Alcohol-Induced Alterations in the Vascular Basement Membrane in the Substantia Nigra of the Adult Human Brain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Autopsy Brain Tissue Collection
2.2. Immunohistochemistry Reactions
2.3. Immunofluorescence Reactions
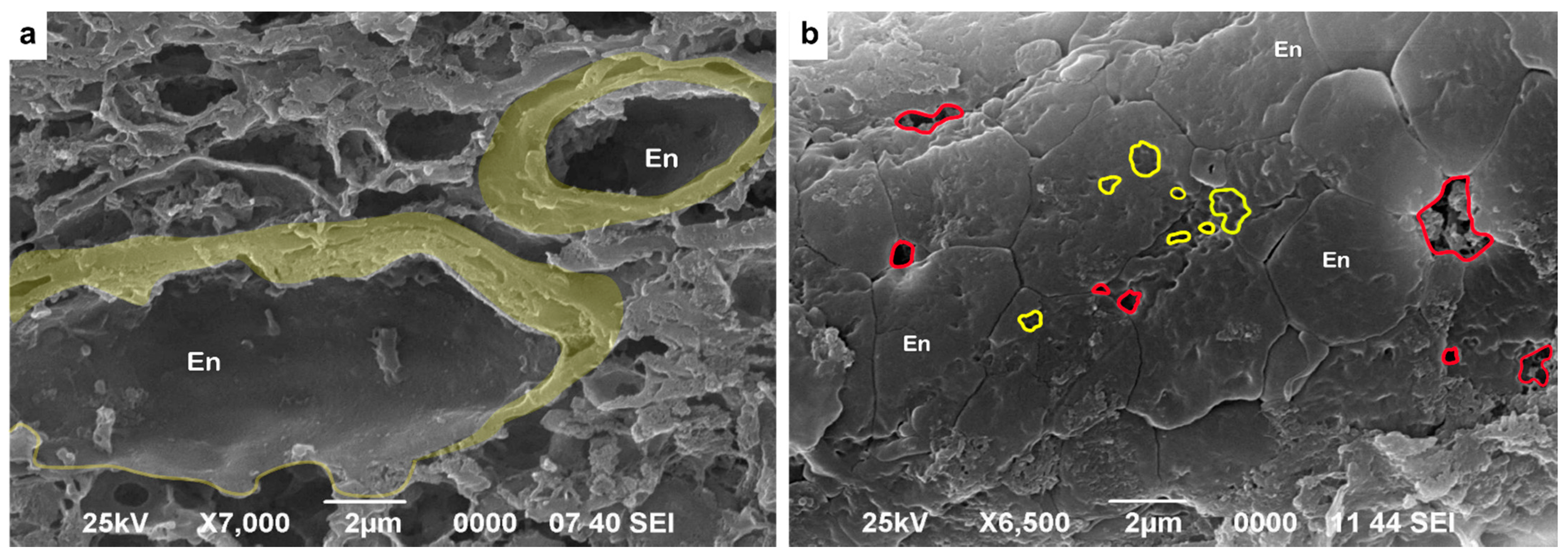
2.4. Transmission (TEM) and Scanning (SEM) Electron Microscopy
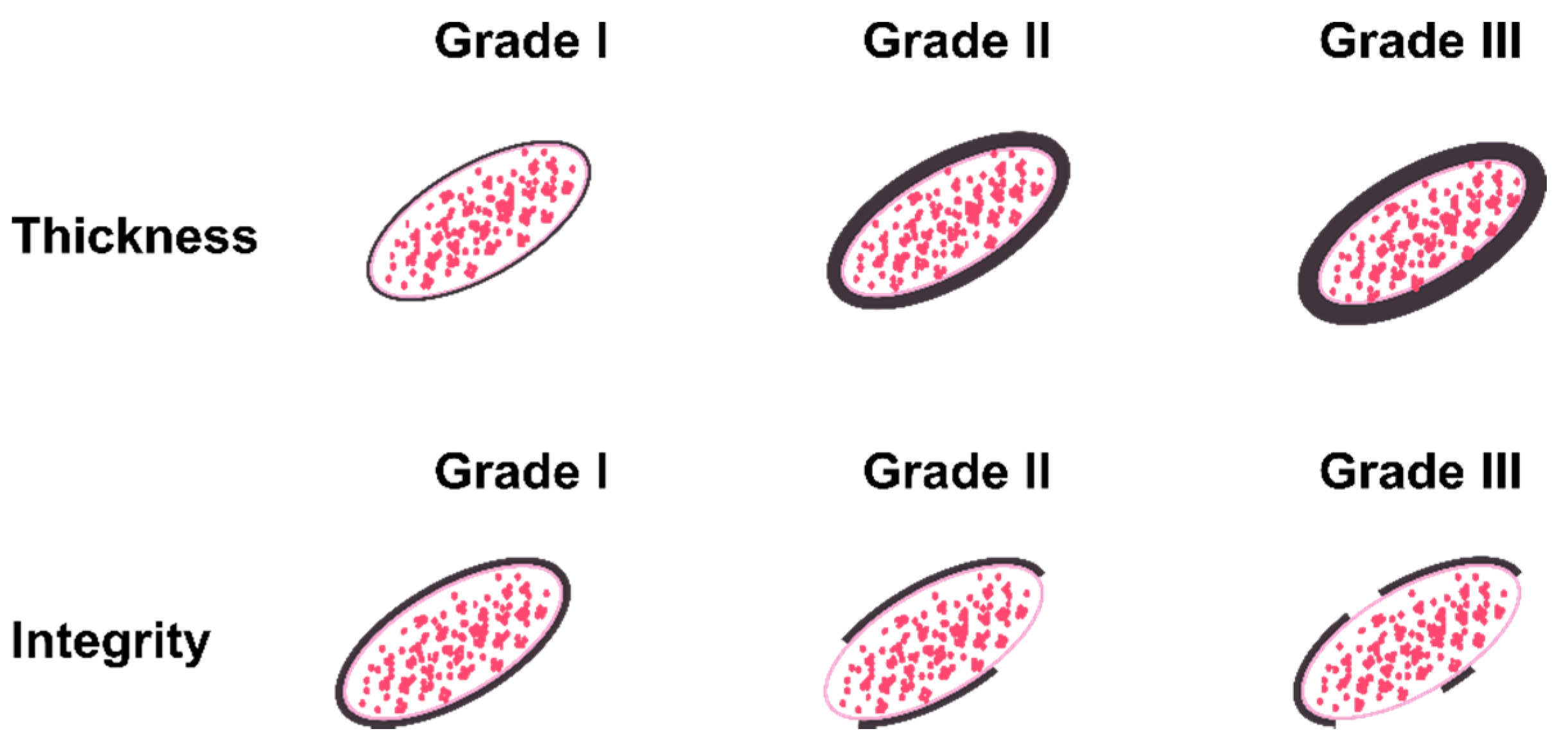
2.5. Scoring System and Statistical Analysis
3. Results
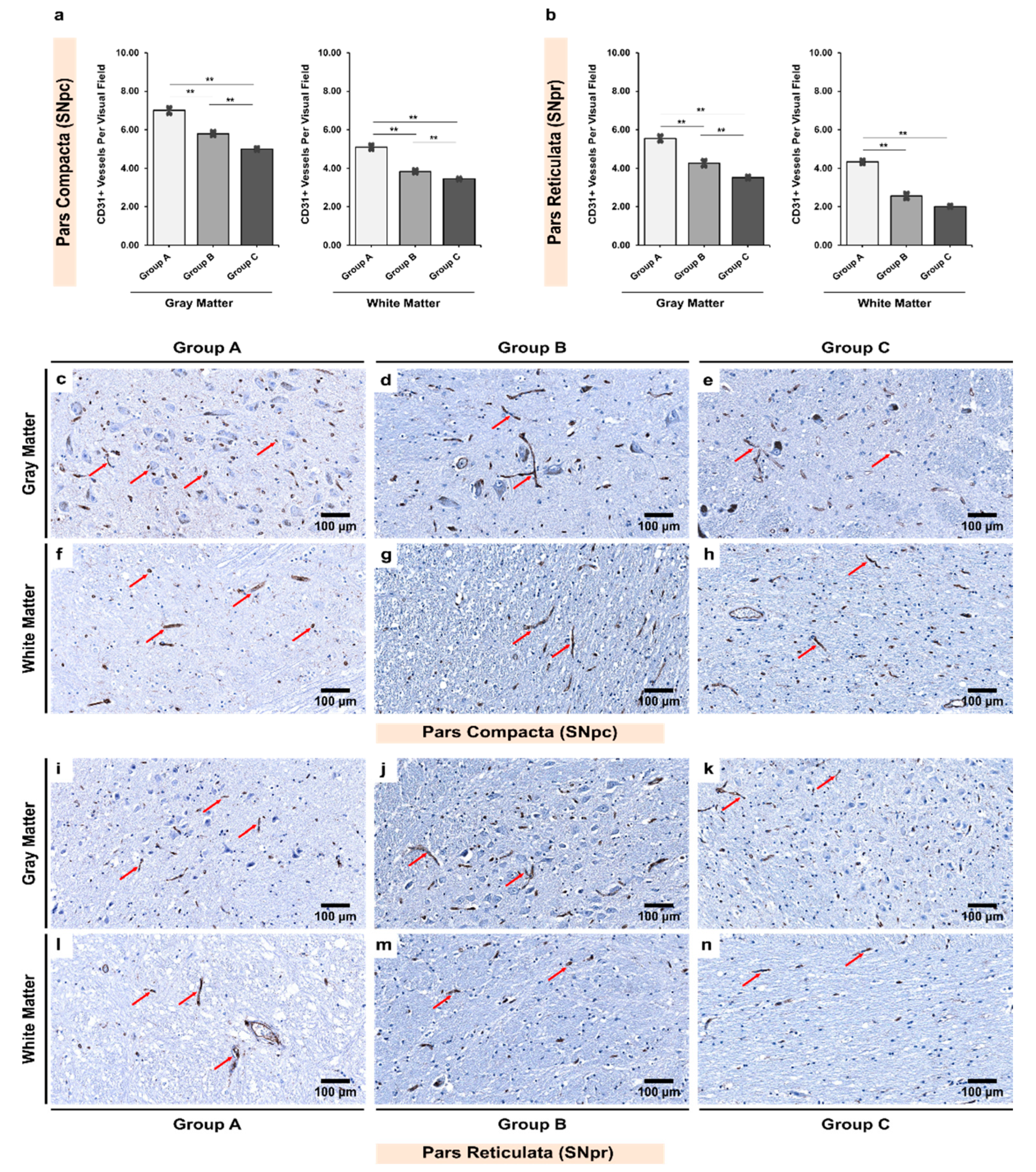
3.1. Alcoholics Showed Significantly Less CD31+ Vessels Than Controls in Both Gray and White Matter
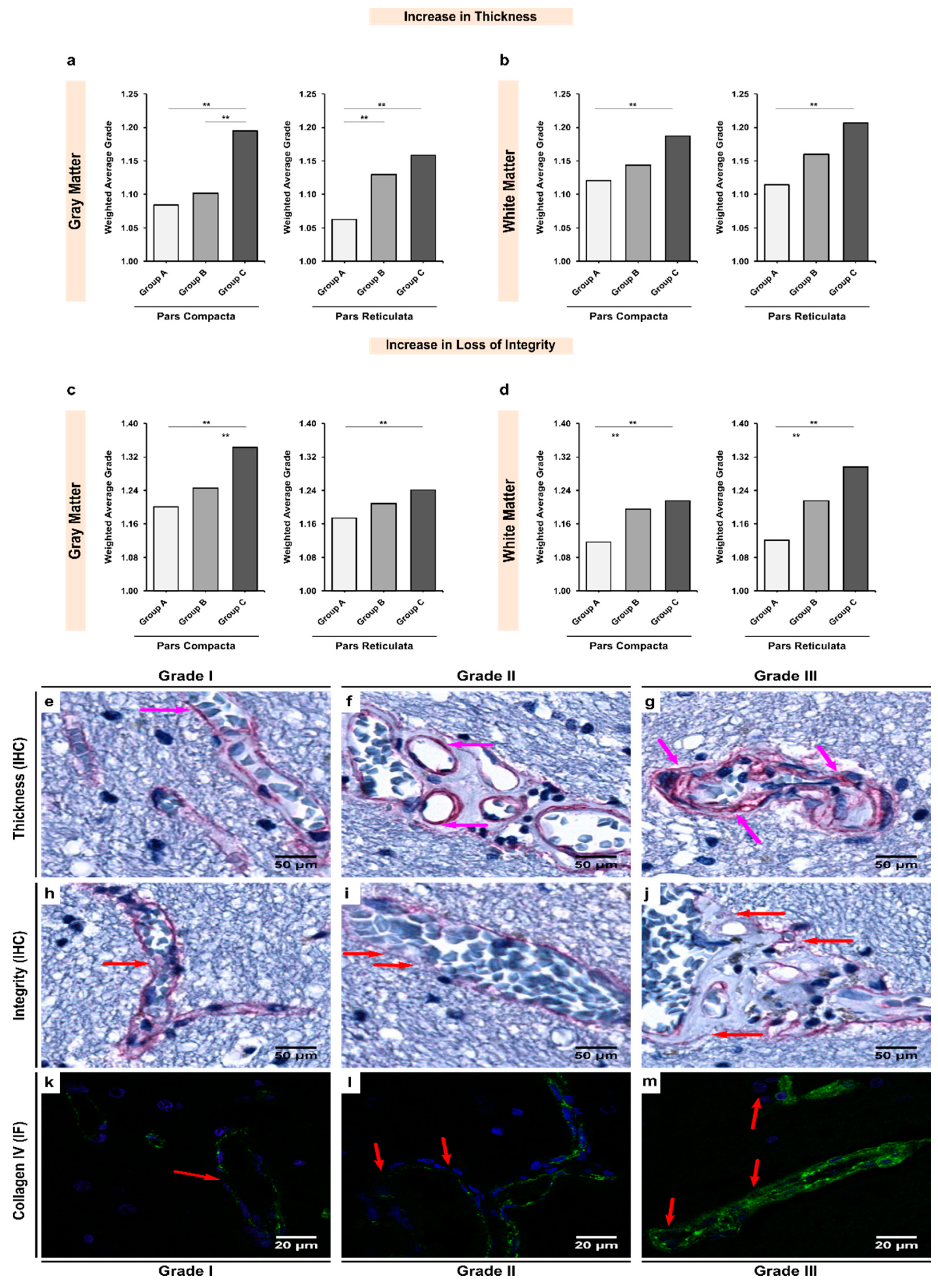
3.2. Alcoholics Showed Significant Increases in Collagen-IV Expression Coupled with Significant Losses of Vessel Integrity in Both Gray and White Matter
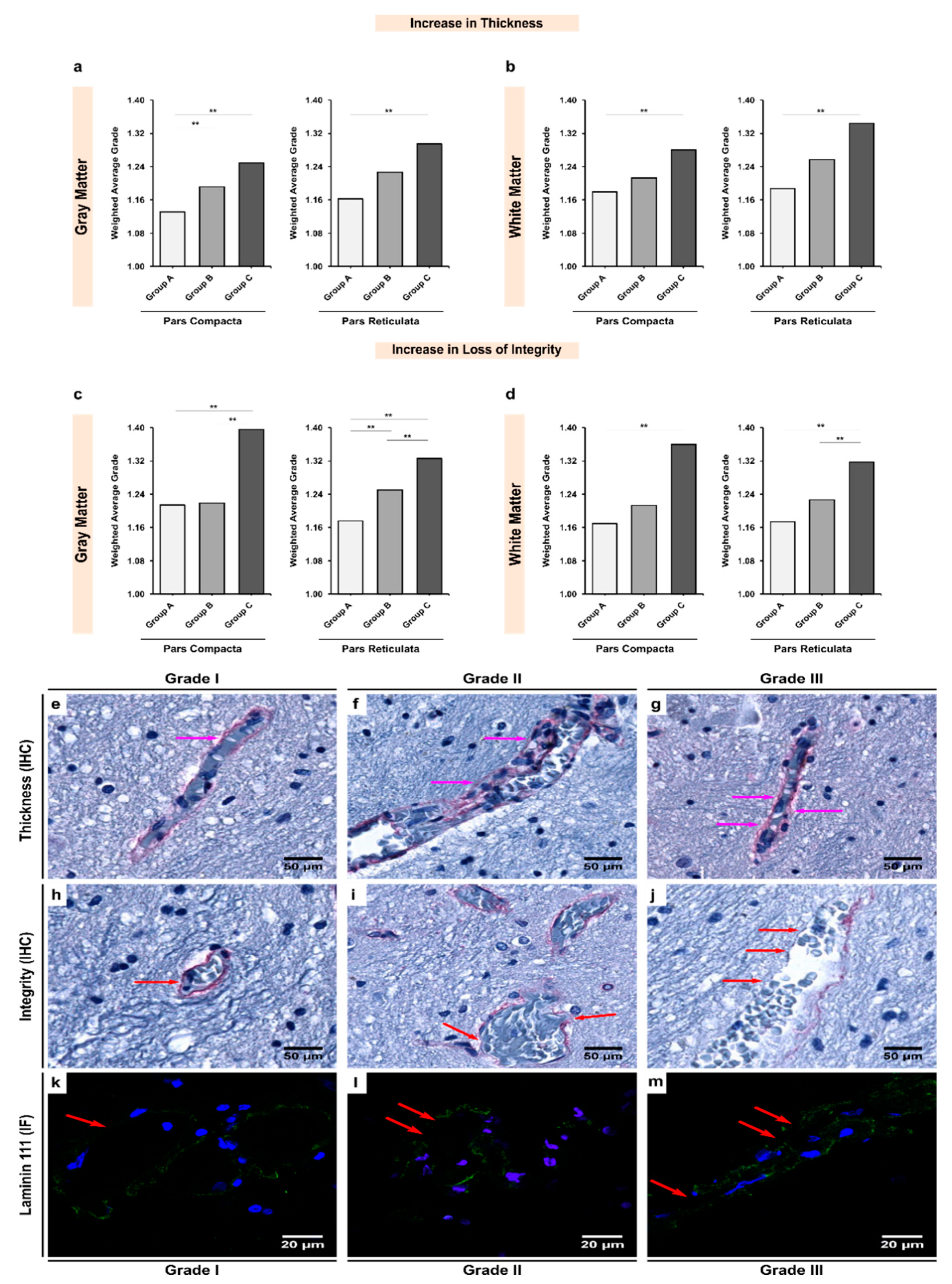
3.3. Expression of Laminin-111 Showed Significant Increases in Alcoholics Coupled with Significant Changes in the Vessel Integrity in Both Gray and White Matter
3.4. Expression of Fibronectin Was Significantly Upregulated in Alcoholics, Which Was Coupled with Significant Loss of Structural Integrity in Both Gray and White Matter
3.5. Increases in the Thickness or Expression of BM Glycoproteins Were Negatively Correlated with the Integrity of the BM
3.6. Ultrastructural Analysis of the Vascular Basement Membrane and BBB
4. Discussion
4.1. White Matter Has Significantly Fewer CD31+ Microvessels Than Gray Matter in Physiological Conditions
4.2. Alcohol Use Aggravates Decreased Microvascular Density in Both Gray and White Matter
4.3. Gray Matter Has Thinner and More Damaged Collagen-Iv-Containing Basement Membrane Than White Matter
4.4. The Dual Role of Upregulated Expression in Laminin-111
4.5. Increased Expression of Fibronectin Might Promote Endothelial Damage
4.6. Potential Diagnostic Applications
4.7. Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardridge, W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999, 5, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The neurovascular unit: Effects of brain insults during the perinatal period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Birková, A.; Hubková, B.; Čižmárová, B.; Bolerázska, B. Current view on the mechanisms of alcohol-mediated toxicity. Int. J. Mol. Sci. 2021, 22, 9686. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, A.-K.; Löfving, S.; Callaghan, R.C.; Allebeck, P. Alcohol use disorders and risk of parkinson’s disease: Findings from a swedish national cohort study 1972–2008. BMC Neurol. 2013, 13, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Yang, Q.; Joshi, R.B.; Liu, Y.; Akbar, M.; Song, B.-J.; Zhou, S.; Wang, X. Role of alcohol drinking in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samokhvalov, A.V.; Irving, H.; Mohapatra, S.; Rehm, J. Alcohol consumption, unprovoked seizures, and epilepsy: A systematic review and meta-analysis: Alcohol & epilepsy. Epilepsia 2010, 51, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.; Branca, J.J.V.; Becatti, M.; Paternostro, F.; Morucci, G.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Alcohol-induced blood-brain barrier impairment: An in vitro study. Int. J. Environ. Res. Public Health 2021, 18, 2683. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, E.; Sivalingam, K.; Doke, M.; Samikkannu, T. Effects of drugs of abuse on the blood-brain barrier: A brief overview. Front. Neurosci. 2020, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharm. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Birkelund, S.; Burkhart, A.; Stensballe, A.; Moos, T. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J. Neurochem. 2017, 140, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Gautam, J.; Zhang, X.; Yao, Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci. Rep. 2016, 6, 36450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Chen, Z.-L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, M.J.; Damodarasamy, M.; Banks, W.A. The Extracellular matrix of the blood–brain barrier: Structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers 2019, 7, 1651157. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, D.N.; Bix, G.J. Roles of blood-brain barrier integrins and extracellular matrix in stroke. Am. J. Physiol.-Cell Physiol. 2019, 316, C252–C263. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Milner, R. Fibronectin Promotes brain capillary endothelial cell survival and proliferation through Alpha5beta1 and Alphavbeta3 integrins via MAP kinase signalling. J. Neurochem. 2006, 96, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R. Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 1989, 180, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.D.; McCullough, L.D.; Urayama, A. The role of basement membranes in cerebral amyloid angiopathy. Front. Physiol. 2020, 11, 601320. [Google Scholar] [CrossRef] [PubMed]
- Michalski, D.; Spielvogel, E.; Puchta, J.; Reimann, W.; Barthel, H.; Nitzsche, B.; Mages, B.; Jäger, C.; Martens, H.; Horn, A.K.E.; et al. Increased immunosignals of collagen IV and fibronectin indicate ischemic consequences for the neurovascular matrix adhesion zone in various animal models and human stroke tissue. Front. Physiol. 2020, 11, 575598. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood–brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snorradottir, A.O.; Isaksson, H.J.; Kaeser, S.A.; Skodras, A.A.; Olafsson, E.; Palsdottir, A.; Bragason, B.T. Deposition of collagen IV and aggrecan in leptomeningeal arteries of hereditary brain haemorrhage with amyloidosis. Brain Res. 2013, 1535, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Hedera, P. Differential degeneration of the cerebral microvasculature in Alzheimer’s disease. NeuroReport 1995, 6, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Ceafalan, L.C.; Fertig, T.E.; Gheorghe, T.C.; Hinescu, M.E.; Popescu, B.O.; Pahnke, J.; Gherghiceanu, M. Age-related ultrastructural changes of the basement membrane in the mouse blood-brain barrier. J. Cell. Mol. Med. 2019, 23, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Larcher, K.M.-H.; Misic, B.; Dagher, A. Anatomical and functional organization of the human substantia nigra and its connections. eLife 2017, 6, e26653. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, D.N.; Salkov, V.N.; Khudoerkov, R.M. Three-dimensional reconstruction of substantia nigra pars compacta of human brain. I.P. Pavlov Russ. Med. Biol. Her. 2018, 26, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Roles for nigrostriatal—Not Just mesocorticolimbic—Dopamine in reward and addiction. Trends Neurosci. 2009, 32, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, Y.; May, P.J. The Substantia nigra pars reticulata modulates error-based saccadic learning in monkeys. Eneuro 2021, 8, ENEURO.0519-20.2021. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-Y.; Kodama, T.; Hsieh, K.-C.; Nguyen, D.; Siegel, J.M. Substantia nigra pars reticulata-mediated sleep and motor activity regulation. Sleep 2021, 44, zsaa151. [Google Scholar] [CrossRef] [PubMed]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Morales, M. The brain on drugs: From reward to addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Östergren, A.; Fredriksson, A.; Brittebo, E.B. Norharman-induced motoric impairment in mice: Neurodegeneration and glial activation in substantia nigra. J. Neural. Transm. 2006, 113, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.A.; Patani, R. Region-specific vulnerability in neurodegeneration: Lessons from normal ageing. Ageing Res. Rev. 2021, 67, 101311. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Smirnovs, M.; Strojeva, S.; Murovska, M.; Skuja, S. Chronic alcoholism and HHV-6 infection synergistically promote neuroinflammatory microglial phenotypes in the substantia nigra of the adult human brain. Biomedicines 2021, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, C.; Villalpando, E.; Steiner, N.; Quach, L.; Fannon, M.; Somkuwar, S. Platelet endothelial cell adhesion molecule-1 and oligodendrogenesis: Significance in alcohol use disorders. Brain Sci. 2017, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood–brain barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finley, K.H. Angio-architecture of the substantia nigra and its pathogenic significance. Arch. Neurol. Psychiatry 1936, 36, 118. [Google Scholar] [CrossRef]
- Paton, A. Alcohol in the body. BMJ 2005, 330, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Ding, R.; Harrison, G.; Hawthorne, E.; King, A.; Gettings, S.; Platten, C.; Stevenson, W.; Craggs, L.J.L.; Kalaria, R.N. White matter capillaries in vascular and neurodegenerative dementias. Acta Neuropathol. Commun. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubíková, T.; Kochová, P.; Tomášek, P.; Witter, K.; Tonar, Z. Numerical and length densities of microvessels in the human brain: Correlation with preferential orientation of microvessels in the cerebral cortex, subcortical grey matter and white matter, pons and cerebellum. J. Chem. Neuroanat. 2018, 88, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Harik, S.; Kalaria, R.; Andersson, L.; Lundahl, P.; Perry, G. Immunocytochemical localization of the erythroid glucose transporter: Abundance in tissues with barrier functions. J. Neurosci. 1990, 10, 3862–3872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moody, D.M.; Bell, M.A.; Challa, V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: An anatomic study. AJNR Am. J. Neuroradiol. 1990, 11, 431–439. [Google Scholar] [PubMed]
- Challa, V.R.; Bell, M.A.; Moody, D.M. A combined hematoxylin-eosin, alkaline phosphatase and high-resolution microradiographic study of lacunes. Clin. Neuropathol. 1990, 9, 196–204. [Google Scholar] [PubMed]
- Moody, D.M.; Santamore, W.P.; Bell, M.A. Does tortuosity in cerebral arterioles impair down-autoregulation in hypertensives and elderly normotensives? A hypothesis and computer model. Clin Neurosurg. 1991, 37, 372–387. [Google Scholar] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr. Res. 2004, 56, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mito, T.; Konomi, H.; Houdou, S.; Takashima, S. Immunohistochemical study of the vasculature in the developing brain. Pediatr. Neurol. 1991, 7, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Tanner, S.F.; Cornette, L.; Ramenghi, L.A.; Miall, L.S.; Ridgway, J.P.; Smith, M.A.; Levene, M.I. Cerebral perfusion in infants and neonates: Preliminary results obtained using dynamic susceptibility contrast enhanced magnetic resonance imaging. Arch. Dis. Child Fetal Neonatal Ed. 2003, 88, F525–F530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolinko, Y.; Cendelin, J.; Kralickova, M.; Tonar, Z. Smaller Absolute quantities but greater relative densities of microvessels are associated with cerebellar degeneration in lurcher mice. Front Neuroanat. 2016, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnieder, T.P.; Zhou Qin, I.D.; Trencevska-Ivanovska, I.; Rosoklija, G.; Stankov, A.; Pavlovski, G.; Mann, J.J.; Dwork, A.J. Blood vessels and perivascular phagocytes of prefrontal white and gray matter in suicide. J. Neuropathol. Exp. Neurol. 2019, 78, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Keiser, J.; Olszewski, B.; Rosebury, W.; Robertson, A.; Kovesdi, I.; Gordon, D. Delayed angiogenesis in aging rats and therapeutic effect of adenoviral gene transfer of VEGF. Int. J. Mol. Med. 2004, 13, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Iemitsu, M.; Maeda, S.; Jesmin, S.; Otsuki, T.; Miyauchi, T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1290–H1298. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cheda, B.; Sousa-Ribeiro, D.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J. Cereb. Blood Flow Metab. 2009, 29, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, D.M.A.; Eaves, N.R.; Marcyniuk, B.; Yates, P.O. Quantitative changes in cerebral cortical microvasculature in ageing and dementia. Neurobiol. Aging 1986, 7, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.W.; Siddiqi, A.; Yusufaly, Y. Altered angioarchitecture in selected areas of brains with Alzheimer’s disease. Acta Neuropathol. 1990, 79, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Tonar, Z.; Kochová, P.; Cimrman, R.; Witter, K.; Janáček, J.; Rohan, V. Microstructure oriented modelling of hierarchically perfused porous media for cerebral blood flow evaluation. Key Eng. Mater. 2011, 465, 286–289. [Google Scholar] [CrossRef]
- Kraszpulski, M.; Tukaj, C.; Wrzołkowa, T. Hippocampal capillaries in different age groups of chronically ethanol-intoxicated rats. morphometrical studies. Folia Morphol. 2000, 59, 9. [Google Scholar] [PubMed]
- Cheung, K.; Ma, L.; Wang, G.; Coe, D.; Ferro, R.; Falasca, M.; Buckley, C.D.; Mauro, C.; Marelli-Berg, F.M. CD31 signals confer immune privilege to the vascular endothelium. Proc. Natl. Acad. Sci. USA 2015, 112, E5815–E5824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataramani, V.; Küffer, S.; Cheung, K.C.P.; Jiang, X.; Trümper, L.; Wulf, G.G.; Ströbel, P. CD31 expression determines redox status and chemoresistance in human angiosarcomas. Clin. Cancer Res. 2018, 24, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, J.; Pavlovic, D.; Dalkie, N.; Waldvogel, H.J.; O’Carroll, S.J.; Green, C.R.; Nicholson, L.F.B. Vascular degeneration in Parkinson’s disease: Vascular degeneration of Parkinson’s disease. Brain Pathol. 2013, 23, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Shulyakov, A.V.; Cenkowski, S.S.; Buist, R.J.; Del Bigio, M.R. Age-dependence of intracranial viscoelastic properties in living rats. J. Mech. Behav. Biomed. Mater. 2011, 4, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Taguchi, T.; Shimada, Y.; Sakuramoto, I.; Kawano, S.; Kawai, S. Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J. Neurotrauma 2001, 18, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Bilston, L.E.; Sinkus, R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008, 21, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Uspenskaia, O.; Liebetrau, M.; Herms, J.; Danek, A.; Hamann, G.F. Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci. 2004, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linka, K.; Reiter, N.; Würges, J.; Schicht, M.; Bräuer, L.; Cyron, C.J.; Paulsen, F.; Budday, S. Unraveling the local relation between tissue composition and human brain mechanics through machine learning. Front. Bioeng. Biotechnol. 2021, 9, 704738. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Sarem, M.; Starck, L.; Sommer, G.; Pfefferle, J.; Phunchago, N.; Kuhl, E.; Paulsen, F.; Steinmann, P.; Shastri, V.P.; et al. Towards microstructure-informed material models for human brain tissue. Acta Biomater. 2020, 104, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ducker, T.B.; Kindt, G.W.; Kempf, L.G. Pathological findings in acute experimental spinal cord trauma. J. Neurosurg. 1971, 35, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Weickenmeier, J.; de Rooij, R.; Budday, S.; Ovaert, T.C.; Kuhl, E. The mechanical importance of myelination in the central nervous system. J. Mech. Behav. Biomed. Mater. 2017, 76, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Araiz, A.; Porcu, F.; Pérez-Hernández, M.; García-Gutiérrez, M.S.; Aracil-Fernández, M.A.; Gutierrez-López, M.D.; Guerri, C.; Manzanares, J.; O’Shea, E.; Colado, M.I. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: Preclinical evidence of TLR4 involvement from a binge-like drinking model: TLR4 Involvement from a binge-like drinking model. Addict. Biol. 2017, 22, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-L.; Yao, Y.; Norris, E.H.; Kruyer, A.; Jno-Charles, O.; Akhmerov, A.; Strickland, S. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J. Cell Biol. 2013, 202, 381–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halder, S.K.; Kant, R.; Milner, R. Chronic mild hypoxia increases expression of laminins 111 and 411 and the laminin receptor A6β1 integrin at the blood-brain barrier. Brain Res. 2018, 1700, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.C.; Tate, M.C.; LaPlaca, M.C. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J. Neurotrauma 2007, 24, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Conejero-Goldberg, C.; Gomar, J.J.; Bobes-Bascaran, T.; Hyde, T.M.; Kleinman, J.E.; Herman, M.M.; Chen, S.; Davies, P.; Goldberg, T.E. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol. Psychiatry 2014, 19, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.Y.; Au, N.P.B.; Ma, C.H.E. The Association between laminin and microglial morphology in vitro. Sci. Rep. 2016, 6, 28580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrogrande, G.; Mabotuwana, N.; Zhao, Z.; Abdolhoseini, M.; Johnson, S.J.; Nilsson, M.; Walker, F.R. Chronic Stress induced disturbances in laminin: A significant contributor to modulating microglial pro-inflammatory tone? Brain Behav. Immun. 2018, 68, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, F.-X.; Mann, D.M.A.; Robinson, A.C.; Pinteaux, E.; Boutin, H. Early changes in extracellular matrix in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017, 43, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, T.; Suzuki, T.; Tanaka, H.; Arisawa, K.; Miyashita, T.; Nekooki-Machida, Y.; Kurosawa, T.; Tega, Y.; Deguchi, Y.; Hagiwara, H. Fibronectin is essential for formation of fenestrae in endothelial cells of the fenestrated capillary. Cell Tissue Res. 2021, 383, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Kuebler, W.M. Normal endothelium. In The Vascular Endothelium I.; Moncada, S., Higgs, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 176/I, pp. 1–40. ISBN 978-3-540-32966-4. [Google Scholar]
- Zarow, C.; Barron, E.; Chui, H.C.; Perlmutter, L.S. Vascular basement membrane pathology and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1997, 826, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Tsvetanov, K.A.; Henson, R.N.A.; Rowe, J.B. Separating vascular and neuronal effects of age on fMRI BOLD signals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190631. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flück, D.; Beaudin, A.E.; Steinback, C.D.; Kumarpillai, G.; Shobha, N.; McCreary, C.R.; Peca, S.; Smith, E.E.; Poulin, M.J. Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front. Physiol. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenders, K.L.; Perani, D.; Lammertsma, A.A.; Heather, J.D.; Buckingham, P.; Healy, M.J.; Gibbs, J.M.; Wise, R.J.; Hatazawa, J.; Herold, S.; et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990, 113, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ambarki, K.; Wåhlin, A.; Zarrinkoob, L.; Wirestam, R.; Petr, J.; Malm, J.; Eklund, A. Accuracy of parenchymal cerebral blood flow measurements using pseudocontinuous arterial spin-labeling in healthy volunteers. AJNR Am. J. Neuroradiol. 2015, 36, 1816–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Primary Antibody * | Antibody Properties ** | Clone | Working Dilutions | Manufacturer | Catalogue No. |
|---|---|---|---|---|---|
| CD31 | Mouse monoclonal AB against human AG | JC70A | 1/30 | DakoCytomation (Glostrup, Denmark) | M0823 |
| Collagen-IV | Mouse monoclonal AB against human AG | PHM-12 | 1/100 | Novocastra (Deer Park, IL, USA) | NCL-COLL-IV |
| Laminin-111 | Rabbit polyclonal AB against human AG | - | 1/1000 | Arigo Biolaboratories (Hsinchu City, Taiwan) | ARG10736 |
| Fibronectin | Rabbit Polyclonal AB against human AG | - | 1/400 | DakoCytomation (Glostrup, Denmark) | A0245 |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 07.00 ± 0.17 | 05.80 ± 0.14 | 05.03 ± 0.09 | <0.001 ** |
| White Matter | 05.09 ± 0.14 | 03.83 ± 0.11 | 03.34 ± 0.07 | <0.001 ** |
| pValue ‡ | <0.001 ** | <0.001 ** | <0.001 ** | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 05.55 ± 0.18 | 04.26 ± 0.16 | 03.52 ± 0.10 | <0.001 ** |
| White Matter | 04.34 ± 0.12 | 02.56 ± 0.17 | 02.02 ± 0.08 | <0.001 ** |
| pValue ‡ | <0.001 ** | <0.001 ** | <0.001 ** | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.084 | 1.102 | 1.195 | <0.001 ** |
| White Matter | 1.121 | 1.143 | 1.187 | 0.009 ** |
| pValue ‡ | 0.072 | 0.031 ** | 0.355 | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.063 | 1.130 | 1.158 | <0.001 ** |
| White Matter | 1.115 | 1.160 | 1.201 | 0.002 ** |
| pValue ‡ | 0.002 ** | 0.991 | 0.187 | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.201 | 1.245 | 1.343 | <0.001 ** |
| White Matter | 1.116 | 1.195 | 1.216 | <0.001 ** |
| pValue ‡ | <0.001 ** | 0.004 ** | <0.001 ** | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.173 | 1.209 | 1.241 | 0.012 ** |
| White Matter | 1.121 | 1.216 | 1.296 | <0.001 ** |
| pValue ‡ | <0.001 ** | 0.939 | 0.452 | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.132 | 1.192 | 1.249 | <0.001 ** |
| White Matter | 1.179 | 1.213 | 1.280 | 0.005 ** |
| pValue ‡ | 0.146 | 0.949 | 0.611 | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.163 | 1.226 | 1.295 | <0.001 ** |
| White Matter | 1.188 | 1.257 | 1.344 | <0.001 ** |
| pValue ‡ | 0.333 | 0.786 | 0.279 | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.214 | 1.219 | 1.396 | <0.001 ** |
| White Matter | 1.169 | 1.213 | 1.360 | 0.003 ** |
| pValue ‡ | 0.011 ** | 0.936 | 0.830 | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.176 | 1.251 | 1.326 | <0.001 ** |
| White Matter | 1.174 | 1.227 | 1.318 | <0.001 ** |
| pValue ‡ | 0.428 | 0.482 | 0.526 | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.145 | 1.158 | 1.217 | <0.001 ** |
| White Matter | 1.185 | 1.317 | 1.380 | <0.001 ** |
| pValue ‡ | 0.010 ** | <0.001 ** | 0.033 ** | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.122 | 1.159 | 1.210 | <0.001 ** |
| White Matter | 1.162 | 1.240 | 1.339 | <0.001 ** |
| pValue ‡ | 0.923 | 0.002 ** | 0.026 ** | - |
| Controls (Group A) | Young Alcoholics (Group B) | Chronic Alcoholics (Group C) | p Value † | |
|---|---|---|---|---|
| Pars Compacta(SNpc) | ||||
| Gray Matter | 1.132 | 1.135 | 1.248 | <0.001 ** |
| White Matter | 1.082 | 1.215 | 1.298 | <0.001 ** |
| pValue ‡ | <0.001 ** | 0.063 | <0.001 ** | - |
| Pars Reticulata(SNpr) | ||||
| Gray Matter | 1.158 | 1.181 | 1.261 | <0.001 ** |
| White Matter | 1.097 | 1.173 | 1.247 | 0.273 |
| pValue ‡ | <0.001 ** | 0.086 | <0.001 ** | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skuja, S.; Jain, N.; Smirnovs, M.; Murovska, M. Alcohol-Induced Alterations in the Vascular Basement Membrane in the Substantia Nigra of the Adult Human Brain. Biomedicines 2022, 10, 830. https://doi.org/10.3390/biomedicines10040830
Skuja S, Jain N, Smirnovs M, Murovska M. Alcohol-Induced Alterations in the Vascular Basement Membrane in the Substantia Nigra of the Adult Human Brain. Biomedicines. 2022; 10(4):830. https://doi.org/10.3390/biomedicines10040830
Chicago/Turabian StyleSkuja, Sandra, Nityanand Jain, Marks Smirnovs, and Modra Murovska. 2022. "Alcohol-Induced Alterations in the Vascular Basement Membrane in the Substantia Nigra of the Adult Human Brain" Biomedicines 10, no. 4: 830. https://doi.org/10.3390/biomedicines10040830
APA StyleSkuja, S., Jain, N., Smirnovs, M., & Murovska, M. (2022). Alcohol-Induced Alterations in the Vascular Basement Membrane in the Substantia Nigra of the Adult Human Brain. Biomedicines, 10(4), 830. https://doi.org/10.3390/biomedicines10040830








