Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update
Abstract
1. Introduction
2. Methods
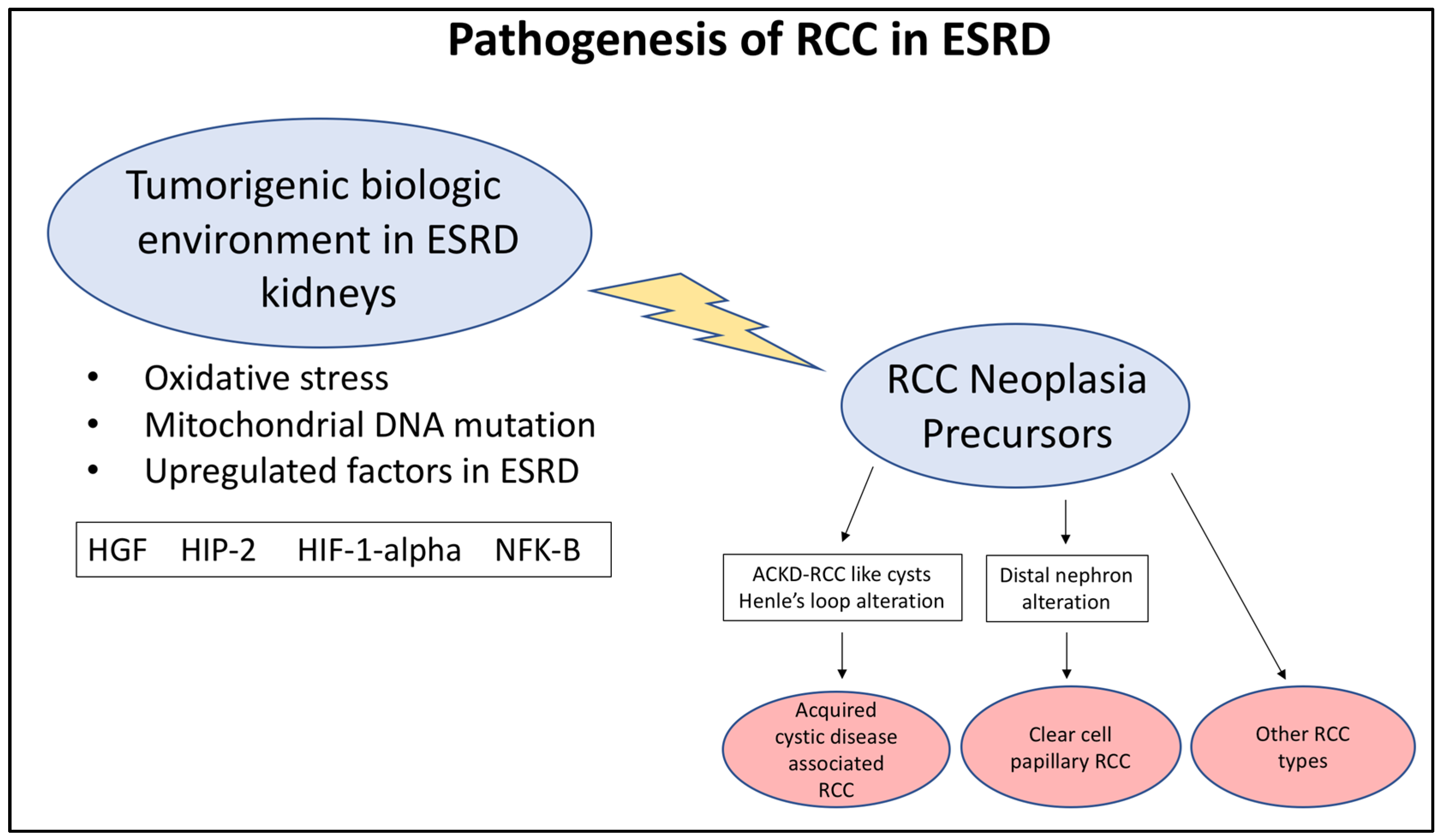
3. Etiology and Pathogenesis of RCC in ESRD
4. Clinical Features of ACKD-RCC and ccpRCC
4.1. Epidemiology and Risk Factors
4.2. Clinical Diagnosis, Prognosis, and Management
5. Pathological Features of RCC Associated with ESRD
5.1. Overview of RCC Pathology in ESRD
5.2. Pathological Features of ACKD-RCC
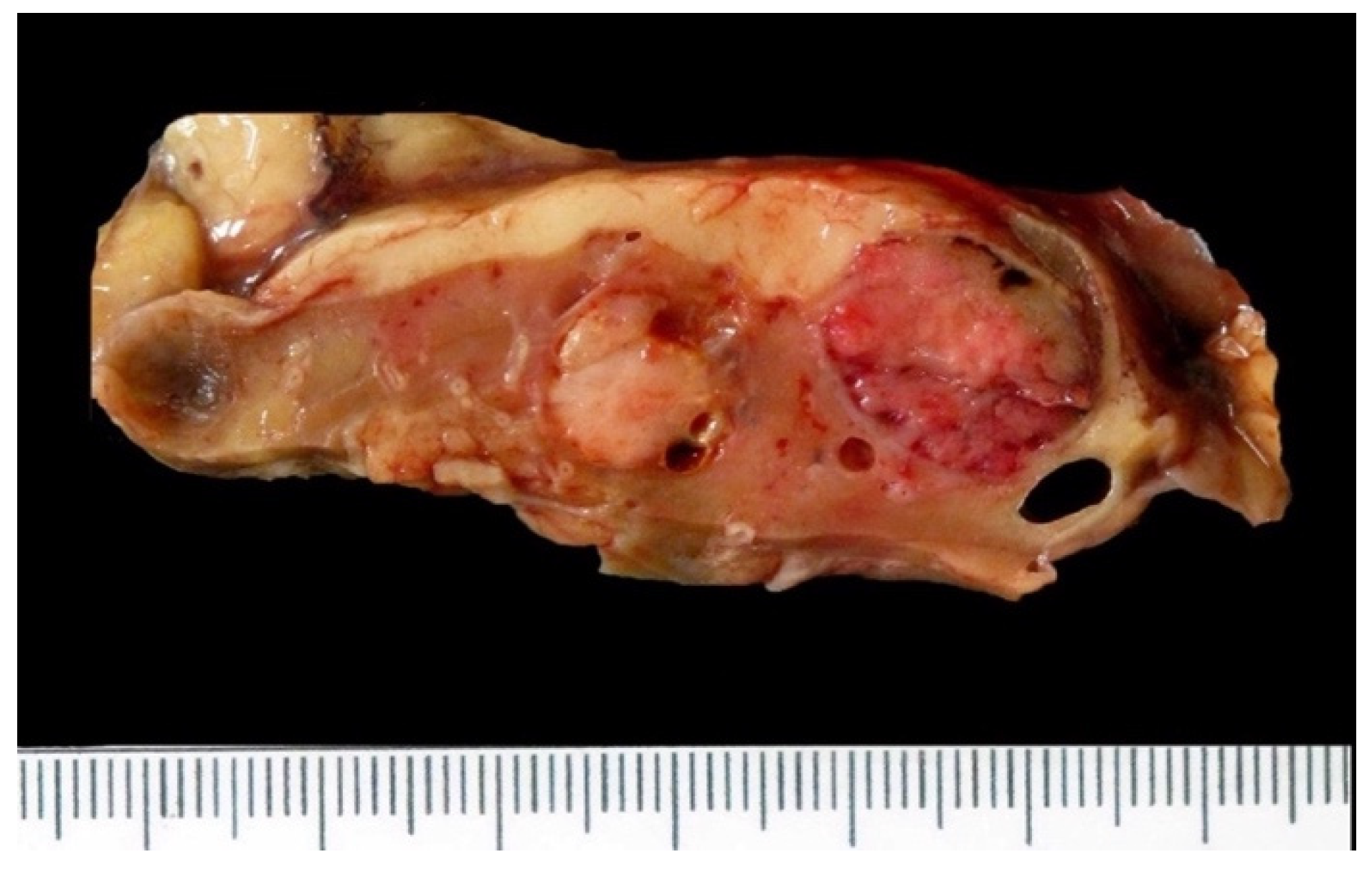
5.2.1. Macroscopic Features
5.2.2. Microscopic Features
5.2.3. Immunophenotype
5.2.4. Molecular Characteristics
5.3. Pathological Features of ccpRCC
5.3.1. Macroscopic Features
5.3.2. Microscopic Features
5.3.3. Immunophenotype
5.3.4. Molecular Characteristics
6. Differential Diagnoses
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Total Study Size | Tumors Assessed by IHC | Patient Population | Tumor Histologies |
|---|---|---|---|---|
| Przybycin 2018 | 40 tumors | 24 tumors | 32 male, 8 female 24–79 years (mean 52 years) | ACKD-RCC (all tumors) |
| Ahn 2013 | 12 tumors | 12 tumors | 10 male, 2 female 35–70 years (mean 48.7 years) | ccpRCC (all tumors) |
| Kuroda 2017 | 7 tumor | 7 tumors | 5 male, 2 female 38–78 years (mean 52.9 years) | ACKD-RCC (all tumors) |
| Zhu 2015 | 159 tumors | 159 tumors | Not documented | ACKD-RCC (2 tumors), ccpRCC (19 tumors), other RCC types (138 tumors) |
| Tajima 2015 | 1 tumor | 1 tumor | 77-year-old male | ACKD-RCC with sarcomatoid component |
| Williamson 2013 | 55 tumors | 34 tumors | 19 male, 15 female 33–87 years (mean 61 years) | ccpRCC (all tumors) |
| Gobbo 2008 | 7 tumors | 7 tumors | 3 male, 2 female 53–64 years (mean 60 years) | ccpRCC (all tumors) |
| Kuroda 2011 | 1 tumor | 1 tumor | 57-year-old male | ccpRCC |
| Kuroda 2011 | 6 tumors | 6 tumors | 6 male, 0 female 44–74 years (mean 59.7 years) | ACKD-RCC, including 1 with sarcomatoid change |
| Rohan 2011 | 9 tumors | 9 tumors | 5 male, 4 female 46–74 years (mean 61.7 years) | ccpRCC (all tumors) |
| Pramick 2013 | 20 tumors | 20 tumors | 12 male, 8 female 27–76 years. (mean 59 years) | ccpRCC (all tumors) |
| Cui 2013 | 20 tumors | 20 tumors | 11 male, 9 female 23–70 years. (mean 55 years.) | ccpRCC (all tumors) |
| Park 2012 | 15 tumors | 15 tumors | 4 male, 11 female 35–70 years (mean 52 years) | ccpRCC (all tumors) |
| Martignoni 2017 | 14 tumors | 14 tumors | 9 male, 5 female 46–77 years (mean 61 years) | ccpRCC (all tumors) |
| Gilani 2012 | 1 tumor | 1 tumor | 70 year old female | ccpRCC |
| Mantilla 2017 | 210 tumors | 210 tumors | 3:1 male/femle ratio, mean age 64 years * | ccpRCC (25 tumors), ccRCC (109 tumors), papillary RCC (62 tumors), others (14 tumors) |
| Leroy 2014 | 42 tumors | 42 tumors | 25 male, 17 female 35–78 years (mean 60.7 years) | ccpRCC (all tumors) |
| Wang 2018 | 26 tumors | 26 tumors | 19 male, 7 female 36–74 years (mean 53.5 years) | ccpRCC (all tumors) |
References
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H.; Vajdic, C.M.; van Leeuwen, M.T.; Amin, J.; Webster, A.C.; Chapman, J.R.; McDonald, S.P.; Grulich, A.E.; McCredie, M.R. The pattern of excess cancer in dialysis and transplantation. Nephrol. Dial. Transplant. 2009, 24, 3225–3231. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.M.; Olshan, A.F.; Kshirsagar, A.V.; Edwards, J.K.; Nielsen, M.E.; Wheeler, S.B.; Brookhart, M.A. Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996–2009. Am. J. Kidney Dis. 2015, 65, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I. Present status of renal cell carcinoma in dialysis patients in Japan: Questionnaire study in 2002. Nephron. Clin. Pract. 2004, 97, c11–c16. [Google Scholar] [CrossRef] [PubMed]
- Sule, N.; Yakupoglu, U.; Shen, S.S.; Krishnan, B.; Yang, G.; Lerner, S.; Sheikh-Hamad, D.; Truong, L.D. Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: A comprehensive study. Am. J. Surg. Pathol. 2005, 29, 443–451. [Google Scholar] [CrossRef]
- Moch, H. WHO Classification of Tumours of the Urinary System and Male Genital Organs; International Agency for Research on Cancer: Lyon, France, 2016. [Google Scholar]
- Tickoo, S.K.; dePeralta-Venturina, M.N.; Harik, L.R.; Worcester, H.D.; Salama, M.E.; Young, A.N.; Moch, H.; Amin, M.B. Spectrum of epithelial neoplasms in end-stage renal disease: An experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am. J. Surg. Pathol. 2006, 30, 141–153. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Alexiev, B.A. Renal-cell carcinomas in end-stage kidneys: A clinicopathological study with emphasis on clear-cell papillary renal-cell carcinoma and acquired cystic kidney disease-associated carcinoma. Int. J. Surg. Pathol. 2012, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sassa, N.; Yamada, H.; Takagi, T.; Iizuka, J.; Kobayashi, H.; Yoshida, K.; Fukuda, H.; Ishihara, H.; Tanabe, K.; et al. Comparable survival outcome between acquired cystic disease associated renal cell carcinoma and clear cell carcinoma in patients with end-stage renal disease: A multi-institutional central pathology study. Pathology 2021, 53, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Steward, J.E.; Kern, S.Q.; Cheng, L.; Boris, R.S.; Tong, Y.; Bahler, C.D.; Masterson, T.A.; Cary, K.C.; Kaimakliotis, H.; Gardner, T.; et al. Clear cell papillary renal cell carcinoma: Characteristics and survival outcomes from a large single institutional series. Urol. Oncol. 2021, 39, 370.e21–370.e25. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.; Srigley, J.R.; Whelan, C.; Cooper, J.; Delahunt, B. Type 2 and clear cell papillary renal cell carcinoma, and tubulocystic carcinoma: A unifying concept. Anticancer. Res. 2010, 30, 641–644. [Google Scholar] [PubMed]
- Bing, Z.; Tomaszewski, J.E. Clear cell papillary renal cell carcinoma in the bilateral native kidneys after 2 years of renal transplantation: Report of a case and review of the literature. Case Rep. Transplant. 2011, 2011, 387645. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Shiotsu, T.; Kawada, C.; Shuin, T.; Hes, O.; Michal, M.; Ohe, C.; Mikami, S.; Pan, C.C. Clear cell papillary renal cell carcinoma and clear cell renal cell carcinoma arising in acquired cystic disease of the kidney: An immunohistochemical and genetic study. Ann. Diagn. Pathol. 2011, 15, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, C.; Suh, J.H.; Moon, K.C. Clear cell papillary renal cell carcinoma: A report of 15 cases including three cases of concurrent other-type renal cell carcinomas. Korean J. Pathol. 2012, 46, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Oda, Y.; Kiyoshima, K.; Yamada, Y.; Nakashima, Y.; Naito, S.; Tsuneyoshi, M. Oxidative stress and DNA hypermethylation status in renal cell carcinoma arising in patients on dialysis. J. Pathol. 2007, 212, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, F.; Taguchi, K.; Izumi, H.; Kohno, K.; Kuwano, M.; Ono, M.; Nakashima, Y.; Takesue, T.; Naito, S.; Oda, Y. Peroxiredoxins, thioredoxin, and Y-box-binding protein-1 are involved in the pathogenesis and progression of dialysis-associated renal cell carcinoma. Virchows Arch. 2013, 463, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Konda, R.; Sato, H.; Hatafuku, F.; Nozawa, T.; Ioritani, N.; Fujioka, T. Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J. Urol. 2004, 171, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Konda, R.; Sugimura, J.; Sohma, F.; Katagiri, T.; Nakamura, Y.; Fujioka, T. Over expression of hypoxia-inducible protein 2, hypoxia-inducible factor-1alpha and nuclear factor kappaB is putatively involved in acquired renal cyst formation and subsequent tumor transformation in patients with end stage renal failure. J. Urol. 2008, 180, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Wilhelm, M.; Kovacs, G. Mutations of mtDNA in renal cell tumours arising in end-stage renal disease. J. Pathol. 2003, 199, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Argani, P.; Tickoo, S.K.; Epstein, J.I. Acquired Cystic Disease-associated Renal Cell Carcinoma (ACKD-RCC)-like Cysts. Am. J. Surg. Pathol. 2018, 42, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Antic, T.; Paner, G.P.; Chang, A. Pathologic spectrum of cysts in end-stage kidneys: Possible precursors to renal neoplasia. Hum. Pathol. 2014, 45, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Kojima, F.; Alaghehbandan, R.; Kuroda, N.; Matsuzaki, I.; Mikasa, Y.; Musangile, F.Y.; Iwamoto, R.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; et al. Paneth-like cells in renal cell carcinomas and in cysts associated with acquired cystic kidney disease: Clinicopathologic analysis, comparative study and description of precursor lesions. Ann. Diagn. Pathol. 2021, 51, 151707. [Google Scholar] [CrossRef] [PubMed]
- Enoki, Y.; Katoh, G.; Okabe, H.; Yanagisawa, A. Clinicopathological features and CD57 expression in renal cell carcinoma in acquired cystic disease of the kidneys: With special emphasis on a relation to the duration of haemodialysis, the degree of calcium oxalate deposition, histological type, and possible tumorigenesis. Histopathology 2010, 56, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.R. Clear cell papillary renal cell carcinoma: An update after 15 years. Pathology 2021, 53, 109–119. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, S.; Truong, L.D.; Ro, J.Y.; Ayala, A.G.; Shen, S.S. Clear cell papillary renal cell carcinoma is the fourth most common histologic type of renal cell carcinoma in 290 consecutive nephrectomies for renal cell carcinoma. Hum. Pathol. 2014, 45, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sasa, N.; Yamada, H.; Takagi, T.; Iizuka, J.; Kobayashi, H.; Yoshida, K.; Fukuda, H.; Ishihara, H.; Tanabe, K.; et al. Acquired cystic disease-associated renal cell carcinoma is the most common subtype in long-term dialyzed patients: Central pathology results according to the 2016 WHO classification in a multi-institutional study. Pathol. Int. 2018, 68, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kuthi, L.; Jenei, A.; Hajdu, A.; Németh, I.; Varga, Z.; Bajory, Z.; Pajor, L.; Iványi, B. Prognostic Factors for Renal Cell Carcinoma Subtypes Diagnosed According to the 2016 WHO Renal Tumor Classification: A Study Involving 928 Patients. Pathol. Oncol. Res. 2017, 23, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Billis, A.; Freitas, L.L.L.; Costa, L.B.E.; Barreto, I.S.; Asato, M.A.; Araujo, K.S.; Losada, D.M.; Herculiani, A.P.; Tabosa, G.V.B.S.; Zaidan, B.C.; et al. Genitourinary Malignancies in Transplant or Dialysis Patients: The Frequency of Two Newly Described 2016 World Health Organization Histopathologic Types. Transplant. Proc. 2017, 49, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Levine, E. Acquired cystic kidney disease. Radiol. Clin. N. Am. 1996, 34, 947–964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Eyzaguirre, E. Clear Cell Papillary Renal Cell Carcinoma. Arch. Pathol. Lab. Med. 2019, 143, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Edo, H.; Suyama, Y.; Sugiura, H.; Ojima, K.; Ito, K.; Miyai, K.; Matsukuma, S.; Shinmoto, H. Acquired Cystic Disease-Associated Renal Cell Carcinoma Extending to the Renal Pelvis Mimicking Urothelial Carcinoma on Computed Tomography (CT): Two Case Reports. Am. J. Case Rep. 2020, 21, e926630. [Google Scholar] [CrossRef]
- Banno, T.; Takagi, T.; Kondo, T.; Yoshida, K.; Iizuka, J.; Okumi, M.; Ishida, H.; Morita, S.; Nagashima, Y.; Tanabe, K. Computed tomography imaging characteristics of clear cell papillary renal cell carcinoma. Int. Braz. J. Urol. 2020, 46, 26–33. [Google Scholar] [CrossRef]
- Tordjman, M.; Dbjay, J.; Chamouni, A.; Morini, A.; Timsit, M.O.; Mejean, A.; Vasiliu, V.; Eiss, D.; Correas, J.M.; Verkarre, V.; et al. Clear Cell Papillary Renal Cell Carcinoma: A Recent Entity With Distinct Imaging Patterns. Am. J. Roentgenol. 2020, 214, 579–587. [Google Scholar] [CrossRef]
- Wang, K.; Zarzour, J.; Rais-Bahrami, S.; Gordetsky, J. Clear Cell Papillary Renal Cell Carcinoma: New Clinical and Imaging Characteristics. Urology 2017, 103, 136–141. [Google Scholar] [CrossRef]
- Weng, S.; DiNatale, R.G.; Silagy, A.; Mano, R.; Attalla, K.; Kashani, M.; Weiss, K.; Benfante, N.E.; Winer, A.G.; Coleman, J.A.; et al. The Clinicopathologic and Molecular Landscape of Clear Cell Papillary Renal Cell Carcinoma: Implications in Diagnosis and Management. Eur. Urol. 2021, 79, 468–477. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Wang, J.; Gu, M.; Wang, Z.; Qin, C.; Han, C.; Li, H.; Liu, X.; Wu, P.; et al. Clinical features and survival analysis of clear cell papillary renal cell carcinoma: A 10-year retrospective study from two institutions. Oncol. Lett. 2018, 16, 1010–1022. [Google Scholar] [CrossRef]
- Gill, S.; Kauffman, E.C.; Kandel, S.; George, S.; Schwaab, T.; Xu, B. Incidence of Clear Cell Papillary Renal Cell Carcinoma in Low-Grade Renal Cell Carcinoma Cases: A 12-Year Retrospective Clinicopathologic Study From a Single Cancer Center. Int. J. Surg. Pathol. 2016, 24, 207–212. [Google Scholar] [CrossRef]
- Williamson, S.R.; Zhang, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; Brunelli, M.; Wang, M.; Gobbo, S.; Baldridge, L.A.; Cheng, L. Clear cell papillary renal cell carcinoma-like tumors in patients with von Hippel-Lindau disease are unrelated to sporadic clear cell papillary renal cell carcinoma. Am. J. Surg. Pathol. 2013, 37, 1131–1139. [Google Scholar] [CrossRef]
- Alexiev, B.A.; Drachenberg, C.B. Clear cell papillary renal cell carcinoma: Incidence, morphological features, immunohistochemical profile, and biologic behavior: A single institution study. Pathol. Res. Pract. 2014, 210, 234–241. [Google Scholar] [CrossRef]
- Diolombi, M.L.; Cheng, L.; Argani, P.; Epstein, J.I. Do Clear Cell Papillary Renal Cell Carcinomas Have Malignant Potential? Am. J. Surg. Pathol. 2015, 39, 1621–1634. [Google Scholar] [CrossRef]
- Yoshida, K.; Takagi, T.; Kondo, T.; Kobayashi, H.; Iizuka, J.; Fukuda, H.; Ishihara, H.; Okumi, M.; Ishida, H.; Tanabe, K. Efficacy of axitinib in patients with metastatic renal cell carcinoma refractory to nivolumab therapy. Jpn. J. Clin. Oncol. 2019, 49, 576–580. [Google Scholar] [CrossRef]
- Foshat, M.; Eyzaguirre, E. Acquired Cystic Disease-Associated Renal Cell Carcinoma: Review of Pathogenesis, Morphology, Ancillary Tests, and Clinical Features. Arch. Pathol. Lab. Med. 2017, 141, 600–606. [Google Scholar] [CrossRef]
- Kuroda, N.; Naroda, T.; Tamura, M.; Yorita, K.; Kojima, F.; Murata, S.I. Acquired cystic disease-associated renal cell carcinoma: A clinicopathological study of seven cases. Pol. J. Pathol. 2017, 68, 306–311. [Google Scholar] [CrossRef]
- Przybycin, C.G.; Harper, H.L.; Reynolds, J.P.; Magi-Galluzzi, C.; Nguyen, J.K.; Wu, A.; Sangoi, A.R.; Liu, P.S.; Umar, S.; Mehra, R.; et al. Acquired Cystic Disease-associated Renal Cell Carcinoma (ACD-RCC): A Multiinstitutional Study of 40 Cases With Clinical Follow-up. Am. J. Surg. Pathol. 2018, 42, 1156–1165. [Google Scholar] [CrossRef]
- Chrabańska, M.; Jakub, R.; Bogna, D. Bilateral and Multifocal Acquired Cystic Disease-Associated Renal Cell Carcinomas in Patient With End-Stage Renal Disease Caused by Systemic Lupus Erythematosus. Int. J. Surg. Pathol. 2021, 29, 198–204. [Google Scholar] [CrossRef]
- Tajima, S.; Waki, M.; Doi, W.; Hayashi, K.; Takenaka, S.; Fukaya, Y.; Kimura, R. Acquired cystic disease-associated renal cell carcinoma with a focal sarcomatoid component: Report of a case showing more pronounced polysomy of chromosomes 3 and 16 in the sarcomatoid component. Pathol. Int. 2015, 65, 89–94. [Google Scholar] [CrossRef]
- Kuroda, N.; Tamura, M.; Hamaguchi, N.; Mikami, S.; Pan, C.C.; Brunelli, M.; Martignoni, G.; Hes, O.; Michal, M.; Lee, G.H. Acquired cystic disease-associated renal cell carcinoma with sarcomatoid change and rhabdoid features. Ann. Diagn. Pathol. 2011, 15, 462–466. [Google Scholar] [CrossRef]
- Ahn, S.; Kwon, G.Y.; Cho, Y.M.; Jun, S.Y.; Choi, C.; Kim, H.J.; Park, Y.W.; Park, W.S.; Shim, J.W. Acquired cystic disease-associated renal cell carcinoma: Further characterization of the morphologic and immunopathologic features. Med. Mol. Morphol. 2013, 46, 225–232. [Google Scholar] [CrossRef]
- Zhu, B.; Rohan, S.M.; Lin, X. Immunoexpression of napsin A in renal neoplasms. Diagn. Pathol. 2015, 10, 4. [Google Scholar] [CrossRef]
- Cossu-Rocca, P.; Eble, J.N.; Zhang, S.; Martignoni, G.; Brunelli, M.; Cheng, L. Acquired cystic disease-associated renal tumors: An immunohistochemical and fluorescence in situ hybridization study. Mod. Pathol. 2006, 19, 780–787. [Google Scholar] [CrossRef]
- Kuroda, N.; Tamura, M.; Taguchi, T.; Tominaga, A.; Hes, O.; Michal, M.; Ohara, M.; Hirouchi, T.; Mizuno, K.; Hayashi, Y.; et al. Sarcomatoid acquired cystic disease-associated renal cell carcinoma. Histol. Histopathol. 2008, 23, 1327–1331. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, Y.J.; Chang, L.C.; Chang, Y.H.; Ho, D.M. Immunohistochemical and molecular genetic profiling of acquired cystic disease-associated renal cell carcinoma. Histopathology 2009, 55, 145–153. [Google Scholar] [CrossRef]
- Kuntz, E.; Yusenko, M.V.; Nagy, A.; Kovacs, G. Oligoarray comparative genomic hybridization of renal cell tumors that developed in patients with acquired cystic renal disease. Hum. Pathol. 2010, 41, 1345–1349. [Google Scholar] [CrossRef]
- Kuroda, N.; Yamashita, M.; Kakehi, Y.; Hes, O.; Michal, M.; Lee, G.H. Acquired cystic disease-associated renal cell carcinoma: An immunohistochemical and fluorescence in situ hybridization study. Med. Mol. Morphol. 2011, 44, 228–232. [Google Scholar] [CrossRef]
- Inoue, T.; Matsuura, K.; Yoshimoto, T.; Nguyen, L.T.; Tsukamoto, Y.; Nakada, C.; Hijiya, N.; Narimatsu, T.; Nomura, T.; Sato, F.; et al. Genomic profiling of renal cell carcinoma in patients with end-stage renal disease. Cancer Sci. 2012, 103, 569–576. [Google Scholar] [CrossRef]
- Shah, A.; Lal, P.; Toorens, E.; Palmer, M.B.; Schwartz, L.; Vergara, N.; Guzzo, T.; Nayak, A. Acquired Cystic Kidney Disease-associated Renal Cell Carcinoma (ACKD-RCC) Harbor Recurrent Mutations in KMT2C and TSC2 Genes. Am. J. Surg. Pathol. 2020, 44, 1479–1486. [Google Scholar] [CrossRef]
- Pena-Llopis, S.; Vega-Rubin-de-Celis, S.; Liao, A.; Leng, N.; Pavia- Jimenez, A.; Wang, S.; Yamasaki, T.; Zhrebker, L.; Sivanand, S.; Spence, P.; et al. BAP1 loss defines a new class of renal cell carcinoma. Nat. Genet. 2012, 44, 751–759. [Google Scholar] [CrossRef]
- Wi, Y.C.; Moon, A.; Jung, M.J.; Kim, Y.; Bang, S.S.; Jang, K.; Paik, S.S.; Shin, S.J. Loss of Nuclear BAP1 Expression Is Associated with High WHO/ISUP Grade in Clear Cell Renal Cell Carcinoma. J. Pathol. Transl. Med. 2018, 52, 378–385. [Google Scholar] [CrossRef]
- Gobbo, S.; Eble, J.N.; Grignon, D.J.; Martignoni, G.; MacLennan, G.T.; Shah, R.B.; Zhang, S.; Brunelli, M.; Cheng, L. Clear cell papillary renal cell carcinoma: A distinct histopathologic and molecular genetic entity. Am. J. Surg. Pathol. 2008, 32, 1239–1245. [Google Scholar] [CrossRef]
- Rohan, S.M.; Xiao, Y.; Liang, Y.; Dudas, M.E.; Al-Ahmadie, H.A.; Fine, S.W.; Gopalan, A.; Reuter, V.E.; Rosenblum, M.K.; Russo, P.; et al. Clear-cell papillary renal cell carcinoma: Molecular and immunohistochemical analysis with emphasis on the von Hippel-Lindau gene and hypoxia-inducible factor pathway-related proteins. Mod. Pathol. 2011, 24, 1207–1220. [Google Scholar] [CrossRef]
- Cui, C.; Ziober, A.; Bing, Z. Expression of parafibromin in clear cell papillary renal cell carcinoma. Appl. Immunohistochem. Mol. Morphol. 2013, 21, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Pramick, M.; Ziober, A.; Bing, Z. Useful immunohistochemical panel for differentiating clear cell papillary renal cell carcinoma from its mimics. Ann. Diagn. Pathol. 2013, 17, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, G.; Brunelli, M.; Segala, D.; Munari, E.; Gobbo, S.; Cima, L.; Borze, I.; Wirtanen, T.; Sarhadi, V.K.; Atanesyan, L.; et al. Validation of 34betaE12 immunoexpression in clear cell papillary renal cell carcinoma as a sensitive biomarker. Pathology 2017, 49, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mantilla, J.G.; Antic, T.; Tretiakova, M. GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics. Hum. Pathol. 2017, 66, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Leroy, X.; Camparo, P.; Gnemmi, V.; Aubert, S.; Flamand, V.; Roupret, M.; Fantoni, J.C.; Comperat, E. Clear cell papillary renal cell carcinoma is an indolent and low-grade neoplasm with overexpression of cyclin-D1. Histopathology 2014, 64, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Qin, C.; Gu, M.; Wang, Z.; Han, C.; Liu, X.; Li, H.; Hua, H. Expression of vitamin D receptor in clear cell papillary renal cell carcinoma. Ann. Diagn. Pathol. 2018, 36, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Reznik, E.; Lee, H.J.; Gundem, G.; Jonsson, P.; Sarungbam, J.; Bialik, A.; Sanchez-Vega, F.; Creighton, C.J.; Hoekstra, J.; et al. Abnormal oxidative metabolism in a quiet genomic background underlies clear cell papillary renal cell carcinoma. eLife 2019, 8, e38986. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xu, W.H.; Wu, J.L.; Gan, H.L.; Wang, H.K.; Gu, W.J.; Qu, Y.Y.; Zhang, H.L.; Ye, D.W. Clear Cell Papillary Renal Cell Carcinoma Shares Distinct Molecular Characteristics and may be Significantly Associated With Higher Risk of Developing Second Primary Malignancy. Pathol. Oncol. Res. 2021, 27, 1609809. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Tickoo, S.K.; Jacobsen, A.; Sarungbam, J.; Sfakianos, J.P.; Sato, Y.; Morikawa, T.; Kume, H.; Fukayama, M.; Homma, Y.; et al. TCEB1-mutated renal cell carcinoma: A distinct genomic and morphological subtype. Mod. Pathol. 2015, 28, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, H.; Kehar, S.I.; Ali, S.; Tariq, N. Expression of Von Hippel—Lindau (VHL) gene mutation in diagnosed cases of renal cell carcinoma. Pak. J. Med. Sci. 2014, 30, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Morlote, D.M.; Harada, S.; Batista, D.; Gordetsky, J.; Rais-Bahrami, S. Clear cell papillary renal cell carcinoma: Molecular profile and virtual karyotype. Hum. Pathol. 2019, 91, 52–60. [Google Scholar] [CrossRef]
- Hong, B.; Zhang, Z.; Zhou, J.; Ma, K.; Zhang, J.; Cai, L.; Zhang, N.; Gong, K. Distinctive clinicopathological features of Von Hippel-Lindau-associated hereditary renal cell carcinoma: A single-institution study. Oncol. Lett. 2019, 17, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Monzon, F.; Jonasch, E.; Matin, S.F.; Tamboli, P. Clear cell papillary renal cell carcinoma in patients with von Hippel-Lindau syndrome—Clinicopathological features and comparative genomic analysis of 3 cases. Hum. Pathol. 2014, 45, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Favazza, L.; Chitale, D.A.; Barod, R.; Rogers, C.G.; Kalyana-Sundaram, S.; Palanisamy, N.; Gupta, N.S.; Williamson, S.R. Renal cell tumors with clear cell histology and intact VHL and chromosome 3p: A histological review of tumors from the Cancer Genome Atlas database. Mod. Pathol. 2017, 30, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H.; Larrea, E.; Larrinaga, G.; Goicoechea, I.; Arestin, M.; Fernandez-Mercado, M.; Hes, O.; Cáceres, F.; Manterola, L.; López, J.I. Targeted next-generation sequencing and non-coding RNA expression analysis of clear cell papillary renal cell carcinoma suggests distinct pathological mechanisms from other renal tumour subtypes. J. Pathol. 2014, 232, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Munari, E.; Marchionni, L.; Chitre, A.; Hayashi, M.; Martignoni, G.; Brunelli, M.; Gobbo, S.; Argani, P.; Allaf, M.; Hoque, M.O.; et al. Clear cell papillary renal cell carcinoma: Micro-RNA expression profiling and comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Hum. Pathol. 2014, 45, 1130–1138. [Google Scholar] [CrossRef]
- Shiomi, E.; Kato, R.; Matsuura, T.; Maekawa, S.; Kato, Y.; Kanehira, M.; Takata, R.; Sugimura, J.; Ishida, K.; Abe, T.; et al. Relationship between miR-155 expression and clear cell papillary renal cell carcinoma in the dialyzed kidney. IJU Case Rep. 2021, 4, 127–131. [Google Scholar] [CrossRef]
- Dhakal, H.P.; McKenney, J.K.; Khor, L.Y.; Reynolds, J.P.; Magi-Galluzzi, C.; Przybycin, C.G. Renal Neoplasms With Overlapping Features of Clear Cell Renal Cell Carcinoma and Clear Cell Papillary Renal Cell Carcinoma: A Clinicopathologic Study of 37 Cases From a Single Institution. Am. J. Surg. Pathol. 2016, 40, 141–154. [Google Scholar] [CrossRef]
- Williamson, S.R.; Gupta, N.S.; Eble, J.N.; Rogers, C.G.; Michalowski, S.; Zhang, S.; Wang, M.; Grignon, D.J.; Cheng, L. Clear Cell Renal Cell Carcinoma With Borderline Features of Clear Cell Papillary Renal Cell Carcinoma: Combined Morphologic, Immunohistochemical, and Cytogenetic Analysis. Am. J. Surg. Pathol. 2015, 39, 1502–1510. [Google Scholar] [CrossRef]
- Canete-Portillo, S.; Rodriguez, M.D.C.P.; Wang, D.; Sanchez, D.F.; Netto, G.J.; Magi-Galluzzi, C. Vascular architectural patterns in clear cell renal cell carcinoma and clear cell papillary renal cell carcinoma. Virchows Arch. 2021, 479, 1187–1196. [Google Scholar] [CrossRef]
- Brimo, F.; Atallah, C.; Li, G.; Srigley, J.R. Cystic clear cell papillary renal cell carcinoma: Is it related to multilocular clear cell cystic neoplasm of low malignant potential? Histopathology 2016, 68, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Tickoo, S.K.; Kumar, S.; Arora, V.K. Xp11 translocation renal cell carcinoma morphologically mimicking clear cell-papillary renal cell carcinoma in an adult patient: Report of a case expanding the morphologic spectrum of Xp11 translocation renal cell carcinomas. Int. J. Surg. Pathol. 2015, 23, 234–237. [Google Scholar] [CrossRef] [PubMed]


| ESRD Only (n = 36) 1 | ESRD and ACKD (n = 91) 1 | All Renal Cell Tumors (WHO 2016) | |
|---|---|---|---|
| ACKD-RCC | 0% | 40% | Not applicable |
| ccpRCC | 25% | 30% | 1–4% |
| Clear-cell RCC | 22% | 14% | 65–70% |
| Papillary RCC | 21% | 17% | 18.5% |
| Chromophobe RCC | 14% | 6% | 5–7% |
| ACKD-RCC | Acquired Cystic Renal Disease (Prerequisite Factor) Long Duration of Dialysis Young Patient Age Male Sex |
| ccpRCC | No known specific risk factors |
| Marker | Number of Positive Cases n (Study) | Total Positive Cases n (%) | Number of Negative Cases n (Study) | Total Negative Cases n (%) | Other Staining Patterns |
|---|---|---|---|---|---|
| AMACR | 24 (Przybycin 2018) | 0 (Przybycin 2018) | - | ||
| 12 (Ahn 2013) | 0 (Ahn 2013) | ||||
| 5 (Kuroda 2017) | 0 (Kuroda 2017) | ||||
| 6 (Kuroda 2011) | 47 (100%) | 0 (Kuroda 2011) | 0 (0%) | ||
| Cytokeratin 7 | 0 (Kuroda 2017) | 5 (Kuroda 2017) | 20 negative to patchy (Przbycin 2018) | ||
| 2 (Kuroda 2011) | |||||
| 1 (Kuroda 2011) | 3 cases focally positive (Kuroda 2011) | ||||
| 1 (13%) | 7 (87%) | ||||
| PAX8 | 19 (Przybycin 2018) | 19 (79%) | 5 (Przybycin 2018) | 5 (21%) | - |
| CD117 | 24 (Przybycin 2018) | 0 (Przybycin 2018) | - | ||
| 0 (Ahn 2013) | 24 (67%) | 12 (Ahn 2013) | 12 (33%) | ||
| CD10 | 12 (Ahn 2013) | 12 (100%) | 0 (Ahn 2013) | 0 (0%) | - |
| Napsin A | - | - | - | - | 2 cases with cytoplasmic dot positivity (Zhu 2015) |
| Fumarate hydratase | - | - | - | - | 24 retained (Przybycin 2018) |
| P53 | - | - | - | - | 1 diffuse in sarcomatoid component (Tajima 2015) |
| Pan cytokeratin | 12 (Ahn 2013) | 12 (100%) | 0 (Ahn 2013) | 0 (0%) | - |
| PTEN | 12 (Ahn 2013) | 12 (100%) | 0 (Ahn 2013) | 0 (0%) | - |
| C-Met | 12 (Ahn 2013) | 12 (100%) | 0 (Ahn 2013) | 0 (0%) | - |
| CA-IX | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | - |
| CD57 | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | |
| CD68 | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | - |
| PDGFR | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | - |
| PAX-2 | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | 3 cases focally positive (Kuroda 2011) |
| 2 (Kuroda 2011) | 2 (13%) | 1 (Kuroda 2010) | 13 (87%) | ||
| VEGFR-2 | 0 (Ahn 2013) | 0 (0%) | 12 (Ahn 2013) | 12 (100%) | - |
| Kidney-Specific Cadherin | - | - | - | - | 12 heterogeneous (Ahn 2013) |
| Antimitoch-ondrial Antibody | 6 (Kuroda 2011) | 6 (100%) | 0 (Kuroda 2011) | 0 (0%) |
| Marker | Number of Positive Cases n (Study) | Total Positive Cases n (%) | Number of Negative Cases n (Study) | Total Negative Cases n (%) | Other Staining Patterns |
|---|---|---|---|---|---|
| Cytokerain 7 | 34 (Williamson 2013) | 0 (Williamson 2013) | - | ||
| 7 (Gobbo 2008) | 0 (Gobbo 2008) | ||||
| 1 (Kuroda 2011) | 0 (Kuroda 2011) | ||||
| 9 (Rohan 2011) | 0 (Rohan 2011) | ||||
| 20 (Pramick 2013) | 0 (Pramick 2013) | ||||
| 20 ** (Cui 2013) | 0 (Cui 2013) | ||||
| 15 *** (Park 2012) | 106 (100%) | 0 (Park 2012) | 0 (0%) | ||
| CAIX | 34 (Williamson 2013) | 0 (Williamson 2013) | - | ||
| 7 (Gobbo 2008) | 0 (Gobbo 2008) | ||||
| 9 (Rohan 2011) | 0 (Rohan 2011) | ||||
| 18 (Pramick 2013) | 68 (100%) | 0 (Pramick 2013) | 0 (0%) | ||
| AMACR | 1 **** (Williamson 2013) | 0 (Williamson 2013) | - | ||
| 0 (Gobbo 2008) | 7 (Gobbo 2008) | ||||
| 0 (Kuroda 2011) | 1 (Kuroda 2011) | ||||
| 0 (Rohan 2011) | 9 (Rohan 2011) | ||||
| 0 (Park 2012) | 15 (Park 2012) | ||||
| 1 (Pramick 2013) | 2 (4%) | 19 (Pramick 2013) | 51 (96%) | ||
| CD10 | 0 (Gobbo 2008) | 7 (Gobbo 2008) | 20 of 34 cases, patchy luminal membranous staining of cystic components only (Williamson 2013) | ||
| 0 (Kuroda 2011) | 1 (Kuroda 2011) | ||||
| 0 (Rohan 2011) | 9 (Rohan 2011) | ||||
| 0 (Park 2012) | 0 (0%) | 15 ***** (Park 2012) | 32 (100%) | ||
| PAX8 | 20 (Pramick 2013) | 20 (100%) | 0 (Pramick 2013) | 0 (0%) | - |
| HMWCK | 12 (Martignoni 2017) | 1 (Martignoni 2017) | - | ||
| 1 (Gilani 2012) | 13 (93%) | 0 (Gilani 2012) | 7 (7%) | ||
| GATA3 | 19 (Mantilla 2017) | 19 (76%) | 6 (Mantilla 2017) | (24%) | - |
| RCC | 0 (Cui 2013) | 0 (0%) | 20 * (Cui 2013) | 20 (100%) | - |
| TFE3 | 0 (Gobbo 2008) | 7 (Gobbo 2008) | - | ||
| 0 (Rohan 2011) | 9 (Rohan 2011) | ||||
| 0 (Park 2012) | 0 (0%) | 15 (Park 2012) | 31 (100%) | ||
| Vimentin | 15 (Park 2012) | 15 (100%) | 0 (Park 2012) | 0 (0%) | - |
| Napsin A | - | - | - | - | 9 out of 19 cases showed cytoplasmic dot positivity (Zhu 2015) |
| Cyclin D1 | 35 (Leroy 2014) | 35 (100%) | 0 (Leroy 2014) | 0 (0%) | 7 cases with focal staining (Leroy 2014) |
| Parafibromin | 20 (Cui 2013) | 20 (100%) | 0 (Cui 2013) | 0 (0%) | - |
| Estrogen Receptor | 0 (Williamson 2013) | 0 (0%) | 29 (Williamson 2013) | 29 (100%) | - |
| Progesterone Receptor | 0 (Williamson 2013) | 0 (0%) | 29 (Williamson 2013) | 29 (100%) | - |
| Vitamin D Receptor | 21 (Wang 2018) | 21 (100%) | 0 (Wang 2018) | 0 (Wang 2018) | 5 cases intermediate (Wang 2018) |
| ACKD-RCC | ccpRCC | |
|---|---|---|
| Background Kidney | Presence of ACKD in background ESRD kidney | Occurs with or without ACKD or ESRD |
| Tumor Histology | “Sieve-like morphology”, calcium oxalate crystals, frequently has high nuclear grade (WHO/ISUP grade 3 or 4) | Tubulopapillary architecture, linearly arranged nuclei with inverted polarity, majority of cases have low nuclear grade (WHO/ISUP 1 or 2) |
| Immunohistochemistry | CK7−, AMACR+ | CK7+, AMACR−, HMWCK+, GATA3+ |
| Molecular | No one specific molecular marker | No one specific molecular marker |
| Differential Diagnosis |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Zaatari, Z.M.; Truong, L.D. Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update. Biomedicines 2022, 10, 657. https://doi.org/10.3390/biomedicines10030657
El-Zaatari ZM, Truong LD. Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update. Biomedicines. 2022; 10(3):657. https://doi.org/10.3390/biomedicines10030657
Chicago/Turabian StyleEl-Zaatari, Ziad M., and Luan D. Truong. 2022. "Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update" Biomedicines 10, no. 3: 657. https://doi.org/10.3390/biomedicines10030657
APA StyleEl-Zaatari, Z. M., & Truong, L. D. (2022). Renal Cell Carcinoma in End-Stage Renal Disease: A Review and Update. Biomedicines, 10(3), 657. https://doi.org/10.3390/biomedicines10030657





