Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Cell Cultures
2.3. Zebrafish
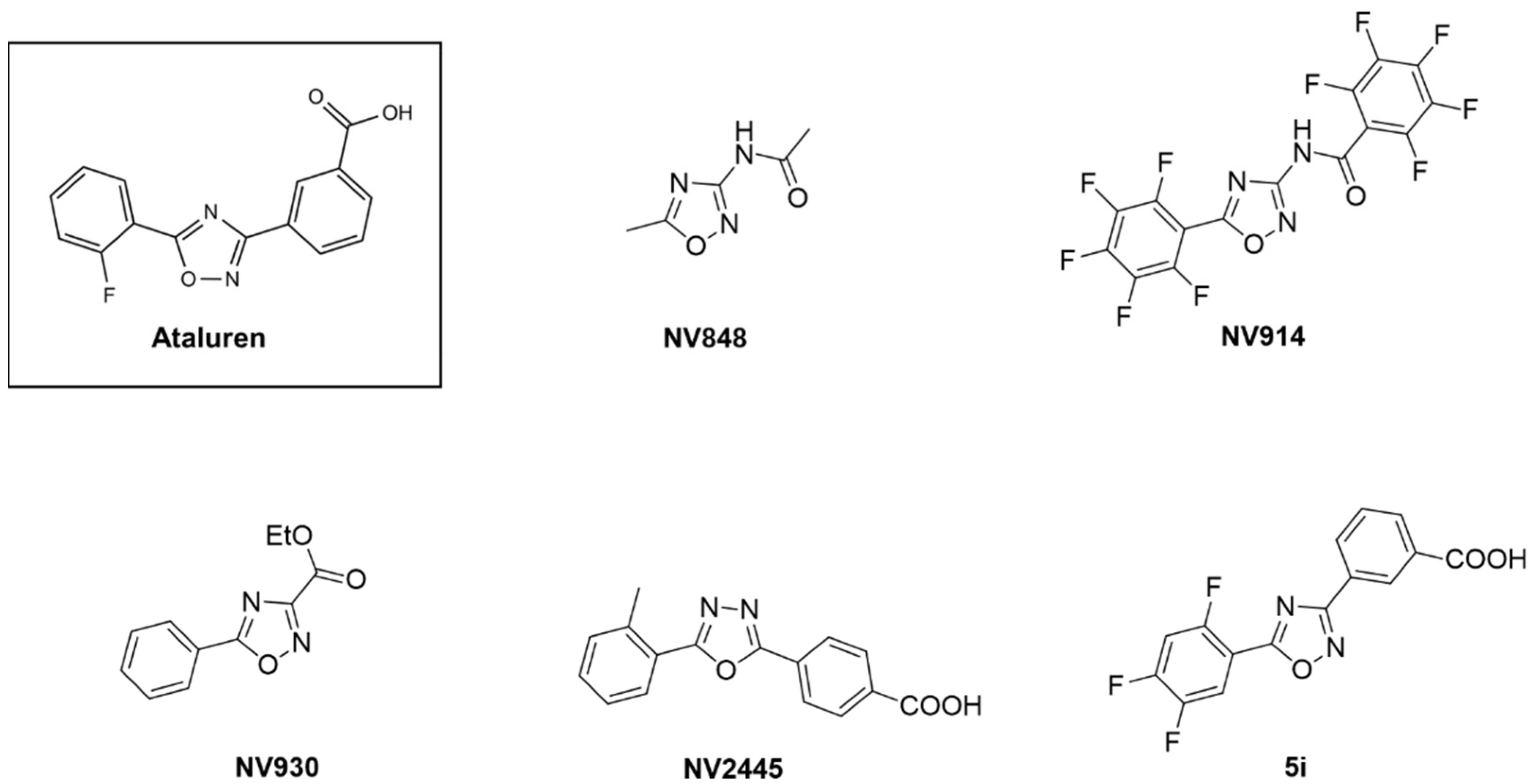
2.4. Synthesis of Ataluren Derivatives
2.5. Western Blot Analysis
2.6. In Vitro Neutrophil Maturation Assay
2.7. Colony Assays
2.8. Orange Acridine Assay
2.9. Locomotor Behavior Assay
3. Results
3.1. Screening of a Panel of Ataluren Derivatives with Established Readthrough Efficacy
3.2. Effect of Ataluren Analogues on Myeloid Differentiation of SDS Bone Marrow Hematopoietic Progenitors
3.3. NV848 Can Improve Neutrophil Maturation In Vitro, Reducing Expression of Dysplastic Markers in Bone Marrow Hematopoietic Progenitors
3.4. NV848 Restores SBDS Protein Expression Also in Primary Non-Hematological Cells
3.5. Toxicity Test in Zebrafish
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, A.S.; Myers, K.C. Diagnosis, treatment, and molecular pathology of Shwachman-Diamond syndrome. Hematol. Oncol. Clin. N. Am. 2018, 32, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, A.; Cannata, E.; Perbellini, O.; Cugno, C.; Balter, R.; Zaccaron, A.; Tridello, G.; Pizzolo, G.; De Bortoli, M.; Krampera, M.; et al. Immunophenotypic analysis of hematopoiesis in patients suffering from Shwachman-Bodian-Diamond syndrome. Eur. J. Haematol. 2015, 95, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Donadieu, J.; Fenneteau, O.; Beaupain, B.; Beaufils, S.; Bellanger, F.; Mahlaoui, N.; Lambilliotte, A.; Aladjidi, N.; Bertrand, Y.; Mialou, V.; et al. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica 2012, 97, 1312–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donadieu, J.; Leblanc, T.; Bader Meunier, B.; Barkaoui, M.; Fenneteau, O.; Bertrand, Y.; Maier-Redelsperger, M.; Micheau, M.; Stephan, J.L.; Phillipe, N.; et al. Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia. Experience of the French severe chronic neutropenia study group. Haematologica 2005, 90, 45–53. [Google Scholar]
- Boocock, G.R.; Morrison, J.A.; Popovic, M.; Richards, N.; Ellis, L.; Durie, P.R.; Rommens, J.M. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 2003, 33, 97–101. [Google Scholar] [CrossRef]
- Carapito, R.; Konantz, M.; Paillard, C.; Miao, Z.; Pichot, A.; Leduc, M.S.; Yang, Y.; Bergstrom, K.L.; Mahoney, D.H.; Shardy, D.L.; et al. Mutations in signal recognition particle SRP54 cause syndromic neutropenia with Shwachman-Diamond-like features. J. Clin. Invest. 2017, 127, 4090–4103. [Google Scholar] [CrossRef]
- Stepensky, P.; Chacon-Flores, M.; Kim, K.H.; Abuzaitoun, O.; Bautista-Santos, A.; Simanovsky, N.; Siliqi, D.; Altamura, D.; Mendez-Godoy, A.; Gijsbers, A.; et al. Mutations in EFL1, an SBDS partner, are associated with infantile pancytopenia, exocrine pancreatic insufficiency and skeletal anomalies in a Shwachman-Diamond like syndrome. J. Med. Genet. 2017, 54, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.L.; Bonnard, C.; Lim, J.Y.; Liew, W.K.; Thoon, K.C.; Thomas, T.; Ali, N.A.B.; Ng, A.Y.J.; Tohari, S.; Phua, K.B.; et al. Heterozygous missense variant in EIF6 gene: A novel form of Shwachman-Diamond syndrome? Am. J. Med. Genet. A 2020, 182, 2010–2020. [Google Scholar] [CrossRef]
- Warren, A.J. Molecular basis of the human ribosomopathy Shwachman-Diamond syndrome. Adv. Biol. Regul. 2018, 67, 109–127. [Google Scholar] [CrossRef]
- Burke, J.F.; Mogg, A.E. Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res. 1985, 13, 6265–6272. [Google Scholar] [CrossRef]
- Manuvakhova, M.; Keeling, K.; Bedwell, D.M. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA 2000, 6, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nudelman, I.; Glikin, D.; Smolkin, B.; Hainrichson, M.; Belakhov, V.; Baasov, T. Repairing faulty genes by aminoglycosides: Development of new derivatives of geneticin (G418) with enhanced suppression of diseases-causing nonsense mutations. Bioorg. Med. Chem. 2010, 18, 3735–3746. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, I.; Altman, R.B.; Djumagulov, M.; Shrestha, J.P.; Urzhumtsev, A.; Ferguson, A.; Chang, C.T.; Yusupov, M.; Blanchard, S.C.; Yusupova, G. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc. Natl. Acad. Sci. USA 2017, 114, E10899–E10908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, C.M.; Campbell, C.; Torricelli, R.E.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Heydemann, P.; Kaminska, A.; Kirschner, J.; Muntoni, F.; et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1489–1498. [Google Scholar] [CrossRef]
- Ryan, N.J. Ataluren: First global approval. Drugs 2014, 74, 1709–1714. [Google Scholar] [CrossRef]
- Bezzerri, V.; Bardelli, D.; Morini, J.; Vella, A.; Cesaro, S.; Sorio, C.; Biondi, A.; Danesino, C.; Farruggia, P.; Assael, B.M.; et al. Ataluren-driven restoration of Shwachman-Bodian-Diamond syndrome protein function in Shwachman-Diamond syndrome bone marrow cells. Am. J. Hematol. 2018, 93, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Finkel, R.S.; Flanigan, K.M.; Wong, B.; Bonnemann, C.; Sampson, J.; Sweeney, H.L.; Reha, A.; Northcutt, V.J.; Elfring, G.; Barth, J.; et al. Phase 2a study of ataluren-mediated dystrophin production in patients with nonsense mutation Duchenne muscular dystrophy. PLoS ONE 2013, 8, e81302. [Google Scholar] [CrossRef]
- McElroy, S.P.; Nomura, T.; Torrie, L.S.; Warbrick, E.; Gartner, U.; Wood, G.; McLean, W.H. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 2013, 11, e1001593. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Friesen, W.J.; Tomizawa, Y.; Leszyk, J.D.; Zhuo, J.; Johnson, B.; Dakka, J.; Trotta, C.R.; Xue, X.; Mutyam, V.; et al. Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc. Natl. Acad. Sci. USA 2016, 113, 12508–12513. [Google Scholar] [CrossRef] [Green Version]
- Tutone, M.; Pibiri, I.; Lentini, L.; Pace, A.; Almerico, A.M. Deciphering the nonsense readthrough mechanism of action of ataluren: An in silico compared study. ACS Med. Chem. Lett. 2019, 10, 522–527. [Google Scholar] [CrossRef]
- Konstan, M.W.; Van Devanter, D.R.; Rowe, S.M.; Wilschanski, M.; Kerem, E.; Sermet-Gaudelus, I.; Di Mango, E.; Melotti, P.; McIntosh, J.; De Boeck, K.; et al. Efficacy and safety of ataluren in patients with nonsense-mutation cystic fibrosis not receiving chronic inhaled aminoglycosides: The international, randomized, double-blind, placebo-controlled Ataluren Confirmatory Trial in Cystic Fibrosis (CF). J. Cyst. Fibros. 2020, 19, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Lentini, L.; Melfi, R.; Gallucci, G.; Pace, A.; Spinello, A.; Barone, G.; Di Leonardo, A. Enhancement of premature stop codon readthrough in the CFTR gene by Ataluren (PTC124) derivatives. Eur. J. Med. Chem. 2015, 101, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Melfi, R.; Tutone, M.; Di Leonardo, A.; Pace, A.; Lentini, L. Targeting nonsense: Optimization of 1,2,4-oxadiazole TRIDs to rescue CFTR expression and functionality in cystic fibrosis cell model systems. Int. J. Mol. Sci. 2020, 21, 6420. [Google Scholar] [CrossRef] [PubMed]
- Bezzerri, V.; Vella, A.; Calcaterra, E.; Finotti, A.; Gasparello, J.; Gambari, R.; Assael, B.M.; Cipolli, M.; Sorio, C. New insights into the Shwachman-Diamond syndrome-related haematological disorder: Hyper-activation of mTOR and STAT3 in leukocytes. Sci. Rep. 2016, 6, 33165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trubiani, O.; Di Primio, R.; Traini, T.; Pizzicannella, J.; Scarano, A.; Piattelli, A.; Caputi, S. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int. J. Immunopathol. Pharmacol. 2005, 18, 213–221. [Google Scholar] [CrossRef]
- Lentini, L.; Melfi, R.; Di Leonardo, A.; Spinello, A.; Barone, G.; Pace, A.; Palumbo Piccionello, A.; Pibiri, I. Toward a rationale for the PTC124 (Ataluren) promoted readthrough of premature stop codons: A computational approach and GFP-reporter cell-based assay. Mol. Pharm. 2014, 11, 653–664. [Google Scholar] [CrossRef]
- Pibiri, I.; Lentini, L.; Tutone, M.; Melfi, R.; Pace, A.; Di Leonardo, A. Exploring the readthrough of nonsense mutations by non-acidic Ataluren analogues selected by ligand-based virtual screening. Eur. J. Med. Chem. 2016, 122, 429–435. [Google Scholar] [CrossRef]
- Van De Loosdrecht, A.A.; Alhan, C.; Bene, M.C.; Della Porta, M.G.; Drager, A.M.; Feuillard, J.; Font, P.; Germing, U.; Haase, D.; Homburg, C.H.; et al. Standardization of flow cytometry in myelodysplastic syndromes: Report from the first European LeukemiaNet working conference on flow cytometry in myelodysplastic syndromes. Haematologica 2009, 94, 1124–1134. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Andersson-Lendahl, M.; Sejersen, T.; Arner, A. Muscle dysfunction and structural defects of dystrophin-null sapje mutant zebrafish larvae are rescued by ataluren treatment. FASEB J. 2014, 28, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Zhang, B.; Gao, H.; Qiu, C. Nonsense suppression induced readthrough of a novel PAX6 mutation in patient-derived cells of congenital aniridia. Mol. Genet. Genom. Med. 2020, 8, e1198. [Google Scholar] [CrossRef] [Green Version]
- Moosajee, M.; Tracey-White, D.; Smart, M.; Weetall, M.; Torriano, S.; Kalatzis, V.; Da Cruz, L.; Coffey, P.; Webster, A.R.; Welch, E. Functional rescue of REP1 following treatment with PTC124 and novel derivative PTC-414 in human choroideremia fibroblasts and the nonsense-mediated zebrafish model. Hum. Mol. Genet. 2016, 25, 3416–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilschanski, M.; Miller, L.L.; Shoseyov, D.; Blau, H.; Rivlin, J.; Aviram, M.; Cohen, M.; Armoni, S.; Yaakov, Y.; Pugatsch, T.; et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur. Respir. J. 2011, 38, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Li, H.; Ghelfi, M.D.; Goldman, Y.E.; Cooperman, B.S. Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms. Proc. Natl. Acad. Sci. USA 2021, 118, e2020599118. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Konstan, M.W.; De Boeck, K.; Accurso, F.J.; Sermet-Gaudelus, I.; Wilschanski, M.; Elborn, J.S.; Melotti, P.; Bronsveld, I.; Fajac, I.; et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2014, 2, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi-Fakhari, D.; Dillmann, U.; Flotats-Bastardas, M.; Poryo, M.; Abdul-Khaliq, H.; Shamdeen, M.G.; Mischo, B.; Zemlin, M.; Meyer, S. Off-label use of ataluren in four non-ambulatory patients with nonsense mutation duchenne muscular dystrophy: Effects on cardiac and pulmonary function and muscle strength. Front. Pediatr. 2018, 6, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gregory-Evans, K.; Wasan, K.M.; Sivak, O.; Shan, X.; Gregory-Evans, C.Y. Efficacy of postnatal in vivo nonsense suppression therapy in a pax6 mouse model of aniridia. Mol. Ther. Nucleic Acids 2017, 7, 417–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eintracht, J.; Forsythe, E.; May-Simera, H.; Moosajee, M. Translational readthrough of ciliopathy genes BBS2 and ALMS1 restores protein, ciliogenesis and function in patient fibroblasts. EBioMedicine 2021, 70, 103515. [Google Scholar] [CrossRef]
- Pibiri, I.; Lentini, L.; Melfi, R.; Tutone, M.; Baldassano, S.; Ricco Galluzzo, P.; Di Leonardo, A.; Pace, A. Rescuing the CFTR protein function: Introducing 1,3,4-oxadiazoles as translational readthrough inducing drugs. Eur. J. Med. Chem. 2018, 159, 126–142. [Google Scholar] [CrossRef]
- Dror, Y.; Freedman, M.H. Shwachman-Diamond syndrome marrow cells show abnormally increased apoptosis mediated through the Fas pathway. Blood 2001, 97, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Westers, T.M.; Ireland, R.; Kern, W.; Alhan, C.; Balleisen, J.S.; Bettelheim, P.; Burbury, K.; Cullen, M.; Cutler, J.A.; Della Porta, M.G.; et al. Standardization of flow cytometry in myelodysplastic syndromes: A report from an international consortium and the European LeukemiaNet Working Group. Leukemia 2012, 26, 1730–1741. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.K.; Cheng, S.H. Cadmium-induced ectopic apoptosis in zebrafish embryos. Arch. Toxicol. 2003, 77, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Muntoni, F.; Osorio, A.N.; Tulinius, M.; Buccella, F.; Morgenroth, L.P.; Gordish-Dressman, H.; Jiang, J.; Trifillis, P.; Zhu, J.; et al. Safety and effectiveness of ataluren: Comparison of results from the STRIDE Registry and CINRG DMD Natural History Study. J. Comp. Eff. Res. 2020, 9, 341–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, L.; Iodice, R.; Esposito, M.; Dubbioso, R.; Tozza, S.; Vitale, F.; Santoro, L.; Manganelli, F. One-year follow up of three Italian patients with Duchenne muscular dystrophy treated with ataluren: Is earlier better? Ther. Adv. Neurol. Disord. 2018, 11, 1756286418809588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerem, E.; Hirawat, S.; Armoni, S.; Yaakov, Y.; Shoseyov, D.; Cohen, M.; Nissim-Rafinia, M.; Blau, H.; Rivlin, J.; Aviram, M.; et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: A prospective phase II trial. Lancet 2008, 372, 719–727. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Boeck, K.D.; Casimir, G.J.; Vermeulen, F.; Leal, T.; Mogenet, A.; Roussel, D.; Fritsch, J.; Hanssens, L.; Hirawat, S.; et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Thada, V.; Miller, J.N.; Kovacs, A.D.; Pearce, D.A. Tissue-specific variation in nonsense mutant transcript level and drug-induced read-through efficiency in the Cln1(R151X) mouse model of INCL. J. Cell. Mol. Med. 2016, 20, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Wangen, J.R.; Green, R. Stop codon context influences genome-wide stimulation of termination codon readthrough by aminoglycosides. Elife 2020, 9, e52611. [Google Scholar] [CrossRef] [Green Version]
- Loken, M.R.; Van De Loosdrecht, A.; Ogata, K.; Orfao, A.; Wells, D.A. Flow cytometry in myelodysplastic syndromes: Report from a working conference. Leuk. Res. 2008, 32, 5–17. [Google Scholar] [CrossRef]
- Stetler-Stevenson, M.; Arthur, D.C.; Jabbour, N.; Xie, X.Y.; Molldrem, J.; Barrett, A.J.; Venzon, D.; Rick, M.E. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood 2001, 98, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Pibiri, I.; Pace, A.; Tutone, M.; Lentini, L.; Melfi, R.; Di Leonardo, A. Oxadiazole Derivatives for the Treatment of Genetic Diseases Due to Nonsense Mutations. U.S. Patent US20210002238A1, 7 January 2021. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezzerri, V.; Lentini, L.; Api, M.; Busilacchi, E.M.; Cavalieri, V.; Pomilio, A.; Diomede, F.; Pegoraro, A.; Cesaro, S.; Poloni, A.; et al. Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome. Biomedicines 2022, 10, 886. https://doi.org/10.3390/biomedicines10040886
Bezzerri V, Lentini L, Api M, Busilacchi EM, Cavalieri V, Pomilio A, Diomede F, Pegoraro A, Cesaro S, Poloni A, et al. Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome. Biomedicines. 2022; 10(4):886. https://doi.org/10.3390/biomedicines10040886
Chicago/Turabian StyleBezzerri, Valentino, Laura Lentini, Martina Api, Elena Marinelli Busilacchi, Vincenzo Cavalieri, Antonella Pomilio, Francesca Diomede, Anna Pegoraro, Simone Cesaro, Antonella Poloni, and et al. 2022. "Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome" Biomedicines 10, no. 4: 886. https://doi.org/10.3390/biomedicines10040886
APA StyleBezzerri, V., Lentini, L., Api, M., Busilacchi, E. M., Cavalieri, V., Pomilio, A., Diomede, F., Pegoraro, A., Cesaro, S., Poloni, A., Pace, A., Trubiani, O., Lippi, G., Pibiri, I., & Cipolli, M. (2022). Novel Translational Read-through–Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome. Biomedicines, 10(4), 886. https://doi.org/10.3390/biomedicines10040886










