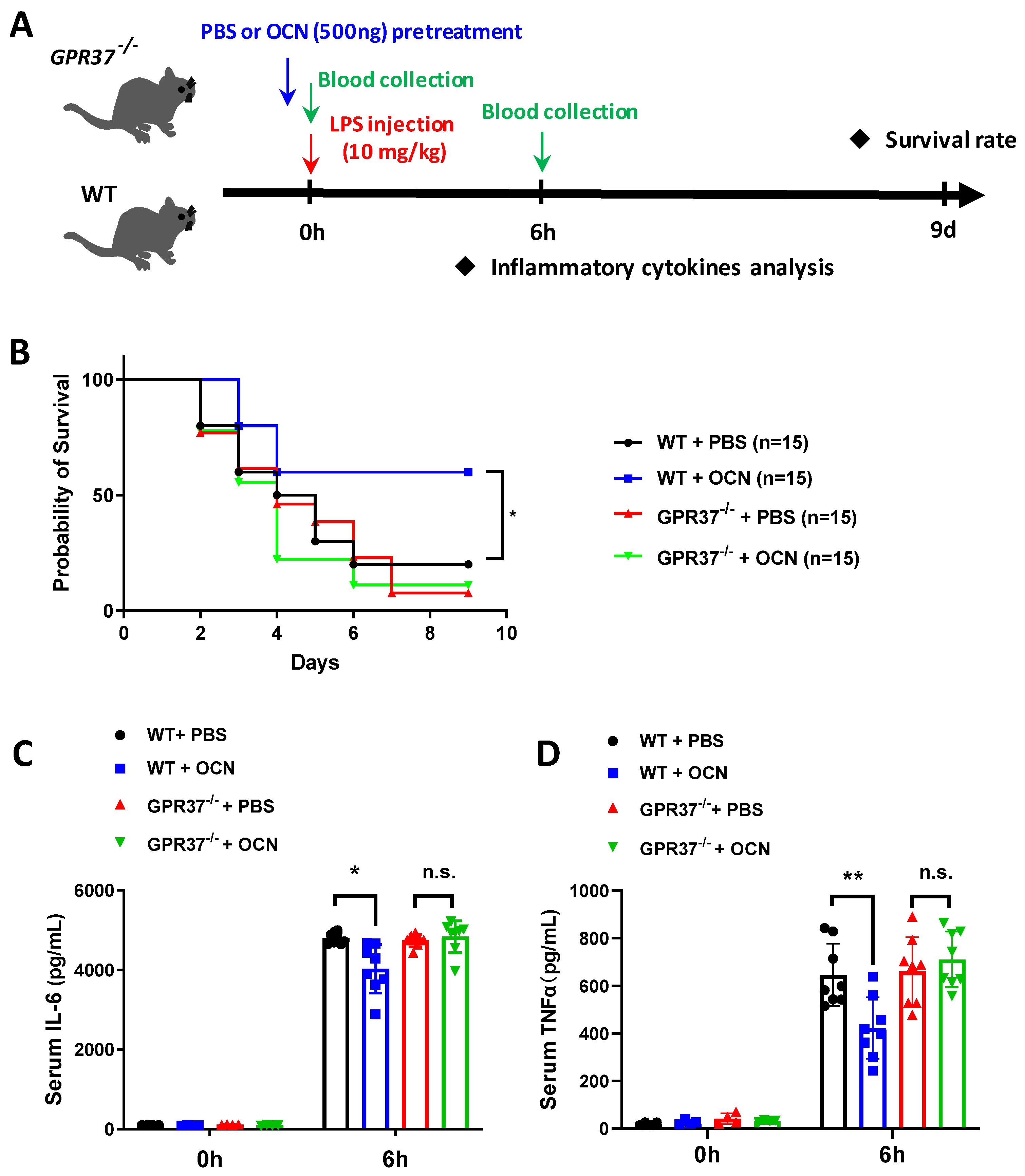
3.1. OCN Attenuates LPS-Induced Acute Inflammation in Both WT and OCN−/− Mice
3.2. OCN Inhibits the Release of Pro-Inflammatory Factors in LPS-Treated Macrophages
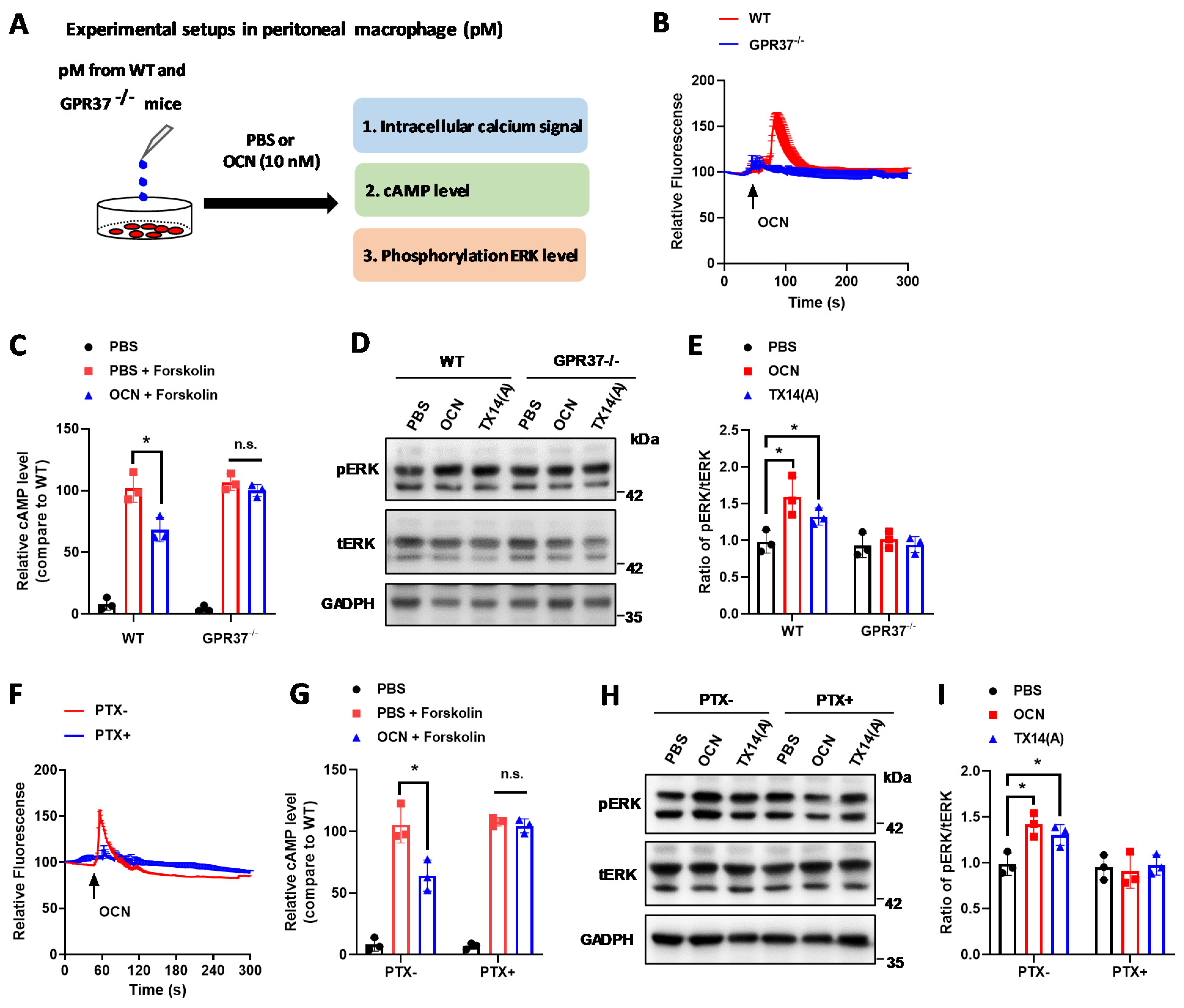
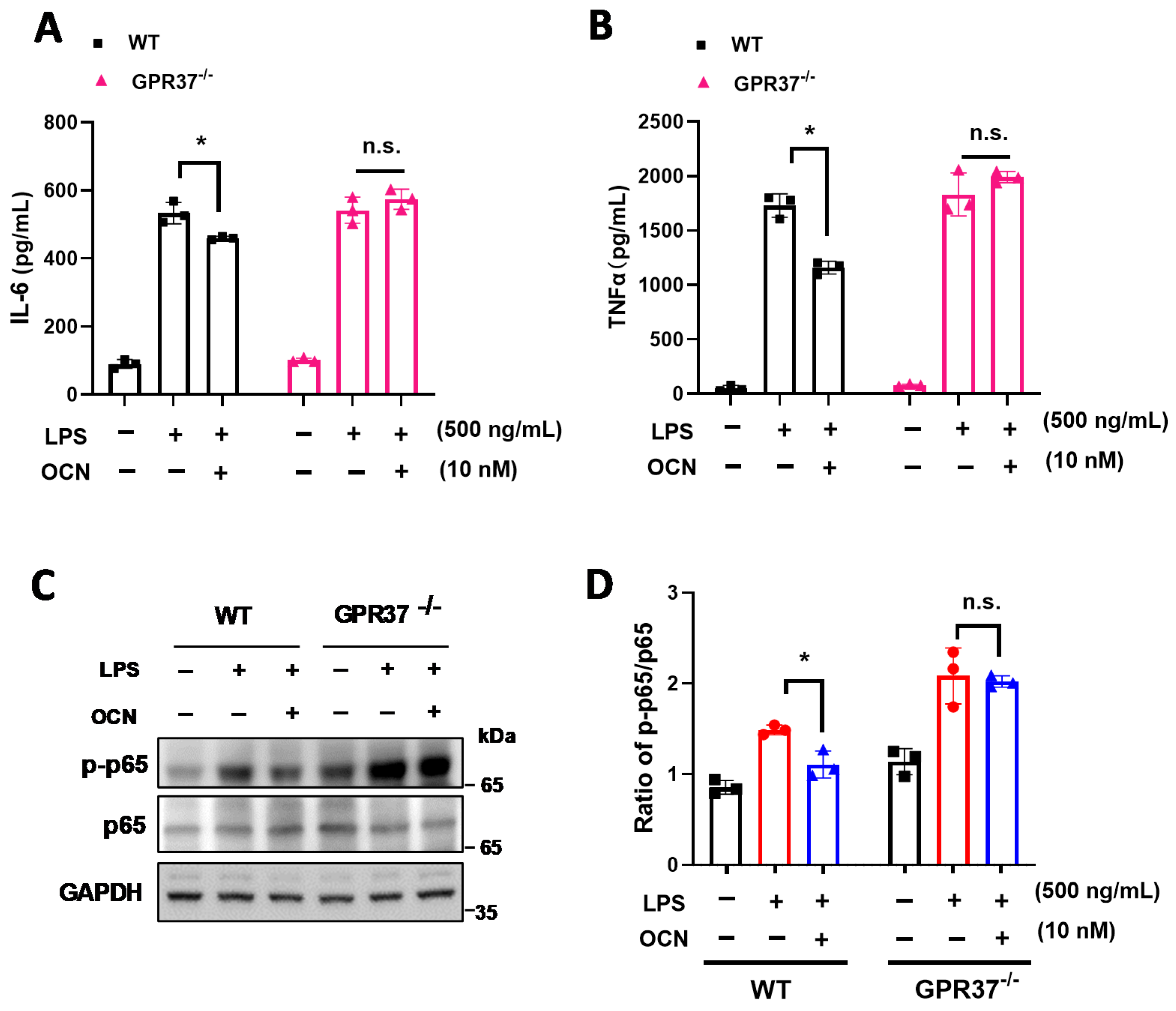
3.3. GPR37 Mediates OCN’s Signal in Macrophages
3.4. GRP37 Is Required for OCN’s Function in Macrophages
3.5. GPR37 Is Required for OCN’s Protective Role against LPS-Induced Acute Inflammation In Vivo
3.6. Adoptive Transfer of OCN-Treated Macrophages Alleviates LPS-Induced Acute Inflammation
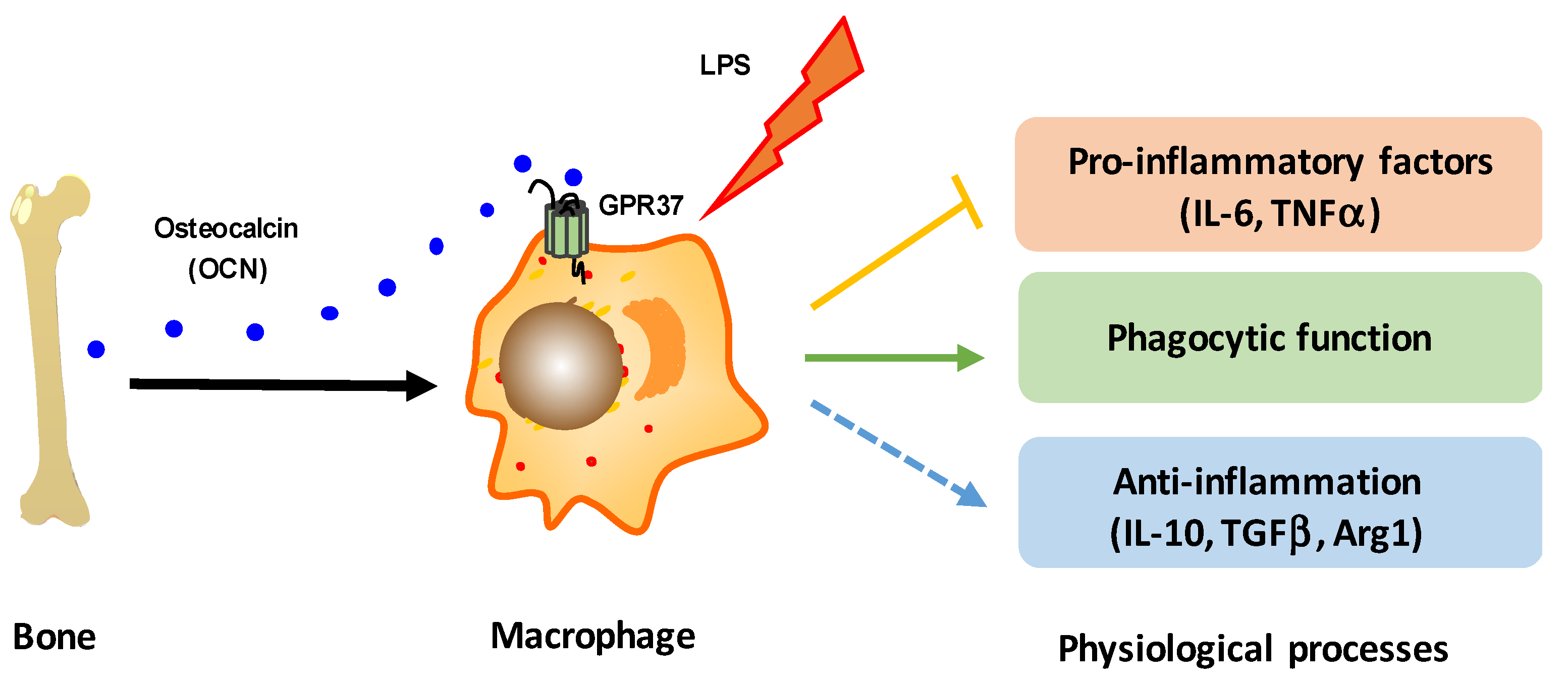
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WT | wild type |
| I.P. | Intraperitoneal |
| OCN | osteocalcin |
| ARU | agonist artesunate |
| IL-6 | interleukin-6 |
| PTX | pertussis toxin |
| CRP | C-reactive protein |
| LPS | lipopolysaccharide |
| TNFα | tumor necrosis factor |
| CNS | central nervous system |
| GPR | G protein coupled receptor |
| TGFβ | transforming growth factor |
| cAMP | cyclic Adenosine monophosphate |
| ELISA | enzyme linked immunosorbent assay |
| qPCR | real-time quantitative polymerase chain reaction |
| SDS-PAGE | sodium dodecyl sulphate-polyacrylamide gel electrophoresis |
References
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Yang, C.; Li, Y.; Dai, Z. An overview of osteocalcin progress. J. Bone Miner. Metab. 2016, 34, 367–379. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Schatz, M.; Saravanan, S.; d’Adesky, N.D.; Bramlett, H.; Perez-Pinzon, M.A.; Raval, A.P. Osteocalcin, ovarian senescence, and brain health. Front. Neuroendocrinol. 2020, 59, 100861. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2018, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Obri, A.; Khrimian, L.; Karsenty, G.; Oury, F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nature reviews. Endocrinology 2018, 14, 174–182. [Google Scholar]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Otani, T.; Mizokami, A.; Kawakubo-Yasukochi, T.; Takeuchi, H.; Inai, T.; Hirata, M. The roles of osteocalcin in lipid metabolism in adipose tissue and liver. Adv. Biol. Regul. 2020, 78, 100752. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Al-Jafary, M.A. Endocrine roles of vitamin K-dependent- osteocalcin in the relation between bone metabolism and metabolic disorders. Rev. Endocr. Metab. Disord. 2020, 21, 117–125. [Google Scholar] [CrossRef]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Kover, K.; Yan, Y.; Tong, P.Y.; Watkins, D.; Li, X.; Tasch, J.; Hager, M.; Clements, M.; Moore, W.V. Osteocalcin protects pancreatic beta cell function and survival under high glucose conditions. Biochem. Biophys. Res. Commun. 2015, 462, 21–26. [Google Scholar] [CrossRef]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Mera, P.; Ferron, M.; Mosialou, I. Regulation of Energy Metabolism by Bone-Derived Hormones. Cold Spring Harb. Perspect. Med. 2018, 8, a031666. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Brennan-Speranza, T.C.; Conigrave, A.D. Osteocalcin: An Osteoblast-Derived Polypeptide Hormone that Modulates Whole Body Energy Metabolism. Calcif. Tissue Int. 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Tacey, A.; Hayes, A.; Zulli, A.; Levinger, I. Osteocalcin and vascular function: Is there a cross-talk? Mol. Metab. 2021, 49, 101205. [Google Scholar] [CrossRef]
- Tacey, A.; Qaradakhi, T.; Brennan-Speranza, T.; Hayes, A.; Zulli, A.; Levinger, I. Potential Role for Osteocalcin in the Development of Atherosclerosis and Blood Vessel Disease. Nutrients 2018, 10, 1426. [Google Scholar] [CrossRef] [Green Version]
- Magni, P.; Macchi, C.; Sirtori, C.R.; Corsi Romanelli, M.M. Osteocalcin as a potential risk biomarker for cardiovascular and metabolic diseases. Clin. Chem. Lab. Med. 2016, 54, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine Regulation of Male Fertility by the Skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef] [Green Version]
- Oury, F.; Ferron, M.; Huizhen, W.; Confavreux, C.; Xu, L.; Lacombe, J.; Srinivas, P.; Chamouni, A.; Lugani, F.; Lejeune, H.; et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J. Clin. Investig. 2013, 123, 2421–2433. [Google Scholar] [CrossRef]
- Desentis-Desentis, M.F.; Rivas-Carrillo, J.D.; Sánchez-Enríquez, S. Protective role of osteocalcin in diabetes pathogenesis. J. Bone Miner. Metab. 2020, 38, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galan-Diez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Li, H.; Yang, H.; Yang, Q.; Lu, Z.; Wang, L.; Chen, Y.; Li, X. Osteocalcin attenuates oligodendrocyte differentiation and myelination via GPR37 signaling in the mouse brain. Sci. Adv. 2021, 7, eabi5811. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.-S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A.; Goeden, N.; Huang, Y.-Y.; Lee, H.; Srinivas, P.; Gao, X.-B.; et al. Maternal and Offspring Pools of Osteocalcin Influence Brain Development and Functions. Cell 2013, 155, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, S.; Polyzos, A.; Harvey, L.; Youssef, M.; Denny, C.A.; Dranovsky, A.; Kandel, E.R. RbAp48 Protein Is a Critical Component of GPR158/OCN Signaling and Ameliorates Age-Related Memory Loss. Cell Rep. 2018, 25, 959. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Ghosh, A.; Guo, X.-Z.; Wang, S.-M.; Hou, Y.-F.; Li, S.-T.; Liu, J.-M. Roles for osteocalcin in brain signalling: Implications in cognition- and motor-related disorders. Mol. Brain 2019, 12, 23. [Google Scholar] [CrossRef]
- Hou, Y.-F.; Shan, C.; Zhuang, S.-Y.; Zhuang, Q.-Q.; Ghosh, A.; Zhu, K.-C.; Kong, X.-K.; Wang, S.-M.; Gong, Y.-L.; Yang, Y.-Y.; et al. Microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, X.; Yang, R.; Wang, F.; Hao, Y.; Dou, J.; He, H.; Jia, W. Inverse relationship between serum osteocalcin levels and visceral fat area in Chinese men. J. Clin. Endocrinol. Metab. 2013, 98, 345–351. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q.; Yang, Z.; Ye, Z.; Huang, Y.; He, M.; Wen, J.; Wang, X.; Lu, B.; Hu, J.; et al. Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese. Diabet. Med. A J. Br. Diabet. Assoc. 2013, 30, 309–317. [Google Scholar] [CrossRef]
- Liao, M.; Huang, L.; Mao, Y.; Jiang, Y.; Yao, Z.; Lin, X.; Lu, Z.; Wu, C.; Qin, X.; Zhang, H.; et al. Serum Osteocalcin Is Associated with Inflammatory Factors in Metabolic Syndrome: A Population-Based Study in Chinese Males. Mediat. Inflamm. 2015, 2015, 683739. [Google Scholar] [CrossRef] [Green Version]
- Lucey, A.J.; Paschos, G.K.; Thorsdottir, I.; Martínéz, J.A.; Cashman, K.D.; Kiely, M. Young overweight and obese women with lower circulating osteocalcin concentrations exhibit higher insulin resistance and concentrations of C-reactive protein. Nutr. Res. 2013, 33, 67–75. [Google Scholar] [CrossRef]
- Sarkar, P.D.; Choudhury, A.B. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1631–1635. [Google Scholar] [PubMed]
- Kanazawa, I.; Tanaka, S.; Sugimoto, T. The Association Between Osteocalcin and Chronic Inflammation in Patients with Type 2 Diabetes Mellitus. Calcif. Tissue Int. 2018, 103, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Costa, M.B.W.; Mathew, A.; Krishnan, S.; Schneider, J.G.; Roomp, K.; Isermann, B.; Biemann, R. Osteocalcin Is Independently Associated with C-Reactive Protein during Lifestyle-Induced Weight Loss in Metabolic Syndrome. Metabolites 2021, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.A.C.; Esteves, J.V.; Morais, M.R.; Zorn, T.M.; Furuya, D.T. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone 2018, 115, 68–82. [Google Scholar] [CrossRef]
- Hill, H.S.; Grams, J.; Walton, R.G.; Liu, J.; Moellering, D.R.; Garvey, W.T. Carboxylated and uncarboxylated forms of osteocalcin directly modulate the glucose transport system and inflammation in adipocytes. Horm. Metab. Res. 2014, 46, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polonskaya, Y.V.; Kashtanova, E.V.; Murashov, I.S.; Volkov, A.M.; Kurguzov, A.V.; Chernyavsky, A.M.; Ragino, Y.I. Associations of Osteocalcin, Osteoprotegerin, and Calcitonin with Inflammation Biomarkers in Atherosclerotic Plaques of Coronary Arteries. Bull. Exp. Biol. Med. 2017, 162, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Zala, I.; Anderson, S.I.; O’Sullivan, S.E. Osteocalcin does not influence acute or chronic inflammation in human vascular cells. J. Cell Physiol. 2020, 235, 3414–3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, J.; Schuon, J.; Davis, J.; Ferguson, T.F.; Welsh, D.A.; Molina, P.E.; Ronis, M.J.J. Reduced Serum Osteocalcin in High-Risk Alcohol Using People Living with HIV Does Not Correlate with Systemic Oxidative Stress or Inflammation: Data from the New Orleans Alcohol Use in HIV Study. Alcohol. Clin. Exp. Res. 2019, 43, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Imai, Y. Pael receptor, endoplasmic reticulum stress, and Parkinson’s disease. J. Neurol. 2003, 250 (Suppl. S3), iii25–iii29. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Soda, M.; Inoue, H.; Hattori, N.; Mizuno, Y.; Takahashi, R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 2001, 105, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mantas, I.; Fridjonsdottir, E.; Andrén, P.E.; Chergui, K.; Svenningsson, P. Deficits in Motor Performance, Neurotransmitters and Synaptic Plasticity in Elderly and Experimental Parkinsonian Mice Lacking GPR37. Front. Aging Neurosci. 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Shoji, M.; Imai, Y.; Inoue, H.; Kawarabayashi, T.; Matsubara, E.; Harigaya, Y.; Sasaki, A.; Takahashi, R.; Abe, K. Pael-R is accumulated in Lewy bodies of Parkinson’s disease. Ann. Neurol. 2004, 55, 439–442. [Google Scholar] [CrossRef]
- Marazziti, D.; Golini, E.; Mandillo, S.; Magrelli, A.; Witke, W.; Matteoni, R.; Tocchini-Valentini, G.P. Altered dopamine signaling and MPTP resistance in mice lacking the Parkinson’s disease-associated GPR37/parkin-associated endothelin-like receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 10189–10194. [Google Scholar] [CrossRef] [Green Version]
- Leinartaité, L.; Svenningsson, P. Folding Underlies Bidirectional Role of GPR37/Pael-R in Parkinson Disease. Trends Pharmacol. Sci. 2017, 38, 749–760. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujita-Jimbo, E.; Momoi, M.Y.; Momoi, T. CASPR2 forms a complex with GPR37 via MUPP1 but not with GPR37(R558Q), an autism spectrum disorder-related mutation. J. Neurochem. 2015, 134, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Fujita-Jimbo, E.; Yu, Z.L.; Li, H.; Yamagata, T.; Mori, M.; Momoi, T.; Momoi, M.Y. Mutation in Parkinson disease-associated, G-protein-coupled receptor 37 (GPR37/PaelR) is related to autism spectrum disorder. PLoS ONE 2012, 7, e51155. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Ziegler, M.E.; Kim, H.B.; Evans, S.J.; Choudary, P.V.; Li, J.Z.; Meng, F.; Dai, M.; Myers, R.M.; Neal, C.R.; et al. G protein-linked signaling pathways in bipolar and major depressive disorders. Front. Genet. 2013, 4, 297. [Google Scholar] [CrossRef] [Green Version]
- Watkins, L.R.; Orlandi, C. Orphan G Protein Coupled Receptors in Affective Disorders. Genes 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.S.; Shamsizadeh, A.; Azhdari-Zarmehri, H.; Roohbakhsh, A. Orphan G protein-coupled receptors: The role in CNS disorders. Biomed. Pharmacother. 2018, 98, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Mandillo, S.; Di Pietro, C.; Golini, E.; Matteoni, R.; Tocchini-Valentini, G.P. GPR37 associates with the dopamine transporter to modulate dopamine uptake and behavioral responses to dopaminergic drugs. Proc. Natl. Acad. Sci. USA 2007, 104, 9846–9851. [Google Scholar] [CrossRef] [Green Version]
- Morato, X.; Lujan, R.; Lopez-Cano, M.; Gandia, J.; Stagljar, I.; Watanabe, M.; Cunha, R.A.; Fernandez-Duenas, V.; Ciruela, F. The Parkinson’s disease-associated GPR37 receptor interacts with striatal adenosine A2A receptor controlling its cell surface expression and function in vivo. Sci. Rep. 2017, 7, 9452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.-J.; Vainshtein, A.; Maik-Rachline, G.; Peles, E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016, 7, 10884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Rezgaoui, M.; Süsens, U.; Ignatov, A.; Gelderblom, M.; Glassmeier, G.; Franke, I.; Urny, J.; Imai, Y.; Takahashi, R.; Schaller, H.C. The neuropeptide head activator is a high-affinity ligand for the orphan G-protein-coupled receptor GPR37. J. Cell Sci. 2006, 119 Pt 3, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.C.; Giddens, M.M.; Schaefer, S.A.; Hall, R.A. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc. Natl. Acad. Sci. USA 2013, 110, 9529–9534. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Hu, L.; Zang, M.; Zhang, B.; Duan, Y.; Fan, Z.; Li, J.; Su, L.; Yan, M.; Zhu, Z.; et al. REG4 promotes peritoneal metastasis of gastric cancer through GPR37. Oncotarget 2016, 7, 27874–27888. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Mosienko, V.; Vaccari Cardoso, B.; Prokudina, D.; Huentelman, M.; Teschemacher, A.G.; Kasparov, S. Glio- and neuro-protection by prosaposin is mediated by orphan G-protein coupled receptors GPR37L1 and GPR37. Glia 2018, 66, 2414–2426. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Donnelly, C.R.; Luo, X.; Toro-Moreno, M.; Tao, X.; Wang, Z.; Chandra, S.; Bortsov, A.V.; Derbyshire, E.R.; Ji, R.R. Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat. Commun. 2021, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Xie, Y.K.; Zhang, Z.J.; Wang, Z.; Xu, Z.Z.; Ji, R.R. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCrary, M.R.; Jiang, M.Q.; Giddens, M.M.; Zhang, J.Y.; Owino, S.; Wei, Z.Z.; Zhong, W.; Gu, X.; Xin, H.; Hall, R.A.; et al. Protective effects of GPR37 via regulation of inflammation and multiple cell death pathways after ischemic stroke in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 10680–10691. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Yang, H.; Li, H.; Liu, C.; Yang, L.; Qu, Z.; Li, X. The Cholinergic Anti-Inflammatory Pathway Attenuates the Development of Atherosclerosis in Apoe(−/−) Mice through Modulating Macrophage Functions. Biomedicines 2021, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappa B and I kappa B proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.T.; Verma, I.M. NF-kappa B regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Olson, E.N. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell 2016, 164, 1248–1256. [Google Scholar] [CrossRef] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]


| Gene Primer Name | Sequence (5′–3′) |
|---|---|
| TNFa-F | GCCTCTTCTCATTCCTGCTT |
| TNFa-R | TGGGAACTTCTCATCCCTTTG |
| IL-6-F | CAAAGCCAGAGTCCTTCAGAG |
| IL-6-R | GTCCTTAGCCACTCCTTCTG |
| Arg1F | AAGAATGGAAGAGTCAGTGTGG |
| Arg1R | GGGAGTGTTGATGTCAGTGTG |
| IL10-F | AGGCGCTGTCATCGATTT |
| IL10-R | CACCTTGGTCTTGGAGCTTAT |
| TGFβ-F | CCTGAGTGGCTGTCTTTTGA |
| TGFβ-R | CGTGGAGTTTGTTATCTTTGCTG |
| GAPDH-F | AACAGCAACTCCCACTCTTC |
| GAPDH-R | CCTGTTGCTGTAGCCGTATT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Liu, C.; Li, H.; Yang, H.; Wu, J.; Liu, J.; Li, Y.; Chen, X.; Xu, J.; Li, X. Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages. Biomedicines 2022, 10, 1006. https://doi.org/10.3390/biomedicines10051006
Qian Z, Liu C, Li H, Yang H, Wu J, Liu J, Li Y, Chen X, Xu J, Li X. Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages. Biomedicines. 2022; 10(5):1006. https://doi.org/10.3390/biomedicines10051006
Chicago/Turabian StyleQian, Zhengjiang, Chunhua Liu, Hongchao Li, Haiyang Yang, Jianhao Wu, Jing Liu, Yanjiao Li, Xuhui Chen, Jianyang Xu, and Xiang Li. 2022. "Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages" Biomedicines 10, no. 5: 1006. https://doi.org/10.3390/biomedicines10051006
APA StyleQian, Z., Liu, C., Li, H., Yang, H., Wu, J., Liu, J., Li, Y., Chen, X., Xu, J., & Li, X. (2022). Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages. Biomedicines, 10(5), 1006. https://doi.org/10.3390/biomedicines10051006