Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review
Abstract
:1. Introduction
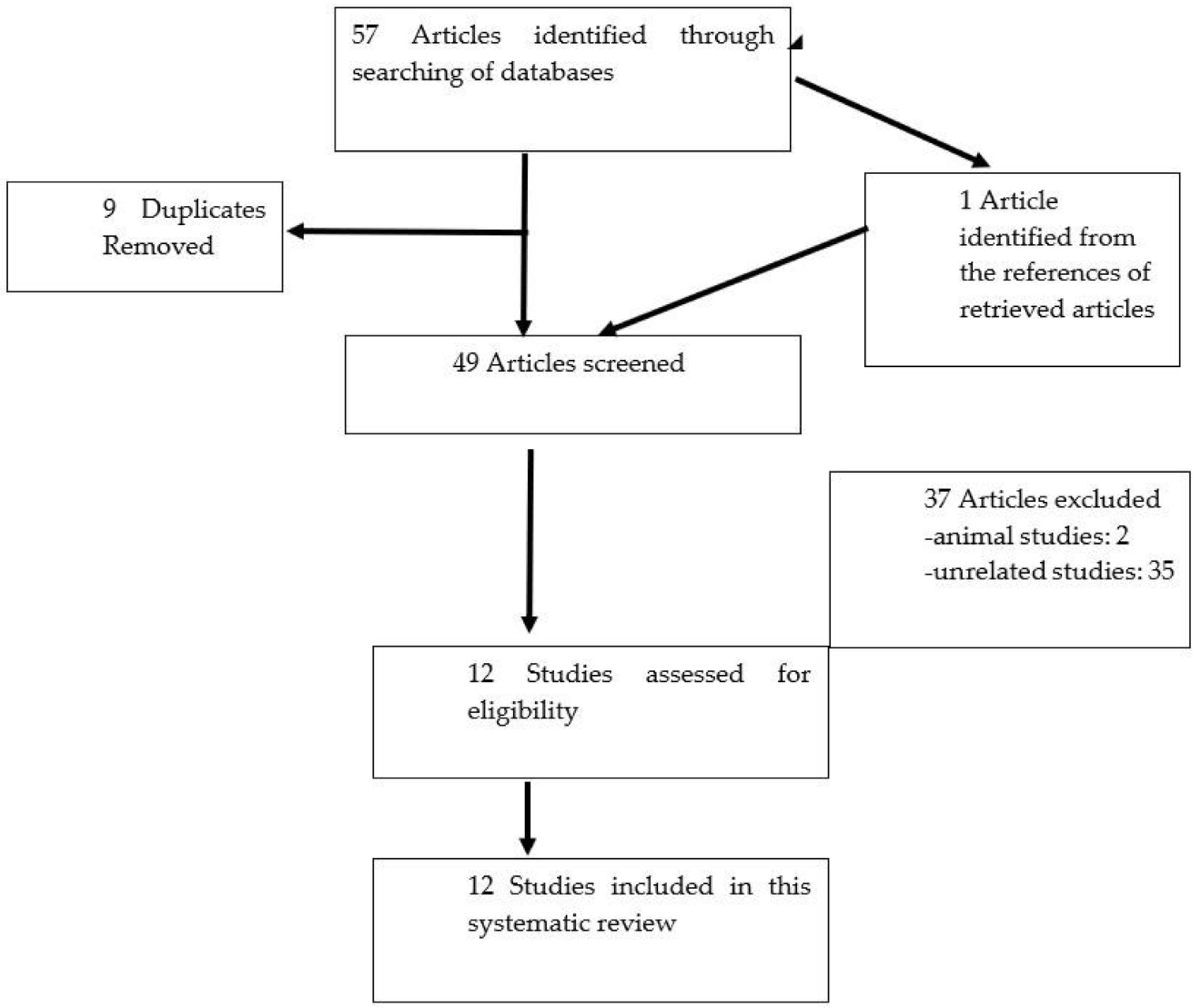
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
3. Results
3.1. Study Characteristics Proteomic Analysis of IgG4
3.2. Frequency of Elevated IgG4 in Rheumatoid Arthritis
3.3. Clinical Significance of IgG4 in Rheumatoid Arthritis
3.3.1. IgG4 and Disease Activity
3.3.2. IgG4 and Treatment Response
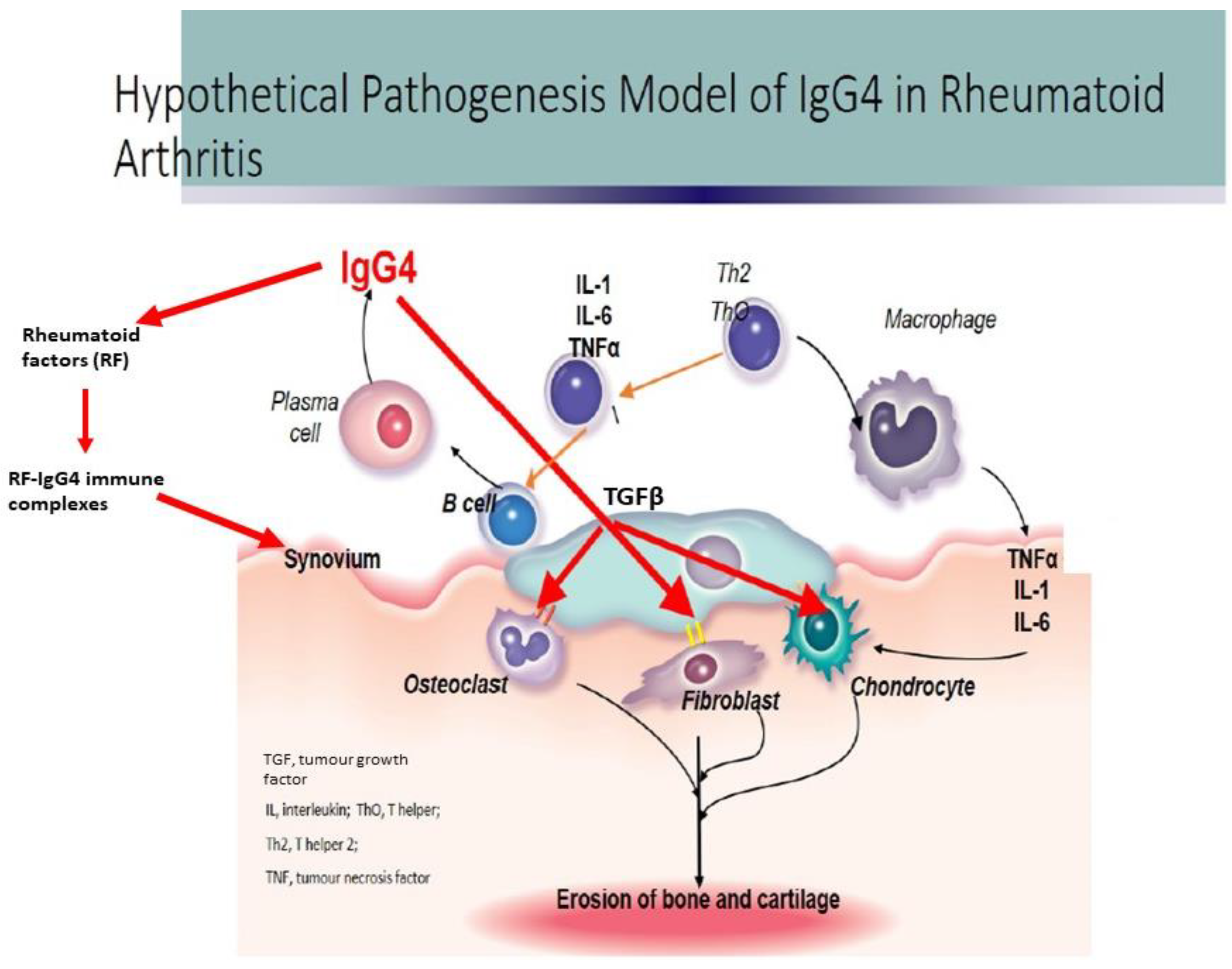
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puissant-Lubrano, B.; Peres, M.; Apoil, P.A.; Congy-Jolivet, N.; Roubinet, F.; Blancher, A. Immunoglobulin IgA, IgD, IgG, IgM and IgG subclass reference values in adults. Clin. Chem. Lab. Med. 2015, 53, e359–e361. [Google Scholar] [CrossRef]
- Corrigall, V.M.; Panayi, G.S. Autoantigens and immune pathways in rheumatoid arthritis. Crit. Rev. Immunol. 2002, 22, 281–293. [Google Scholar]
- Wang, Y.; Lu, Q.; Wu, S.L.; Karger, B.L.; Hancock, W.S. Characterization and comparison of disulfide linkages and scrambling patterns in therapeutic monoclonal antibodies: Using LC-MS with electron transfer dissociation. Anal. Chem. 2011, 83, 3133–3140. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.H.; Khosroshahi, A.; Deshpande, V.; Chan, J.K.; Heathcote, J.G.; Aalberse, R.; Azumi, A.; Bloch, D.B.; Brugge, W.R.; Carruthers, M.N.; et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012, 64, 3061–3067. [Google Scholar] [CrossRef]
- Masaki, Y.; Dong, L.; Kurose, N.; Kitagawa, K.; Morikawa, Y.; Yamamoto, M.; Takahashi, H.; Shinomura, Y.; Imai, K.; Saeki, T.; et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: Analysis of 64 cases of IgG4-related disorders. Ann. Rheum. Dis. 2009, 68, 1310–1315. [Google Scholar] [CrossRef]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-related disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef]
- Cohen, S.; Emery, P. The American College of Rheumatology/European League Against Rheumatism Criteria for the classification of rheumatoid arthritis: A game changer. Ann. Rheum. Dis. 2010, 69, 1575–1576. [Google Scholar] [CrossRef] [Green Version]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 Fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martinez-Martinez, P.; Vermeulen, E.; den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [Green Version]
- Muraki, T.; Hamano, H.; Ochi, Y.; Komatsu, K.; Komiyama, Y.; Arakura, N.; Yoshizawa, K.; Ota, M.; Kawa, S.; Kiyosawa, K. Autoimmune pancreatitis and complement activation system. Pancreas 2006, 32, 16–21. [Google Scholar] [CrossRef]
- Padigel, U.M.; Hess, J.A.; Lee, J.J.; Lok, J.B.; Nolan, T.J.; Schad, G.A.; Abraham, D. Eosinophils act as antigen-presenting cells to induce immunity to Strongyloides stercoralis in mice. J. Infect. Dis. 2007, 196, 1844–1851. [Google Scholar] [CrossRef] [Green Version]
- Rock, B.; Martins, C.R.; Theofilopoulos, A.N.; Balderas, R.S.; Anhalt, G.J.; Labib, R.S.; Futamura, S.; Rivitti, E.A.; Diaz, L.A. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem). N. Engl. J. Med. 1989, 320, 1463–1469. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.; Chen, C.; Ma, J.D.; Mo, Y.; Lin, J.; Zou, Y.Y.; Zheng, H.; Dai, L. Clinical Characteristics of Rheumatoid Arthritis Patients with Igg4-Related Synovitis. Ann. Rheum. Dis. 2021, 80, 1091–1092. [Google Scholar] [CrossRef]
- Chen, L.F.; Mo, Y.Q.; Ma, J.D.; Luo, L.; Zheng, D.H.; Dai, L. Elevated serum IgG4 defines specific clinical phenotype of rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 635293. [Google Scholar] [CrossRef]
- McDonagh, M.; Peterson, K.; Raina, P.; Chang, S.; Shekelle, P. Avoiding Bias in Selecting Studies. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Jeong, H.J.; Kim, J.M.; Jun, J.B.; Son, C.N. Clinical Significance of Elevated Serum Immunoglobulin G4 Levels in Patients With Rheumatoid Arthritis. J. Rheumat. Dis. 2020, 27, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.G.; Li, J.M. Elevation of serum IgG subclass concentration in patients with rheumatoid arthritis. Rheumatol. Int. 2010, 30, 837–840. [Google Scholar] [CrossRef]
- Engelmann, R.; Nekarda, S.; Kuthning, D.; Kneitz, C.; Muller-Hilke, B. Decreased IgG4 ACPA levels in responders and increased CD1c(+) classical dendritic cells in non-responders of patients with rheumatoid arthritis under therapy. Clin. Rheumatol. 2018, 37, 1783–1790. [Google Scholar] [CrossRef]
- Engelmann, R.; Kneitz, C.; Muller-Hilke, B. The dynamics of IgG1 and IgG4 ACPA levels during therapy in rheumatoid arthritis. Z. Rheumatol. 2014, 73, 12. [Google Scholar]
- Farboud, A.; Choy, E. Serological investigation of IgG levels and subclasses in rheumatoid arthritis patients following ingestion of bovine type II collagen: Results of a double blind, randomised controlled trial. Clin. Rheumatol. 2011, 30, 193–199. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Nogueira, L.; Clavel, C.; Sebbag, M.; Vincent, C.; Serre, G. IgG subclass distribution of the rheumatoid arthritis-specific autoantibodies to citrullinated fibrin. Clin. Exp. Immunol. 2005, 139, 542–550. [Google Scholar] [CrossRef]
- van Schouwenburg, P.A.; Krieckaert, C.L.; Nurmohamed, M.; Hart, M.; Rispens, T.; Aarden, L.; Wouters, D.; Wolbink, G.J. IgG4 production against adalimumab during long term treatment of RA patients. J. Clin. Immunol. 2012, 32, 1000–1006. [Google Scholar] [CrossRef]
- Bos, W.H.; Bartelds, G.M.; Vis, M.; van der Horst, A.R.; Wolbink, G.J.; de Stadt, R.J.V.; van Schaardenburg, D.; Dijkmans, B.A.C.; Lems, W.F.; Nurmohamed, M.T.; et al. Preferential decrease in IgG4 anti-citrullinated protein antibodies during treatment with tumour necrosis factor blocking agents in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2009, 68, 558–563. [Google Scholar] [CrossRef]
- Martinsson, K.; Johansson, A.; Kastbom, A.; Skogh, T. Immunoglobulin (Ig)G1 and IgG4 anti-cyclic citrullinated peptide (CCP) associate with shared epitope, whereas IgG2 anti-CCP associates with smoking in patients with recent-onset rheumatoid arthritis (the Swedish TIRA project). Clin. Exp. Immunol. 2017, 188, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Carbone, G.; Wilson, A.; Diehl, S.A.; Bunn, J.; Cooper, S.M.; Rincon, M. Interleukin-6 Receptor Blockade Selectively Reduces IL-21 Production by CD4 T Cells and IgG4 Autoantibodies in Rheumatoid Arthritis. Int. J. Biol. Sci. 2013, 9, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Fransen, J.; van Riel, P.L. The Disease Activity Score and the EULAR response criteria. Clin. Exp. Rheumatol. 2005, 23, S93–S99. [Google Scholar] [CrossRef]
- Thewjitcharoen, Y.; Krittiyawong, S.; Porramatikul, S.; Wanothayaroj, E.; Lekpittaya, N.; Jeamjiraprasert, J.; Nakasatien, S.; Himathongkam, T. A study of serum IgG4 levels in the clinical metamorphosis of autoimmune thyroid disease. J. Clin. Transl. Endocrinol. 2017, 8, 35–40. [Google Scholar] [CrossRef]
- Ryu, J.H.; Horie, R.; Sekiguchi, H.; Peikert, T.; Yi, E.S. Spectrum of Disorders Associated with Elevated Serum IgG4 Levels Encountered in Clinical Practice. Int. J. Rheumatol. 2012, 2012, 232960. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Takahashi, H.; Suzuki, C.; Tabeya, T.; Ohara, M.; Naishiro, Y.; Yamamoto, H.; Imai, K.; Shinomura, Y. Analysis of serum IgG subclasses in Churg-Strauss syndrome--the meaning of elevated serum levels of IgG4. Intern. Med. 2010, 49, 1365–1370. [Google Scholar] [CrossRef] [Green Version]
- Hao, M.; Liu, M.; Fan, G.; Yang, X.; Li, J. Diagnostic Value of Serum IgG4 for IgG4-Related Disease: A PRISMA-compliant Systematic Review and Meta-analysis. Medicine 2016, 95, e3785. [Google Scholar] [CrossRef]
- Dienz, O.; Eaton, S.M.; Bond, J.P.; Neveu, W.; Moquin, D.; Noubade, R.; Briso, E.M.; Charland, C.; Leonard, W.J.; Ciliberto, G.; et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med. 2009, 206, 69–78. [Google Scholar] [CrossRef]
- Ettinger, R.; Sims, G.P.; Fairhurst, A.M.; Robbins, R.; da Silva, Y.S.; Spolski, R.; Leonard, W.J.; Lipsky, P.E. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005, 175, 7867–7879. [Google Scholar] [CrossRef]
- Nishimoto, N.; Kishimoto, T. Interleukin 6: From bench to bedside. Nat. Clin. Pract. Rheumatol. 2006, 2, 619–626. [Google Scholar] [CrossRef]
- Zen, Y.; Fujii, T.; Harada, K.; Kawano, M.; Yamada, K.; Takahira, M.; Nakanuma, Y. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007, 45, 1538–1546. [Google Scholar] [CrossRef]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Kloppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Lan, Q.; Peng, Y.; Cai, J.; Zheng, J.; Dickerson, C.; Xiao, H.; Liu, H.F. Nature, functions, and clinical implications of IgG4 autoantibodies in systemic lupus erythematosus and rheumatoid arthritis. Discov. Med. 2017, 23, 169–174. [Google Scholar]
- Aversa, G.; Punnonen, J.; de Vries, J.E. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J. Exp. Med. 1993, 177, 1575–1585. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; af Klint, E.; Lundberg, I.E.; Lofberg, R.; Ulfgren, A.K.; Klareskog, L.; Catrina, A.I. Citrullination is an inflammation-dependent process. Ann. Rheum. Dis. 2006, 65, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.; Dorner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Greenberg, J.D.; Spruill, T.M.; Shan, Y.; Reed, G.; Kremer, J.M.; Potter, J.; Yazici, Y.; Ogedegbe, G.; Harrold, L.R. Racial and ethnic disparities in disease activity in patients with rheumatoid arthritis. Am. J. Med. 2013, 126, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
| Studies | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed Truly Representative of Average | Selection of Non Exposed from the Same Community | Exposure Ascertained by Secure Record or Interview | Demonstration of Outcome of Interest Not Present at the Start of the Study | Study Controls for Other Variables | Follow Up Long Enough for Outcome to Occur | Complete Follow Up for All Subjects Accounted for | Subjects Lost to Follow Up Unlikely to Introduce Bias | ||
| Chen et al. (2014) | ✓ | ✓ | ✓ | x | x | x | x | x | 3 |
| Lin et al. (2010) | ✓ | x | ✓ | x | x | x | x | x | 2 |
| Kim et al. (2020) | ✓ | x | ✓ | x | x | x | x | x | 2 |
| Chen et al. (2021) | ✓ | ✓ | ✓ | x | x | x | x | x | 3 |
| K. Martinsson et al. (2016) | ✓ | x | x | x | x | x | x | x | 1 |
| Carbone et al. (2013) | ✓ | x | ✓ | x | x | ✓ | ✓ | X | 4 |
| Engelmann et al. (2008) | ✓ | x | x | x | x | x | x | x | 1 |
| Bos et al. (2009) | ✓ | x | ✓ | x | x | ✓ | ✓ | x | 4 |
| Engelmann et al. (2018) | ✓ | x | ✓ | x | x | x | ✓ | x | 3 |
| Chapuy-Regaud et al. (2005) | ✓ | x | x | x | x | x | x | x | 1 |
| van Schouwenburg et al. (2012) | ✓ | x | ✓ | x | x | ✓ | x | x | 3 |
| Year | Authors | Country | Study Design & Study Population | Frequency of RA Patients with Raised IgG4 | Mean/Median IgG4 Levels in RA | IgG4 Detection Method | Key Findings |
|---|---|---|---|---|---|---|---|
| 2014 | Chen et al. [15] | China | Cross sectional 136 RA patients | 46% | 1.52 ± 1.27 g/L | Immunonephelometry | 51% patients had elevated IgG4/IgG ratio. The mean IgG4 of the untreated patients was 1.82 ± 1.39 g/L, which was significantly higher than that of the treated patients (1.39 ± 1.20 g/L; p = 0.044) IgG4 of high disease activity group was significantly higher than that of the remission group (p = 0.003). CRP and ESR, of the elevated IgG4 group were significantly higher than those of the normal IgG4 group (CRP: 38 ± 42 mg/L vs. 25 ± 33 mg/L; ESR: 70 ± 42 mm/h vs. 48 ± 32 mm/h; all p < 0.05). IgG4 level correlated positively with CRP (r = 0.426) and ESR (r = 0.315; both p < 0.05). RF and anti-CCP Ab levels of the elevated IgG4 group were significantly higher than those of the normal IgG4 group (RF: 513 ± 636 IU/mL vs. 245 ± 392 IU/mL; anti-CCP Ab: 256 ± 243 U/mL vs. 162 ± 199 U/mL; both p < 0.05), IgG4 correlated positively and significantly with synovial IgG4-positive plasma cells but no significant correlation of IgG4 with total synovitis score or subscores. |
| 2021 | Chen et al. [14] | China | Cross sectional 96 active RA patients | 49 (51.0%) | 1.38 (0.86–2.42) g/L (median) | Immunonephelometry. | RA patients with elevated IgG4 had significantly higher levels of ESR [90 (64–116) mm/h vs. 61 (38–75) mm/h], CRP [46.20 (17.20–74.20) mg/L vs. 18.90 (9.46–49.20) mg/L], DAS28-ESR [6.3 (5.6–7.4) vs. 5.7 (4.7–6.4)], SDAI [34.2 (25.3–48.8) vs. 27.8 (18.9–35.9)] all p < 0.05]. They also showed significantly higher synovial counts of IgG4+ plasma cells [106 (39–249) /mm2 vs. 68 (3–123) /mm2], and higher ratio of IgG4+/IgG+ plasma cells [26.3 (15.5–38.0)% vs. 15.2 (0.9–24.7)%, all p < 0.05]. There were 10 (10.4%) patients showing elevated serum IgG4 and IgG4-related synovitis based on synovial biopsy. |
| 2010 | Lin et al. [20] | China | Cross sectional 72 RA patients | 365.5 (72.85–1377.5) mg/L (median) | Immunonephelometry. | When the patients were divided according to the clinical activity, the IgG subclass concentrations were similar between the two groups (p > 0.05). | |
| 2020 | Kim et al. [19] | Korea | Cross sectional 128 participants (RA: 96; healthy controls: 17; Osteoarthritis: 11; and IgG4-related disease: 4) | 6.3% | 48.0 ± 45.4 mg/dL (mean) | Immunonephelometry. | The mean serum IgG4/IgG ratio in patients with RA was 3.5 ± 2.8% (range 0.2–16.9%). None of the healthy controls or patients with osteoarthritis had elevated serum IgG4 levels. A significant correlation was found between serum IgG4 levels and the Disease Activity Score-28 with erythrocyte sedimentation rate (r = 0.245; p = 0.016). |
| 2010 | Farboud et al. [23] | United Kingdom | Longitudinal (24 weeks) 55 RA patients | ELISA | For IgG1, IgG2, IgG3 and IgG4 subclasses, in the 0.5-mg of oral bovine type II collagen group in which the best clinical response was observed, there was statistically significant decreases observed in the IgG2 and IgG3 subclasses (p = 0.047, p = 0.046) but not in IgG4. |
| Year | Authors | Country | Study Design & Study Population | Frequency of RA Patients with Raised IgG4 | Type of IgG4 | IgG4 Detection Method | Key Findings |
|---|---|---|---|---|---|---|---|
| 2016 | K. Martinsson et al. [27] | Sweden | Cross sectional 504 with recent onset RA (untreated) | 59% | IgG4 anti-CCP | ELISA | Among those who were RF positive, 79% subjects tested positive for IgG4. IgG anti-CCP subclasses that associate with HLA-DRB1/SE are IgG1 and IgG4. Increased proportion of IgG4 anti-CCP-positive cases that were not associated with smoking. The fractions of IgG4 anti-CCP did not differ significantly between ever and never-smokers. |
| 2013 | Carbone et al. [28] | USA | Longitudinal 8 patients with active RA were treated with tocilizumab (TCZ) monotherapy or in combination with non-biologic DMARDs over 6 months; serum samples were obtained at (0 month), 1 month, 3 months, and 6 months | IgG4 anti-CCP | ELISA | Over the 6 months of treatment, there was a prominent four-fold reduction in the levels of IgG4 (p = 0.06). The levels of IgG4 were markedly decreased in all but one patient. Pronounced reduction (2–3 fold) in the serum levels of IgG4-specific anti-CCP Abs in all patients (p = 0.011), but no statistically significant reduction in the levels of IgG1-anti-CCP Abs (p = 0.185). | |
| 2014 | Engelmann et al. [22] | Germany | Cross sectional 77 RA patients | 33 (42.86%) patients with anti-CCP antibodies are positive for the IgG4 subclass | IgG4 anti-CCP | ELISA | Even though IgG1 is the predominant subclass among antibodies against CCP and anti-citrullinated vimentin (MCV) in RA, IgG4 was conspicuously elevated. Elevated IgG4 titers among auto-antibodies in RA are indicative of a Th2-biased environment. |
| 2018 | Engelmann et al. [21] | Germany | Longitudinal 34 ACPA-positive RA were monitored for 3 months after therapy | IgG4 anti-CCP | ELISA | 3 months after therapy, the responders showed a significant decrease in IgG4 ACPA levels, and this was independent of the individual treatment regimen. | |
| 2009 | Bos et al. [26] | Netherlands | Longitudinal 180 patients treated with adalimumab for 28 weeks | IgG4 ACF | ELISA | The median reduction in anti-citrullinated fibrinogen (ACF) levels was 31% for total IgG, 29% for IgG1, 40% for IgG4, and 22% for the IgG4/IgG1 ACF ratio in the infliximab cohort. In adalimumab-treated patients, ACF levels declined 14% for total IgG and IgG1, and 36% for IgG4 ACF; the IgG4: IgG1 ratio was reduced by 24%. European League Against Rheumatism good responders had the greatest decline in antibody levels, and this effect was most pronounced for IgG4 (48% reduction). The IgG4/IgG1 ACF ratio preferentially decreased in patients with adequate therapeutic adalimumab levels. | |
| 2005 | Chapuy-Regaud et al. [24] | France | Cross sectional 186 RA | 21.3% (30/141) had IgG1-AhFibA in combination with IgG4-AhFibA | IgG4 ACF | ELISA | IgG4-Antihuman fibrinogen (AhFibA) observed much more frequently and at higher titers than IgG3- or IgG2-AhFibA. AhFibA were mainly IgG1 and, to a lesser extent, IgG4. |
| 2012 | van Schouwenburg et al. [25] | Netherlands | Longitudinal 271 RA patients monitored for 3 years of adalimumab treatment | 29% of the patients had detectable IgG4 | IgG4 against adalimumab | Radio immunoassay | The proportion IgG4 of total IgG against adalimumab varied widely between patients. IgG4 was found to contribute significantly to the anti-drug antibody (ADA) response in some patients. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthiswary, R.; Shaharir, S.S.; Wahab, A.A. Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review. Biomedicines 2022, 10, 558. https://doi.org/10.3390/biomedicines10030558
Sakthiswary R, Shaharir SS, Wahab AA. Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review. Biomedicines. 2022; 10(3):558. https://doi.org/10.3390/biomedicines10030558
Chicago/Turabian StyleSakthiswary, Rajalingham, Syahrul Sazliyana Shaharir, and Asrul Abdul Wahab. 2022. "Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review" Biomedicines 10, no. 3: 558. https://doi.org/10.3390/biomedicines10030558
APA StyleSakthiswary, R., Shaharir, S. S., & Wahab, A. A. (2022). Frequency and Clinical Significance of Elevated IgG4 in Rheumatoid Arthritis: A Systematic Review. Biomedicines, 10(3), 558. https://doi.org/10.3390/biomedicines10030558






