Aloperine: A Potent Modulator of Crucial Biological Mechanisms in Multiple Diseases
Abstract
1. Introduction
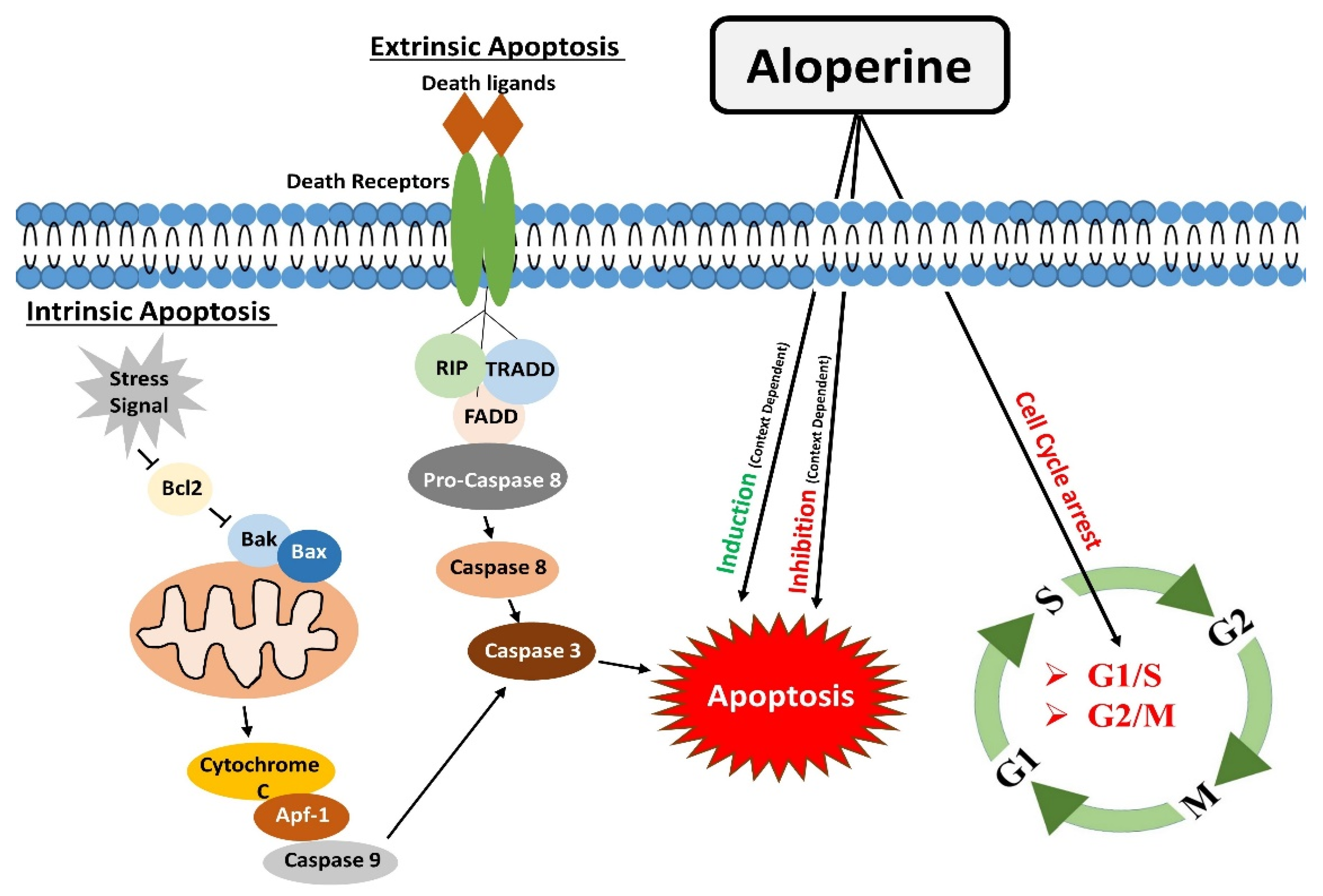
2. Regulation of Apoptosis
3. Modulatory Effects on the Cell Cycle
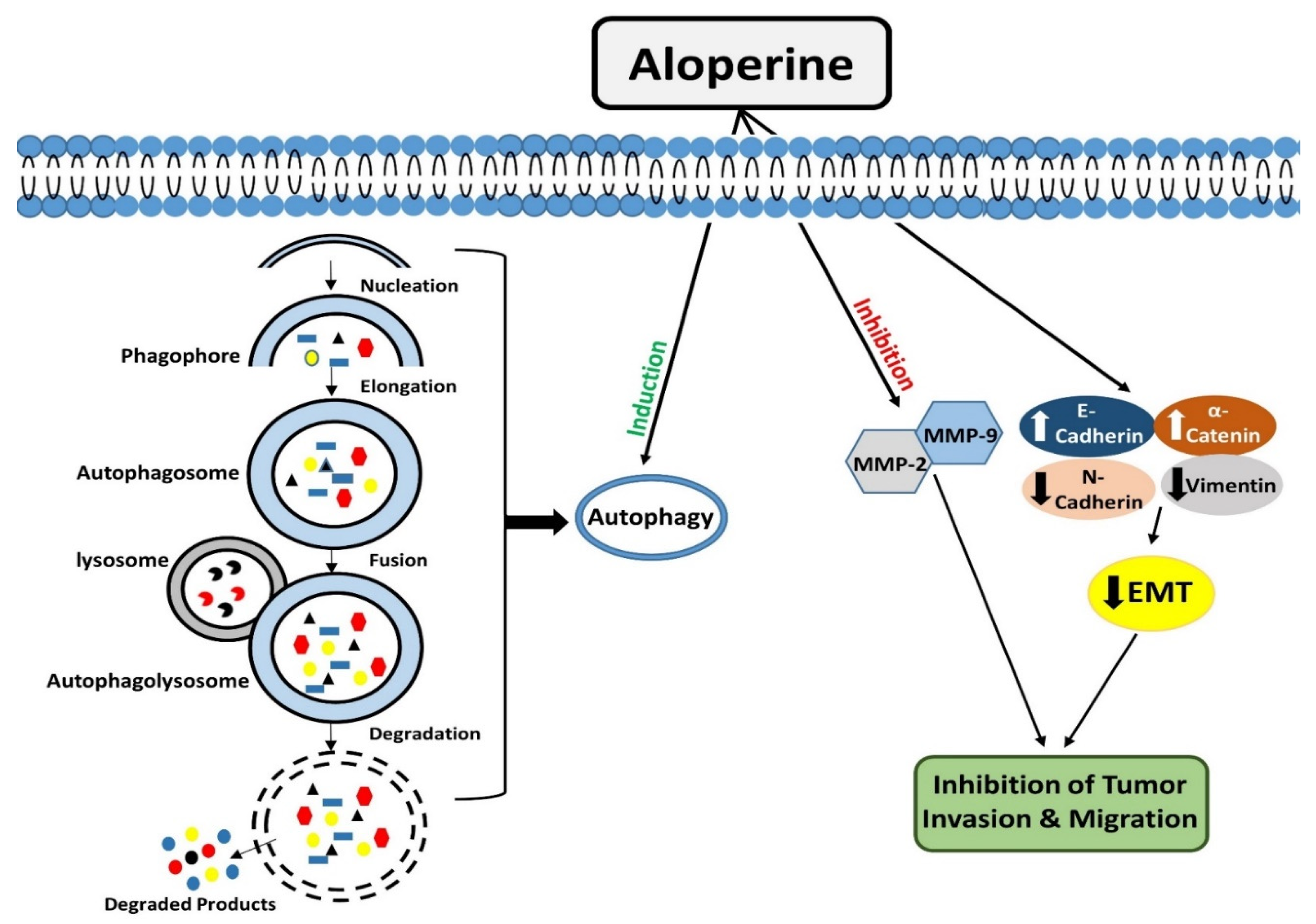
4. Modulation of Autophagy
5. Inhibitory Effects of Aloperine on Tumor Cell Invasion and Migration
| Apoptosis | ||||||
|---|---|---|---|---|---|---|
| Pathological Conditions | Cell Lines | Animal Model | Dosage | Regulatory Effects of Aloperine | Ref. | |
| In Vitro (µM) | In Vivo | |||||
| Multiple Myeloma | U266 and MM.1S | SCID NOD mice | 50/100/250/500 | 20 mg/kg | Induced Caspase-dependent apoptosis | [12] |
| Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | Induced Caspase dependent apoptosis | [22] |
| Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | Induced Mitochondria-dependent apoptosis | [23] |
| Osteosarcoma | MG-63 and U2OS | --------- | 100/200 | --------- | Induced Mitochondria-dependent apoptosis | [11] |
| Colon cancer | HCT116 | --------- | 250/500 | -------- | Induced Mitochondria-dependent apoptosis | [14] |
| Breast cancer | MCF-7 and MDA-MB-231 | --------- | 100/200/400 | --------- | Induced Mitochondria-dependent apoptosis | [26] |
| I/R-Induced Renal Injury | RAW264.7 and HK2 | C57BL/6 mice | 500 | 50 mg/kg | Inhibition of Apoptosis | [15] |
| Thyroid Cancer | IHH-4,8505c and KMH-2 | --------- | 100/200 | --------- | Induced Caspase-dependent apoptosis | [30] |
| Leukemia | HL-60 | --------- | 50/100 | --------- | Induced Mitochondria-dependent apoptosis | [7] |
| Alzheimer’s disease | N2a/Swe.D9 | --------- | 100 | --------- | Induced Mitochondria-dependent apoptosis | [43] |
| Non-small cell lung cancer | H1944 and NCI-H1869 | BALB/C nude mice | 250 | 30 mg/kg | Induced Mitochondria-dependent apoptosis | [24] |
| Intervertebral disc degeneration | Nucleus Pulposus cells | Sprague-Dawley rats | 100 | --------- | Inhibition of Apoptosis | [44] |
| Bladder Cancer | EJ cells | --------- | 25/50/100 | --------- | Induced Mitochondria-dependent apoptosis | [59] |
| OGD/RP neuronal injury | Hippocampal Neuronal cells | Sprague-Dawley rats | 100/200/400 | --------- | Inhibition of Apoptosis | [60] |
| Colorectal Cancer | SW480 and HT29 | --------- | 200/400/800/1000 | --------- | Induced Mitochondria-dependent apoptosis | [40] |
| Early brain injury | --------- | Sprague-Dawley rats | --------- | 75/150 mg/kg | Inhibition of Apoptosis | [17] |
| I/R-Induced Cerebral injury | --------- | Sprague-Dawley rats | --------- | 2/25/50 mg/kg | Inhibition of Apoptosis | [16] |
| Retinal pigment epithelial cells injury | ARPE-19 | --------- | 6.25/12.5/25 | --------- | Inhibition of Apoptosis | [19] |
| DSS-Induced Colitis | Jurkat Cells | C57BL/6 mice | 250/500 | 40 mg/kg | Inhibition of Apoptosis | [29] |
| Microembolisation-Induced cardiac Injury | --------- | Sprague-Dawley rats | --------- | 200 mg/kg | Inhibition of Apoptosis | [61] |
| Cell Cycle | ||||||
| Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | G1 phase arrest | [22] |
| Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | G2 phase arrest | [23] |
| Colon cancer | HCT116 | --------- | 250/500 | --------- | G2 phase arrest | [14] |
| Thyroid Cancer | IHH-4,8505c and KMH-2 | --------- | 100/200 | --------- | No impact on Cell Cycle | [30] |
| Non-small cell lung cancer | H1944 and NCI-H1869 | BALB/C nude mice | 250 | 30 mg/kg | G1 phase arrest | [24] |
| Liver cancer | SNU-182 | --------- | 5 | --------- | G2 phase arrest | [21] |
| Autophagy | ||||||
| Thyroid Cancer | KMH-2 and IHH-4 | --------- | 200 | --------- | Autophagy induction | [27] |
| Thyroid Cancer | 8505c | --------- | 200 | --------- | Autophagy inhibition | [27] |
| Leukaemia | HL-60 | --------- | 50/100 | --------- | Autophagy induction | [7] |
| Migration and Invasion | ||||||
| Breast cancer | MCF-7 and MDA-MB-231 | --------- | 100/200/400 | --------- | Inhibition of Migration and Invasion | [26] |
| Liver cancer | SNU-182 | --------- | 5 | --------- | Inhibition of Migration and Invasion | [21] |
6. Modulatory Effects on PI3K/Akt/mTOR Signaling
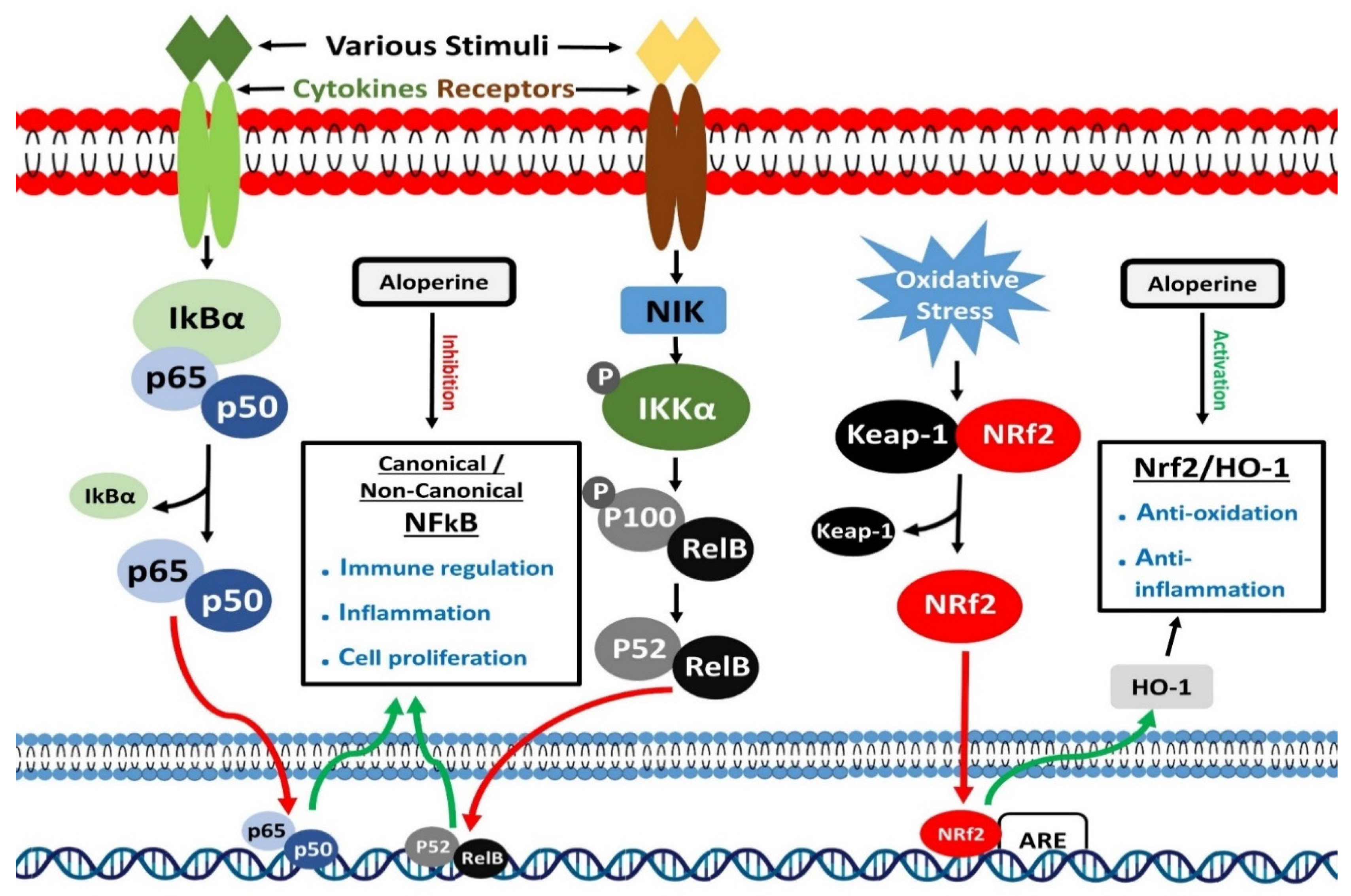
7. Inhibition of NF-κB Signaling
8. Activation of Nrf2 Signaling
9. Inhibition of Ras Signaling
| PI3K/Akt and Other Downstream Molecules Signaling | ||||||
|---|---|---|---|---|---|---|
| Pathological Conditions | Cell Lines | Animal Model | Dosage | Regulatory Effects of Aloperine | Ref. | |
| In Vitro (µM) | In Vivo | |||||
| Prostate cancer | PC3, DU145 and LNCaP | BALB/C mice | 100/200 | 30 mg/kg | Inhibition of Akt/ERK signaling | [22] |
| Hepatocellular carcinoma | Hep3B and Huh7 | Zebrafish embryo | 200/350/500 | 100 µM, 150 µM | Inhibition of PI3K/Akt signaling | [23] |
| Osteosarcoma | MG-63 and U2OS | --------- | 100/200 | --------- | Inhibition of PI3K/Akt signaling | [11] |
| Colon cancer | HCT116 | --------- | 250/500 | --------- | Inhibition of PI3K/Akt signaling | [14] |
| I/R-Induced Renal Injury | RAW264.7 and HK2 | C57BL/6 mice | 500 | 50 mg/kg | Inhibition of PI3K/Akt/mTOR signaling | [15] |
| Thyroid Cancer | KMH-2 and IHH-4 | --------- | 200 | --------- | Inhibition of Akt/mTOR signaling | [27] |
| Thyroid Cancer | IHH-4,8505c and KMH-2 | -------- | 100/200 | ------- | Inhibition of Akt signaling | [30] |
| DSS-Induced Colitis | Jurkat Cells | C57BL/6 mice | 250/500 | 40 mg/kg | Inhibition of PI3K/Akt/mTOR signaling | [29] |
| Microembolisation-Induced cardiac Injury | --------- | Sprague-Dawley rats | --------- | 200 mg/kg | Activation of the PI3K/Akt signaling | [61] |
| I/R-Induced Cerebral injury | --------- | Sprague-Dawley rats | --------- | 2/25/50 mg/kg | Activation of the PI3K/Akt signaling | [16] |
| NF-κB Signaling | ||||||
| Allergic airway inflammation | --------- | BALB/c mice | --------- | 100/200 mg/kg | Inhibition of NF-κB signaling | [18] |
| Neuropathic pain | --------- | ICR mice | --------- | 80 mg/kg | Inhibition of NF-κB signaling | [31] |
| Intervertebral disc degeneration | Nucleus Pulposus cells | Sprague-Dawley rats | 100 | ------- | Inhibition of NF-κB signaling | [44] |
| Pulmonary arterial hypertension | --------- | Sprague-Dawley rats | --------- | 25/50/100 mg/kg | Inhibition of NF-κB signaling | [124] |
| Osteoporosis | RAW264.7 | C57BL/6 mice | 20 | 30 mg/Kg | Inhibition of NF-κB signaling | [121] |
| LPS-induced macrophage activation | RAW264.7 | --------- | 50/100 | --------- | Inhibition of NF-κB signaling | [111] |
| Nrf2/HO-1 Signaling | ||||||
| Allergic airway inflammation | --------- | BALB/c mice | --------- | 100/200 mg/kg | Activation of Nrf2/HO-1 Signaling | [18] |
| Retinal pigment epithelial cells injury | ARPE-19 | --------- | 6.25/12.5/25 | --------- | Activation of Nrf2/HO-1 Signaling | [19] |
| High Glucose induced Schwann cells injury | RSC96 cells | --------- | 1/10/50 | --------- | Activation of Nrf2/HO-1 Signaling | [21] |
| CCl4 induced mouse hepatic injury | --------- | C57BL/6 mice | --------- | 50/100 mg/kg | Activation of Nrf2/HO-1 Signaling | [147] |
| Ras Signaling | ||||||
| Breast cancer | MCF-7 and MDA-MB-231 | --------- | 100/200/400 | --------- | Inhibition of Ras signaling | [26] |
| Bladder Cancer | EJ cells | ---------- | 25/50/100 | --------- | Inhibition of Ras signaling | [59] |

10. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHR | Airway hyper-responsiveness |
| ALP | Alkaline phosphatase |
| ALT | Alanine transaminase |
| AMD | Age-related macular degeneration |
| Apaf-1 | Protease activating factor-1 |
| ARE | Antioxidant response element |
| AST | Aspartate aminotransferase |
| ATP | Adenosine triphosphate |
| BAFFR | B-cell activating factor receptor |
| Bax | Bcl2-associated X protein |
| Bcl2 | B-cell lymphoma 2 |
| BMM | Bone Marrow-Derived Macrophages |
| CCI | Chronic constriction injury (CCI) |
| CCl4 | Carbon Tetrachloride |
| CD40 | Cluster of differentiation 40 |
| Cdc2 | Cell-Division Cycle 2 |
| Cdc25C | Cell division cycle 25 |
| CDK | Cyclin-dependent protein kinase |
| cFLIP | Cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein |
| CL | Clearance |
| CME | Coronary micro-embolization |
| CNC | Cap ‘n’ collar |
| CO | Carbon monoxide |
| DSS | Dextran sodium sulfate |
| E2F1 | E2F Transcription Factor 1 |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| FADD | Fas-associated death domain |
| GRO1 | Growth Regulated Oncogene 1 |
| GST | Glutathione S-transferase |
| GTP | guanosine 5’-triphosphate |
| H2O2 | Hydrogen peroxide |
| HO-1 | Heme oxygenase-1 |
| IkBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IKK | IκB kinase |
| IL-13 | Interleukin-13 |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IR | Ischemia and reperfusion |
| LC3 | 1A/1B-light chain 3 |
| LTβR | Lymphotoxin beta receptor |
| MAPK | Mitogen-activated protein kinase |
| MCAO | Middle cerebral artery occlusion |
| MDA | Malondialdehyde |
| MEK | Mitogen-activated protein kinase kinase |
| MEK1/2 | Mitogen-activated protein kinase kinases 1 and 2 |
| MMP | Matrix metalloproteinases |
| mTOR | Mammalian target of rapamycin |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIK | NF-κB-inducing kinase |
| NQO1 | NAD(P)H: quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer. |
| OGD-RP | Oxygen-glucose deprivation-reperfusion |
| PAH | Pulmonary arterial hypertension |
| PARP | Poly ADP ribose polymerase |
| PI | Propidium Iodide |
| PI3K | Phosphatidylinositol-3 kinase and PI3 kinase. |
| Rb | Retinoblastoma Tumor Suppressor Protein |
| PRR | Pattern recognition receptor |
| RANK | Receptor activator of nuclear factor κB |
| RANKL | Receptor activator of nuclear factor κB ligand |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| RTK | Receptor tyrosine kinase |
| SFDA | Chinese state food and drug administration |
| Snail | Zinc finger protein SNAI1 |
| Th17 | T helper cell 17 |
| T1/2 | Half-life |
| Tmax | Time to reach maximum concentration |
| TNFR | TNF receptors TNFR1 |
| TNF-α | Tumor Necrosis Factor alpha |
| TRAIL | Tumor Necrosis Factor-Alpha-Related Apoptosis-Inducing Ligand |
| TRAIL-R1/2 | Tumor Necrosis Factor-related Apoptosis-inducing Ligand Receptor 1/2 |
| Tregs | Regulatory T-cells |
| Twist1 | Twist-related protein 1 |
| Vd | Apparent volume of distribution |
| XAR | Xenobiotic-activated receptor (XAR) |
References
- Chen, X.; Yi, C.; Yang, X.; Wang, X. Liquid chromatography of active principles in Sophora fla-vescens root. J. Chromatogr. B 2004, 812, 149–163. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Qian, D.; Qian, Y.; Duan, J.-A. Comparative analysis of quinolizidine alkaloids from different parts of Sophora alopecuroides seeds by UPLC–MS/MS. J. Pharm. Biomed. Anal. 2012, 67–68, 16–21. [Google Scholar] [CrossRef]
- Kangli, M.; Jianzhong, Z.; Ying, D.; Yufei, X. Research progress on the chemical compounds and pharmacology of Sophora flavescens. Nat. Prod. Res. Dev. 2001, 13, 69–73. [Google Scholar]
- Tolkachev, O.; Monakhova, T.; Sheichenko, V.; Kabanov, V.; Fesenko, O.; Proskurnina, N. Alka-loids of a new type from Sophora alopecuroides L. Chem. Nat. Compd. 1975, 11, 29–34. [Google Scholar] [CrossRef]
- Brosius, A.; Ziller, J.; Zhang, Q. Relative and absolute configuration of aloperine. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 1510–1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, J.; Lin, Y. Clinical observation on the efficacy of mateling injection combined with radiotherapy in treating nasopharyngeal tumors. Strait Pharm. J. 1996, 8, 41–43. [Google Scholar]
- Lin, Z.; Huang, C.-F.; Liu, X.-S.; Jiang, J. In Vitro Anti-Tumour Activities of Quinolizidine Alkaloids Derived from Sophora Flavescens Ait. Basic Clin. Pharmacol. Toxicol. 2010, 108, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Gao, H.B.; Sun, X.B.; Shi, H.B.; Liu, W.; Yuan, H.N.; Wang, Z.X. Anti-inflammatory and anti-allergic action of aloperine. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1989, 10, 360–365. [Google Scholar]
- Li Fan, S.; Zhang, S. Antiviral effect of aloperine. J. Zhong Cao Yao 1998, 29, 253–254. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jin, Z.; Dai, L.; Wu, H.; Wang, J.; Wang, L.; Zhou, Z.; Yang, L.; Gao, W. Aloperine in-duces apoptosis and inhibits invasion in MG-63 and U2OS human osteosarcoma cells. Biomed. Pharmacother. 2018, 97, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, S.; Zhou, H.; Sun, M.; Du, L.; Wei, M.; Luo, M.; Huang, J.; Deng, H.; Feng, Y. Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mecha-nisms. J. Hematol. Oncol. 2015, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, Y.; Zeng, S.; Qian, L.; Dai, S.; Xiao, L.; Wang, L.; Yang, X.; Xiao, Y.; Gong, Z. Re-ducing autophagy and inducing G1 phase arrest by aloperine enhances radio-sensitivity in lung cancer cells. Oncol. Rep. 2017. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Deng, H.; Liang, L.; Peng, J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int. J. Mol. Med. 2014, 33, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Y.; Zhang, M.; Guo, Y.; Yang, P.; Zhang, S.; Simsekyilmaz, S.; Xu, J.-F.; Li, J.; Xiang, X. Aloperine protects mice against ischemia-reperfusion (IR)-induced renal injury by regu-lating PI3K/AKT/mTOR signaling and AP-1 activity. Mol. Med. 2015, 21, 912–923. [Google Scholar] [CrossRef]
- Li, Z.; Cao, X.; Xiao, L.; Zhou, R. Aloperine protects against cerebral ischemia/reperfusion injury via activating the PI3K/AKT signaling pathway in rats. Exp. Ther. Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Song, S.; Chen, Y.; Han, F.; Dong, M.; Xiang, X.; Sui, J.; Li, Y.; Yang, H.; Liu, J. Aloperine acti-vates the Nrf2-ARE pathway when ameliorating early brain injury in a subarachnoid hemorrhage model. Exp. Ther. Med. 2018, 15, 3847–3855. [Google Scholar]
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int. Immunopharmacol. 2018, 65, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, H.; Chen, J.; Lv, X.; Liu, H. Aloperine protects human retinal pigment epithelial cells against hydrogen peroxide–induced oxidative stress and apoptosis through activation of Nrf2/HO-1 pathway. J. Recept. Signal Transduct. 2022, 42, 88–94. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Crosariol, M.; Loro, E.; Li, Z.; Pestell, R.G. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012, 3, 649–657. [Google Scholar] [CrossRef]
- Huang, H.; Cao, Y.; Huang, L.; Lu, R.; Wang, J.; Zhou, Y. Aloperine suppresses the proliferation, migration and invasion of human liver cancer cells via induction of G2/M cell cycle arrest and inhibition of GROα expression. All Life 2021, 14, 392–400. [Google Scholar] [CrossRef]
- Ling, Z.; Guan, H.; You, Z.; Wang, C.; Hu, L.; Zhang, L.; Wang, Y.; Chen, S.; Xu, B.; Chen, M. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer n vitro and in vivo. OncoTargets Ther. 2018, 11, 2735. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-S.; Huo, C.-Y.; Cao, H.-H.; Fan, C.-L.; Hu, J.-Y.; Deng, L.-J.; Lu, Z.-B.; Yang, H.-Y.; Yu, L.-Z.; Mo, Z.-X. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine 2019, 61, 152843. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Sakhawat, A.; Khan, A.A.; Huang, H.; Khan, H.R.; Huang, Y.; Wang, J. Aloperine in combination with therapeutic adenoviral vector synergistically suppressed the growth of non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, Q.; An, J.; Song, X. Aloperine prevents hypoxia-induced epithelial-mesenchymal transition in bladder cancer cells through regulating the mTOR/p70S6K/4E-BP1 pathway. Preprint 2020. [Google Scholar] [CrossRef]
- Tian, D.; Li, Y.; Li, X.; Tian, Z. Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol. Med. Rep. 2018, 18, 3699–3710. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-I.; Shen, H.-C.; Chen, S.-H.; Lim, Y.-P.; Chuang, H.-H.; Tai, T.-S.; Kung, F.-P.; Lu, C.-H.; Hou, C.-Y.; Lee, Y.-R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019, 20, 5315. [Google Scholar] [CrossRef] [PubMed]
- Holz, R.W.; Fisher, S.K. Synaptic transmission and cellular signaling: An overview. Basic Neurochem. 2012, 235–257. [Google Scholar]
- Fu, X.; Sun, F.; Wang, F.; Zhang, J.; Zheng, B.; Zhong, J.; Yue, T.; Zheng, X.; Xu, J.-F.; Wang, C.-Y. Aloperine protects mice against DSS-induced colitis by PP2A-mediated PI3K/Akt/mTOR signaling suppression. Mediat. Inflamm. 2017, 2017, 5706152. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Chen, S.-H.; Lin, C.-Y.; Chao, W.-Y.; Lim, Y.-P.; Yu, H.-I.; Lu, C.-H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19, 312. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Jin, S.-J.; Liu, N.; Li, Y.-X.; Zheng, J.; Ma, L.; Du, J.; Zhou, R.; Zhao, C.-J.; Niu, Y. Aloperine attenuated neuropathic pain induced by chronic constriction injury via anti-oxidation activity and suppression of the nuclear factor kappa B pathway. Biochem. Biophys. Res. Commun. 2014, 451, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, T.; Wang, Z.; Jia, L.; Zhang, X.; He, Q.; Liu, S. Aloperine attenuates high glucose-induced oxidative injury in Schwann cells via activation of NRF2/HO-1 pathway. Trop. J. Pharm. Res. 2020, 19, 1147–1152. [Google Scholar] [CrossRef]
- Shin, S.-S.; Park, Y.-J.; Hwang, B.; Park, S.L.; Han, S.-W.; Park, S.-S.; Choi, Y.H.; Kim, W.-J.; Moon, S.-K. Triacanthine exerts antitumor effects on bladder cancer in vitro and in vivo. Phytomedicine 2019, 64, 153069. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.-E.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Chen, X.; Zeng, S.; Qian, L.; Wei, J.; Gong, Z.; Yan, Y. Identification of Aloperine as an anti-apoptotic Bcl2 protein inhibitor in glioma cells. PeerJ 2019, 7, e7652. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Han, W.; Kong, D.; Lu, Q.; Zhang, W.; Fan, Z. Aloperine inhibits proliferation and promotes apoptosis in colorectal cancer cells by regulating the circNSUN2/miR-296-5p/STAT3 pathway. Drug Des. Dev. Ther. 2021, 15, 857. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Wang, W. Acute Renal Failure and Sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Liang, H.L.; Arsenault, J.; Mortensen, J.; Park, F.; Johnson, C.P.; Nilakantan, V. Partial attenuation of cytotoxicity and apoptosis by SOD1 in ischemic renal epithelial cells. Apoptosis 2009, 14, 1176–1189. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; Li, M.; Luo, Q.; Leng, Y.; Liu, X. Neuro-protective effects of aloperine in an Alzheimer’s disease cellular model. Biomed. Pharmacother. 2018, 108, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ma, W.; Guo, B.; Wang, S. Aloperine attenuates hydrogen peroxide-induced injury via anti-apoptotic activity and suppression of the nuclear factor-κB signaling pathway. Exp. Ther. Med. 2017, 13, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, L.H.; Kastan, M.B. Cell cycle control and cancer. Science 1994, 266, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Boward, B.; Wu, T.; Dalton, S. Concise review: Control of cell fate through cell cycle and pluripo-tency networks. Stem Cell. 2016, 34, 1427–1436. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ohsumi, Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007, 581, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Badadani, M. Autophagy Mechanism, Regulation, Functions, and Disorders. ISRN Cell Biol. 2012, 2012, 927064. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2020, 17, 1–382. [Google Scholar] [CrossRef]
- Cheung, K.J.; Ewald, A.J. A collective route to metastasis: Seeding by tumor cell clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef]
- Curran, S.; Murray, G.I. Matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 1999, 189, 300–308. [Google Scholar] [CrossRef]
- Curran, S.; Murray, G.I. Matrix metalloproteinases: Molecular aspects of their roles in tumour in-vasion and metastasis. Eur. J. Cancer 2000, 36, 1621–1630. [Google Scholar] [CrossRef]
- Hollestelle, A.; Peeters, J.K.; Smid, M.; Timmermans, M.; Verhoog, L.C.; Westenend, P.J.; Heine, A.A.J.; Chan, A.; Sieuwerts, A.M.; Wiemer, E.; et al. Loss of E-cadherin is not a necessity for epithelial to mesenchymal transition in human breast cancer. Breast Cancer Res. Treat. 2013, 138, 47–57. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Bonnitcha, P.; Grieve, S.; Figtree, G. Clinical imaging of hypoxia: Current status and future directions. Free. Radic. Biol. Med. 2018, 126, 296–312. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Liu, X.; Wu, J.; Tan, D.; Hu, W. Aloperine Exerts Antitumor Effects on Bladder Cancer in vitro. OncoTargets Ther. 2020, 13, 10351–10360. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.-T.; Zhou, R.; Chang, R.-Y.; Hao, Y.-J.; Ma, L.; Jin, S.-J.; Du, J.; Zheng, J.; Zhao, C.-J.; Niu, Y.; et al. Protective effects of aloperine on neonatal rat primary cultured hippocampal neurons injured by oxygen–glucose deprivation and reperfusion. J. Nat. Med. 2015, 69, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Guo, F.; Liang, X.; Wu, Y.; Lu, Y. Aloperine activates the PI3K/Akt pathway and protects against coronary micro-embolisation-induced myocardial injury in rats. Pharmacology 2019, 104, 90–97. [Google Scholar] [CrossRef]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; German, P.; Bai, S.; Barnes, S.; Guo, W.; Qi, X.; Lou, H.; Liang, J.; Jonasch, E.; Mills, G.B.; et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J. Genet. Genom. 2015, 42, 343–353. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Auger, K.R.; Serunian, L.A.; Soltoff, S.P.; Libby, P.; Cantley, L.C. PDGF-dependent tyrosine phosphorylation stimulates pro-duction of novel polyphosphoinositides in intact cells. Cell 1989, 57, 167–175. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Kapeller, R.; White, M.F.; Cantley, L.C. Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl. Acad. Sci. USA 1990, 87, 1411–1415. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic tar-geting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Hua, H.; Zhu, Y.; Song, Y.-H. Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomed. Pharmacother. 2018, 101, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Tian, X.-Y.; An, Q.-M.; Guan, X.-Y.; Hao, C.-Y. LINC00963 promotes hepatocellular carcinoma progression by activating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1645–1652. [Google Scholar]
- Psyrri, A.; Arkadopoulos, N.; Vassilakopoulou, M.; Smyrniotis, V.; Dimitriadis, G. Pathways and targets in hepatocellular carcinoma. Expert Rev. Anticancer Ther. 2012, 12, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.S. Osteosarcoma: Better treatment through better trial design. Lancet Oncol. 2015, 16, 12–13. [Google Scholar] [CrossRef]
- Walkley, C.R.; Qudsi, R.; Sankaran, V.G.; Perry, J.A.; Gostissa, M.; Roth, S.I.; Rodda, S.J.; Snay, E.; Dunning, P.; Fahey, F.H.; et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008, 22, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Naishadham, D.; Jemal, A. Cancer statistics for African Americans, 2013. CA Cancer J. Clin. 2013, 63, 151–166. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W.; Poole, B.; Mitra, A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J. Clin. Investig. 2004, 114, 5–14. [Google Scholar] [CrossRef]
- Amura, C.R.; Renner, B.; Lyubchenko, T.; Faubel, S.; Simonian, P.L.; Thurman, J.M. Complement activation and toll-like re-ceptor-2 signaling contribute to cytokine production after renal ischemia/reperfusion. Mol. Immunol. 2012, 52, 249–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, L.-H.; He, L.; Cao, Z.-Q.; Xiang, J.; Liu, L. Effect of ischemia preconditioning on renal ischemia/reperfusion injury in rats. Int. Braz. J. Urol. 2012, 38, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Kusch, A.; Hoff, U.; Bubalo, G.; Zhu, Y.; Fechner, M.; Schmidt-Ullrich, R.; Marko, L.; Müller, D.; Schmidt-Ott, K.; Gürgen, D. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol. 2013, 208, 25–40. [Google Scholar] [CrossRef]
- Sabbahy, M.E.; Vaidya, V.S. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Miller, K.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.L.; Hague, A. The PI3K/Akt Pathway in Tumors of Endocrine Tissues. Front. Endocrinol. 2016, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Mirlekar, B.; Ghorai, S.; Khetmalas, M.; Bopanna, R.; Chattopadhyay, S. Nuclear matrix protein SMAR1 control regulatory T-cell fate during inflammatory bowel disease (IBD). Mucosal Immunol. 2015, 8, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Chi, H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012, 12, 325–338. [Google Scholar] [CrossRef]
- Huynh, A.; DuPage, M.; Priyadharshini, B.; Sage, P.T.; Quiros, J.; Borges, C.M.; Townamchai, N.; Gerriets, V.; Rathmell, J.C.; Sharpe, A.H.; et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 2015, 16, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death–apoptosis, autophagy and senes-cence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Strozyk, E.; Kulms, D. The role of AKT/mTOR pathway in stress response to UV-irradiation: Implication in skin carcinogene-sis by regulation of apoptosis, autophagy and senescence. Int. J. Mol. Sci. 2013, 14, 15260–15285. [Google Scholar] [CrossRef]
- Heusch, G.; Skyschally, A.; Kleinbongard, P. Coronary microembolization and microvascular dysfunction. Int. J. Cardiol. 2018, 258, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Sun, Y.; He, W.; Wang, X.; Su, Q. The protective effect of activating Nrf2/HO-1 signaling pathway on cardi-omyocyte apoptosis after coronary microembolization in rats. BMC Cardiovasc. Disord. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G. A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Wang, Z.; Zhou, W.; Dong, H.; Ma, X.; He, Z. Dexmedetomidine pretreatment inhibits cerebral ischemia/reperfusion-induced neuroinflammation via activation of AMPK. Mol. Med. Rep. 2018, 18, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, G.S.; Chen, H.; Maier, C.M.; Narasimhan, P.; Song, Y.S.; Niizuma, K.; Katsu, M.; Okami, N.; Yoshioka, H.; et al. Reperfusion and Neurovascular Dysfunction in Stroke: From Basic Mechanisms to Potential Strategies for Neuroprotection. Mol. Neurobiol. 2010, 41, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, J. Sanggenon C ameliorates cerebral ischemia-reperfusion injury by inhibiting inflammation and oxidative stress through regulating RhoA-ROCK signaling. Inflammation 2020, 43, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Miraghazadeh, B.; Cook, M.C. Nuclear factor-kappaB in autoimmunity: Man and mouse. Front. Immunol. 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.B.; Zapf, S.; Kristina, D.; Strobl, D.C.; Weih, F.; Blum, H.; Weigert, O.; Strobl, L.J.; Ursula, Z.-S. The non-canonical NF-kappaB Signaling Pathway Contributes to the Expansion and Lymphomagenesis of CD40-activated B Cells. Blood 2018, 132, 1340. [Google Scholar] [CrossRef]
- Beinke, S.; Ley, S.C. Functions of NF-kappaB 1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004, 382, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.-C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Delhase, M. The IκB kinase (IKK) and NF-κB: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C.; Liu, Z.-G. A special issue on NF-κB signaling and function. Cell Res. 2011, 21, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a1651. [Google Scholar] [CrossRef]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, W.; Zhang, X.; Chen, X. Neocryptotanshinone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm. Sin. B 2015, 5, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, H.; Li, Y.; Meng, X.; Yan, L.; Zhang, D.; Wu, S.; Zhou, H.; Peng, L.; Xie, Q. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3 pathways. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-Y.; Jeon, B.-H.; Kim, Y.-C.; Lee, S.H.; Sohn, D.H.; Seo, G.S. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int. Immunopharmacol. 2013, 16, 160–164. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Yang, Y.; Tao, L. Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflamm. Res. 2020, 69, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Medoff, B.D.; Thomas, S.Y.; Luster, A.D. T Cell Trafficking in Allergic Asthma: The Ins and Outs. Annu. Rev. Immunol. 2008, 26, 205–232. [Google Scholar] [CrossRef]
- Beitha, I.D.; Kemp, A.; Kenyon, J.; Prout, M.; Chestnut, T.J. Identifying neuropathic back and leg pain: A cross-sectional study. Pain 2011, 152, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Luo, J.; Jia, M.; Li, H.; Li, K.; Fu, Z. Small interfering RNA-mediated knockdown of NF-κBp65 attenuates neuro-pathic pain following peripheral nerve injury in rats. Eur. J. Pharmacol. 2012, 682, 79–85. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Samartzis, D. Clarifying the nomenclature of intervertebral disc degeneration and displacement: From bench to bedside. Int. J. Clin. Exp. Pathol. 2014, 7, 1293–1298. [Google Scholar] [PubMed]
- Jackson, R.D.; Mysiw, W.J. Insights into the epidemiology of postmenopausal osteoporosis: The Women’s Health Initiative. Semin. Reprod. Med. 2014, 32, 454–462. [Google Scholar] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoclast differentiation and activation. Clin. Calcium 2007, 17, 484–492. [Google Scholar] [PubMed]
- Hu, R.; Chen, L.; Chen, X.; Xie, Z.; Xia, C.; Chen, Y. Aloperine improves osteoporosis in ovariectomized mice by inhibiting RANKL-induced NF-κB, ERK and JNK approaches. Int. Immunopharmacol. 2021, 97, 107720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, J.; Wang, X.; Zhao, J. Protection against monocrotaline-induced pulmonary arterial hypertension and caveo-lin-1 downregulation by fluvastatin in rats. Mol. Med. Rep. 2018, 17, 3944–3950. [Google Scholar] [PubMed]
- Liu, A.; Philip, J.; Vinnakota, K.C.; Van den Bergh, F.; Tabima, D.M.; Hacker, T.; Beard, D.A.; Chesler, N.C. Estrogen maintains mitochondrial content and function in the right ventricle of rats with pulmonary hypertension. Physiol. Rep. 2017, 5, e13157. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, F.; Dong, J.; Dong, Q.; Luan, H.; Li, L.; Hao, Y. Therapeutic effects of aloperine on the pulmonary arterial hyper-tension. Farmacia 2019, 67, 691–701. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD (P) H: Quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant re-sponse element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Ma, Q. Xenobiotic-Activated Receptors: From Transcription to Drug Metabolism to Disease. Chem. Res. Toxicol. 2008, 21, 1651–1671. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE path-way. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W.D. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A.T. Cancer Chemoprevention Mechanisms Mediated Through the Keap1–Nrf2 Pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yao, W.; Xia, H.; Jin, Y.; Li, X.; Cai, J.; Hei, Z. Elevation of HO-1 Expression Mitigates Intestinal Ischemia-Reperfusion Injury and Restores Tight Junction Function in a Rat Liver Transplantation Model. Oxidative Med. Cell. Longev. 2015, 2015, 986075. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary me-tabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Papp, D.; Lenti, K.; Modos, D.; Fazekas, D.; Dúl, Z.; Turei, D.; Földvári-Nagy, L.; Nussinov, R.; Csermely, P.; Korcsmáros, T. The NRF2-related interactome and regulome contain multifunctional proteins and fine-tuned autoregulatory loops. FEBS Lett. 2012, 586, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzi, M.; Lotery, A.J. Epigenetics in age-related macular degeneration: New discoveries and future perspectives. Cell. Mol. Life Sci. 2020, 77, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Cheung, C.M.G.; Panda-Jonas, S. Updates on the Epidemiology of Age-Related Macular Degeneration. Asia-Pacific J. Ophthalmol. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degenera-tion. Retin. Degener. Dis. 2016, 854, 67–72. [Google Scholar]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin. Exp. Ophthalmol. 2018, 46, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The role of oxi-dative stress in diabetic neuropathy: Generation of free radical species in the glycation reaction and gene polymorphisms en-coding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [PubMed]
- Fang, X.; Zhang, C.; Zhang, C.; Cai, Y.; Yu, Z.; Huang, Z.; Li, W.; Zhang, W. Reactivation of Denervated Schwann Cells by Embryonic Spinal Cord Neurons to Promote Axon Regeneration and Remyelination. Stem Cells Int. 2019, 2019, 7378594. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef]
- Xiong, R.; Shan, S.; Wang, X.; Zhang, X.; Yu, H.; Shi, H.; Wang, X. Aloperine attenuates carbon tetrachloride-induced mouse hepatic injury via Nrf2/HO-1 pathway. Trop. J. Pharm. Res. 2020, 19, 983–988. [Google Scholar] [CrossRef]
- Liu, L.; Shang, Y.; Li, M.; Han, X.; Wang, J.; Wang, J. Curcumin ameliorates asthmatic airway inflammation by activating nuclear factor-E2-related factor 2/haem oxygenase (HO)-1 signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Lowy, D.R.; Willumsen, B.M. Function and regulation of ras. Annu. Rev. Biochem. 1993, 62, 851–891. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. ras oncogenes in human cancer: A review. Cancer Res. 1989, 49, 4682–4689. [Google Scholar] [PubMed]
- Campbell, S.L.; Khosravi-Far, R.; Rossman, K.L.; Clark, G.J.; Der, C.J. Increasing complexity of Ras signaling. Oncogene 1998, 17, 1395–1413. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef]
- Chung, E.; Kondo, M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol. Res. 2010, 49, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, X.; Shen, H.; Wang, D.; Wang, Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: Evidence from an in vitrostudy. BMC Med. 2009, 7, 41. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.Y.; Lee, J.S.; Hong, Y.S.; Kim, J.E.; Kim, K.P.; Kim, J.; Jang, S.J.; Yoon, Y.-K.; Kim, T.W. Primary tumor loca-tion predicts poor clinical outcome with cetuximab in RAS wild-type metastatic colorectal cancer. BMC Gastroenterol. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, S.; Chen, X.; Zheng, X.; Yao, Y.; Lu, G.; Zhou, J. Palbociclib, a selective CDK4/6 inhibitor, enhances the effect of selumetinib in RAS-driven non-small cell lung cancer. Cancer Lett. 2017, 408, 130–137. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, R.; Banu, H.; Ma, L.; Li, H. Inhibition of isoprenylcysteine carboxylmethyltransferase sensitizes common chemo-therapies in cervical cancer via Ras-dependent pathway. Biomed. Pharmacother. 2018, 99, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.; Wang, J.; Wu, Y. Identification of the key factors related to bladder cancer by lncRNA-miRNA-mRNA three-layer network. Front. Genet. 2020, 10, 1398. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Liu, W.; Zhang, P.; Wang, R.-Y.; Guo, J.-Y. Effects and mechanisms of aloperine on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. Eur. J. Pharmacol. 2010, 629, 147–152. [Google Scholar] [CrossRef]
- Dang, Z.; Xie, H.; Zhu, L.; Zhang, Q.; Li, Z.; Huang, L.; Chen, C.-H. Structure Optimization of Aloperine Derivatives as HIV-1 Entry Inhibitors. ACS Med. Chem. Lett. 2017, 8, 1199–1203. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lin, J.-Y. Five Bitter Compounds Display Different Anti-inflammatory Effects through Modulating Cytokine Secretion Using Mouse Primary Splenocytes in Vitro. J. Agric. Food Chem. 2010, 59, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Zhang, Y.J.; Wang, X.F.; Zhang, W.J.; Wang, Z.; Zhang, F.; Zhang, Y.J.; Lu, J.H.; Mei, J.W.; Hu, Y.P. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Han, J.; Zhang, Z.; Han, Z.; Wang, S. Aloperine protects mice against bleomycin-induced pulmonary fibrosis by at-tenuating fibroblast proliferation and differentiation. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, X.Q.; Tang, S.; Mei, L.; Li, Y.H.; Zhang, J.P.; Jiang, J.D.; Peng, Z.G.; Song, D.Q. Discovery and evolution of aloperine derivatives as a new family of HCV inhibitors with novel mechanism. Eur. J. Med. Chem. 2018, 143, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, J.-E.; Lim, D.Y.; Huang, Z.; Chen, H.; Langfald, A.; Lubet, R.A.; Grubbs, C.J.; Dong, Z.; Bode, A.M. Naproxen Induces Cell-Cycle Arrest and Apoptosis in Human Urinary Bladder Cancer Cell Lines and Chemically Induced Cancers by Targeting PI3K. Cancer Prev. Res. 2014, 7, 236–245. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental changes in pharmacokinetics and pharmacodynamics. J. Clin. Pharmacol. 2018, 58, S10–S25. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Zhang, Y.; Liu, J.; Liu, Z.; Wang, X. Establishment of LC-MS/MS method for determination of aloperine in rat plasma and its application in preclinical pharmacokinetics. J. Chromatogr. B 2021, 1173, 122671. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, M.; Ali, S.; Zhang, W.; Lv, B.; Qiu, W.; Wang, J. Aloperine: A Potent Modulator of Crucial Biological Mechanisms in Multiple Diseases. Biomedicines 2022, 10, 905. https://doi.org/10.3390/biomedicines10040905
Tahir M, Ali S, Zhang W, Lv B, Qiu W, Wang J. Aloperine: A Potent Modulator of Crucial Biological Mechanisms in Multiple Diseases. Biomedicines. 2022; 10(4):905. https://doi.org/10.3390/biomedicines10040905
Chicago/Turabian StyleTahir, Muhammad, Sakhawat Ali, Wenting Zhang, Boqiang Lv, Wenge Qiu, and Juan Wang. 2022. "Aloperine: A Potent Modulator of Crucial Biological Mechanisms in Multiple Diseases" Biomedicines 10, no. 4: 905. https://doi.org/10.3390/biomedicines10040905
APA StyleTahir, M., Ali, S., Zhang, W., Lv, B., Qiu, W., & Wang, J. (2022). Aloperine: A Potent Modulator of Crucial Biological Mechanisms in Multiple Diseases. Biomedicines, 10(4), 905. https://doi.org/10.3390/biomedicines10040905






