Critical Evaluation of Specific Efficacy of Preparations Produced According to European Pharmacopeia Monograph 2371
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Preparation of Test Samples
2.3. Cleaning of Glass Vessels
2.4. Experimental Setting
2.5. Systematic Negative Control (SNC) Experiments
2.6. Missing Data
2.7. Statistical Analysis and Software
3. Results
3.1. Measure for Arsenic Stress, Variance Coefficient
3.2. Water Controls C0, C1
3.3. Systematic Negative Control (SNC) Experiments
3.4. Experimental Series 1
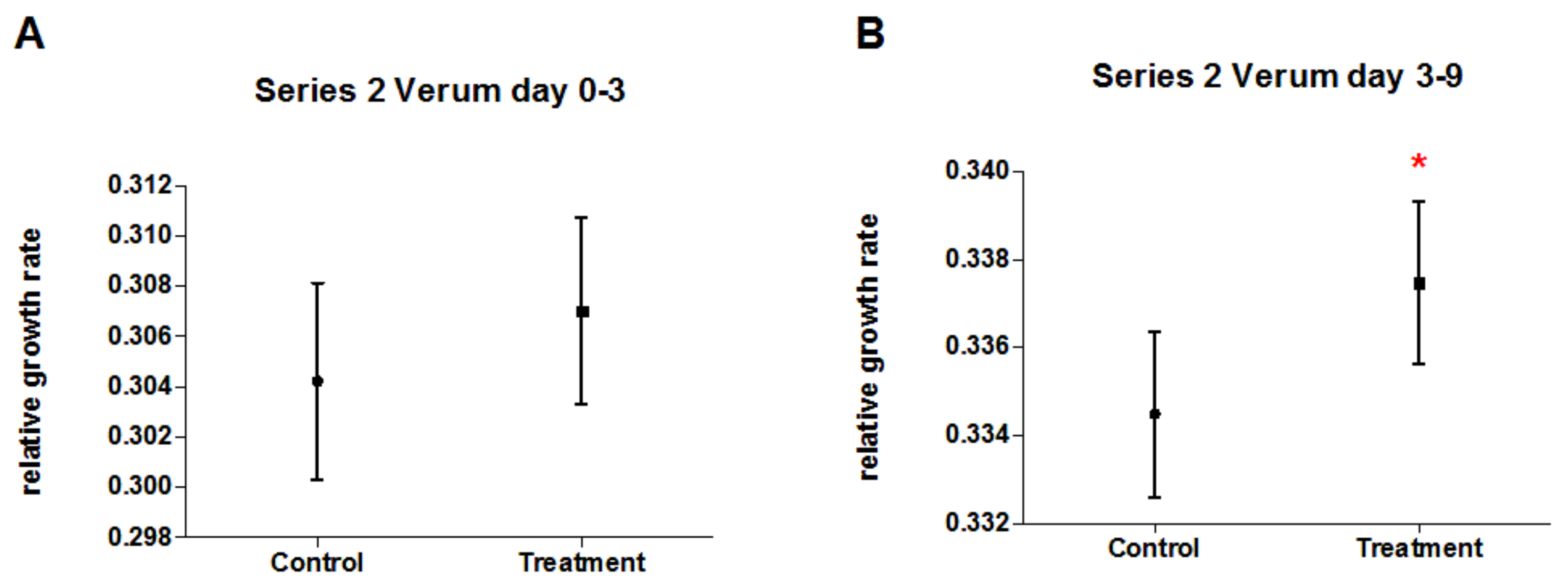
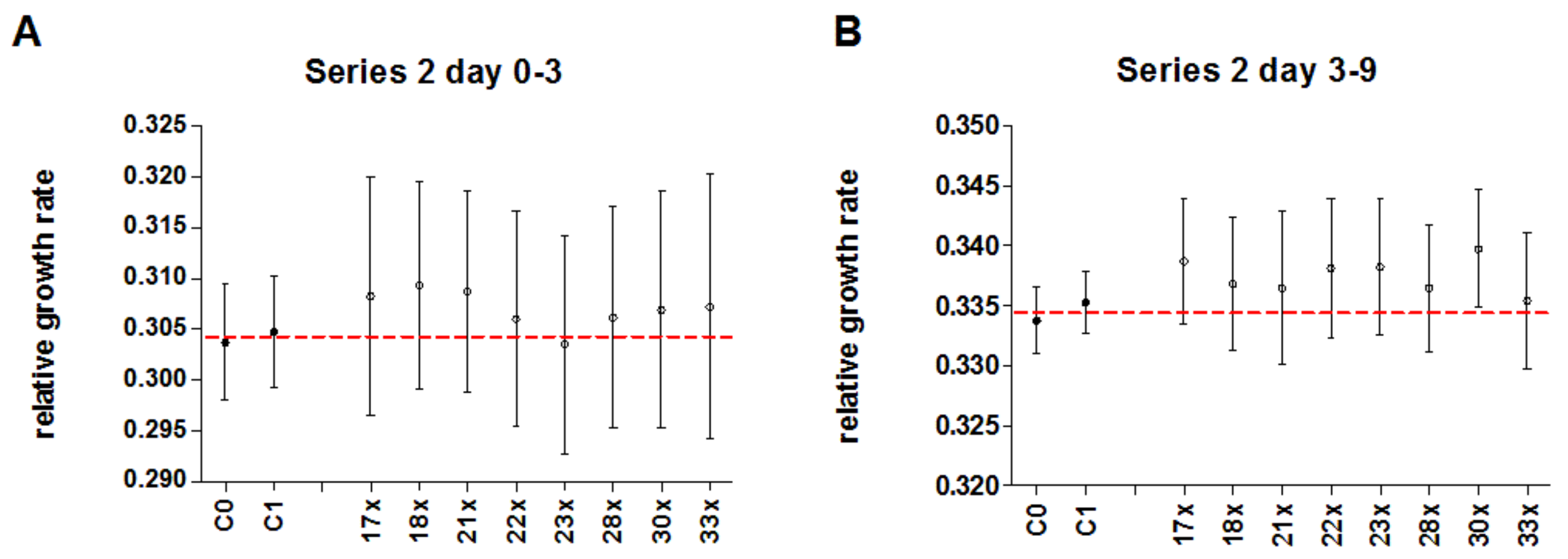
3.5. Experimental Series 2
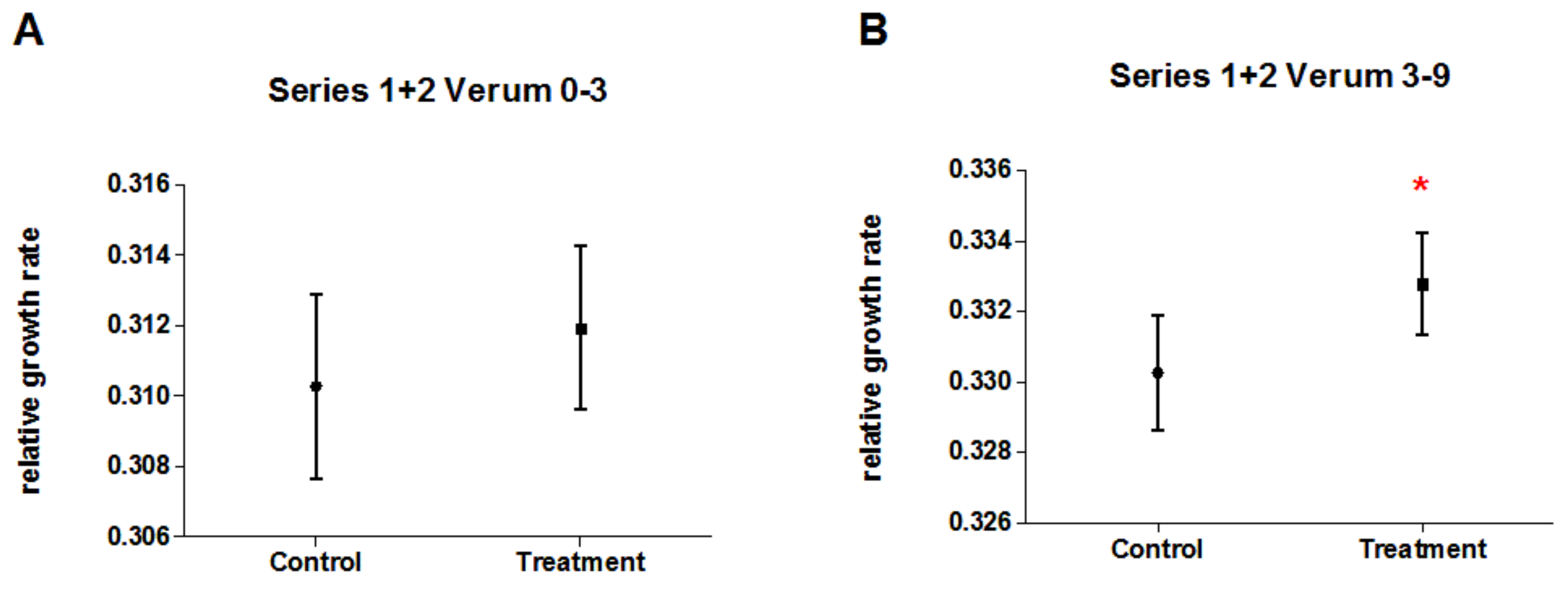
3.6. Pooled Data of Experimental Series 1 and 2
4. Discussion
4.1. Influence of Arsenic Stress Level in Series 1 and 2
4.1.1. Morphology of Plants and Stress Level
4.1.2. Stress Level and Effect Size
4.1.3. Variability and Stress Induction
4.2. Stability of the Experimental Set-Up
4.3. Comparison of Series 1 and 2 to the Original Experiments of 2010
4.3.1. Cultivation Conditions and Growth Chambers
4.3.2. Image Analysis Software
4.3.3. Further Possible Influences on Effect Size
4.4. Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EDQM. European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2019. [Google Scholar]
- Linde, K.; Scholz, M.; Ramirez, G.; Clausius, N.; Melchart, D.; Jonas, W.B. Impact of Study Quality on Outcome in Placebo-Controlled Trials of Homeopathy. J. Clin. Epidemiol. 1999, 52, 631–636. [Google Scholar] [CrossRef]
- Mathie, R.T.; Fok, Y.Y.; Viksveen, P.; To, A.K.; Davidson, J.R. Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Non-Individualised Homeopathic Treatment. Homeopathy 2019, 108, 088–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathie, R.T.; Ulbrich-Zürni, S.; Viksveen, P.; Roberts, E.R.; Baitson, E.S.; Legg, L.A.; Davidson, J.R.T. Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Individualised Homeopathic Treatment. Homeopathy 2018, 107, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Huwiler-Müntener, K.; Nartey, L.; Jüni, P.; Dörig, S.; Sterne, J.; Pewsner, D.; Egger, M. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet 2005, 366, 726–732. [Google Scholar] [CrossRef]
- Cukaci, C.; Freissmuth, M.; Mann, C.; Marti, J.; Sperl, V. Against all odds—the persistent popularity of homeopathy. Wien. Klin. Wochenschr. 2020, 132, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Grimes, D.R. Proposed mechanisms for homeopathy are physically impossible. Focus Altern. Complement. Ther. 2012, 17, 149–155. [Google Scholar] [CrossRef]
- Hopff, W.H. Der Neomystizismus in der Medizin. Beispiel Homöopathie [Neomysticism in medicine. The example of homeopathy]. Wien Med. Wochenschr. 1987, 137, 542–548. [Google Scholar] [PubMed]
- Schmacke, N. Homeopathy: Insubstantial doctrine of salvation. Bundesgesundheitsblatt Gesundh. Gesundh. 2020, 63, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endler, P.C.; Bellavite, P.; Bonamin, L.; Jäger, T.R.; Mazon, S. Replications of fundamental research models in ultra high dilutions 1994 and 2015—Update on a bibliometric study. Homeopathy 2015, 104, 234–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, T.; Scherr, C.; Shah, D.; Majewsky, V.; Wolf, U.; Betti, L.; Baumgartner, S. The use of plant-based bioassays in homeopathic basic research. Homeopathy 2015, 104, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ücker, A.; Baumgartner, S.; Sokol, A.; Huber, R.; Doesburg, P.; Jäger, T. Systematic Review of Plant-Based Homeopathic Basic Research: An Update. Homeopathy 2018, 107, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Jäger, T.; Scherr, C.; Simon, M.; Heusser, P.; Baumgartner, S. Effects of Homeopathic Arsenicum album, Nosode, and Gibberellic Acid Preparations on the Growth Rate of Arsenic-Impaired Duckweed (Lemna gibba L.). Sci. World J. 2010, 10, 2112–2129. [Google Scholar] [CrossRef] [Green Version]
- DIN-ISO20079; Water Quality. Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna Minor). Duckweed Growth Inhibition Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- Baumgartner, S.; Heusser, P.; Thurneysen, S. Methodological Standards and Problems in Preclinical Homoeopathic Potency Research. Forsch. Komplement. 1998, 5, 27–32. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Qiu, G.; Ilyas, M.; Kaye, P. Biomarker Detection in Whole Slide Imaging based on Statistical Color Models. MIDAS J. 2010. [Google Scholar] [CrossRef]
- Landolt, E. 5.5.1 Characteristics of Lemnaceae communities and principles of classification. In The Family of Lemnaceae—A Monographic Study; Geobotanisches Institut der Eidg, Techn. Hochschule, Stiftung Rübel, Zürich: Zürich, Switzerland, 1986; Volume 1. [Google Scholar]
- Finnegan, P.M.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [Green Version]
- Mkandawire, M.; Lyubun, Y.V.; Kosterin, P.V.; Dudel, E.G. Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ. Toxicol. 2004, 19, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Mkandawire, M.; Taubert, B.; Dudel, E.G. Limitations of growth-parameters in Lemna gibba bioassays for arsenic and uranium under variable phosphate availability. Ecotoxicol. Environ. Saf. 2006, 65, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.; Ozturk, F.; Aydin, Z. Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): Effects of concentration and duration of exposure. Ecotoxicology 2010, 19, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Uroic, M.K.; Xie, W.-Y.; Zhu, Y.-G.; Chen, B.-D.; McGrath, S.P.; Feldmann, J.; Zhao, F.-J. Phytochelatins play a key role in arsenic accumulation and tolerance in the aquatic macrophyte Wolffia globosa. Environ. Pollut. 2012, 165, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Leão, G.; Oliveira, J.; Farnese, F.; Gusman, G.; Felipe, R. Sulfur metabolism: Different tolerances of two aquatic macrophytes exposed to arsenic. Ecotoxicol. Environ. Saf. 2014, 105, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hribar-Marko, S.; Graunke, H.; Scherer-Pongratz, W.; Lothaller, H.; Endler, P.C. Prestimulation of wheat seedlings with gibberellic acid followed by application of an agitated high dilution of the same hormone. Int. J. High Dilution Res. 2013, 12, 26–39. [Google Scholar] [CrossRef]
- Jäger, T.; Würtenberger, S.; Baumgartner, S. Effects of Homeopathic Preparations of Mercurius corrosivus on the Growth Rate of Severely Mercury-Stressed Duckweed Lemna gibba L. Homeopathy 2019, 108, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, T.; Würtenberger, S.; Baumgartner, S. Effects of Homeopathic Preparations of Mercurius corrosivus on the Growth Rate of Moderately Mercury-Stressed Duckweed Lemna gibba L. Homeopathy 2021, 110, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Lüdtke, R.; Weisshuhn, T.E.; Quint, P.; Willich, S.N. The Role of Trace Elements in Homeopathic Preparations and the Influence of Container Material, Storage Duration, and Potentisation. Complement. Med. Res. 2006, 13, 15–21. [Google Scholar] [CrossRef]
- Muranaka, T.; Oyama, T. Heterogeneity of cellular circadian clocks in intact plants and its correction under light-dark cycles. Sci. Adv. 2016, 2, e1600500. [Google Scholar] [CrossRef] [Green Version]
- Stock-Schröer, B. Reporting Experiments in Homeopathic Basic Research (REHBaR). Homeopathy 2015, 104, 333–336. [Google Scholar] [CrossRef]
- Hamman, B.; Koning, G.; Lok, K.H. Homeopathically prepared gibberellic acid and barley seed germination. Homeopathy 2003, 92, 140–144. [Google Scholar] [CrossRef]
- Brizzi, M.; Nani, D.; Peruzzi, M.; Betti, L. Statistical analysis of the effect of high dilutions of arsenic in a large dataset from a wheat germination model. Br. Homeopath. J. 2000, 89, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Brizzi, M.; Lazzarato, L.; Nani, D.; Borghini, F.; Peruzzi, M.; Betti, L. A Biostatistical Insight into the As2O3 High Dilution Effects on the Rate and Variability of Wheat Seedling Growth. Complement. Med. Res. 2005, 12, 277–283. [Google Scholar] [CrossRef]
- Binder, M.; Baumgartner, S.; Thurneysen, A. The Effects of a 45x Potency of Arsenicum album on Wheat Seedling Growth—A Reproduction Trial. Complement. Med. Res. 2005, 12, 284–291. [Google Scholar] [CrossRef]
- Lahnstein, L.; Binder, M.; Thurneysen, A.; Frei-Erb, M.; Betti, L.; Peruzzi, M.; Heusser, P.; Baumgartner, S. Isopathic treatment effects of Arsenicum album 45x on wheat seedling growth—Further reproduction trials. Homeopathy 2009, 98, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Endler, P.C.; Scherer-Pongratz, W.; Lothaller, H.; Stephen, S. Wheat and ultra high diluted gibberellic acid—Further experiments and re-analysis of data. Homeopathy 2015, 104, 257–262. [Google Scholar] [CrossRef]
- Scherer-Pongratz, W.; Endler, P.C.; Lothaller, H.; Stephen, S. Wheat and ultra high diluted silver nitrate—Further experiments and re-analysis of data. Homeopathy 2015, 104, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Majewsky, V.; Scherr, C.; Arlt, S.P.; Kiener, J.; Frrokaj, K.; Schindler, T.; Klocke, P.; Baumgartner, S. Reproducibility of effects of homeopathically potentised gibberellic acid on the growth of Lemna gibba L. in a randomised and blinded bioassay. Homeopathy 2014, 103, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Majewsky, V.; Scherr, C.; Schneider, C.; Arlt, S.P.; Baumgartner, S. Reproducibility of the effects of homeopathically potentised Argentum nitricum on the growth of Lemna gibba L. in a randomised and blinded bioassay. Homeopathy 2017, 106, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Elzenga, J.T.M.; De Lange, L.; Pieterse, A.H. Further indications that ethylene is the gibbosity regulator of the Lemna gibba/Lemna minor complex in natural waters. Acta Bot. Neerl. 1980, 29, 225–229. [Google Scholar] [CrossRef]
- Sandler, G.; Bartkowska, M.; Agrawal, A.F.; Wright, S.I. Estimation of the SNP Mutation Rate in Two Vegetatively Propagating Species of Duckweed. G3 Genes Genomes Genet. 2020, 10, 4191–4200. [Google Scholar] [CrossRef]
- Xu, S.; Stapley, J.; Gablenz, S.; Boyer, J.; Appenroth, K.J.; Sree, K.S.; Gershenzon, J.; Widmer, A.; Huber, M. Low genetic variation is associated with low mutation rate in the giant duckweed. Nat Commun. 2019, 10, 1243. [Google Scholar] [CrossRef] [Green Version]

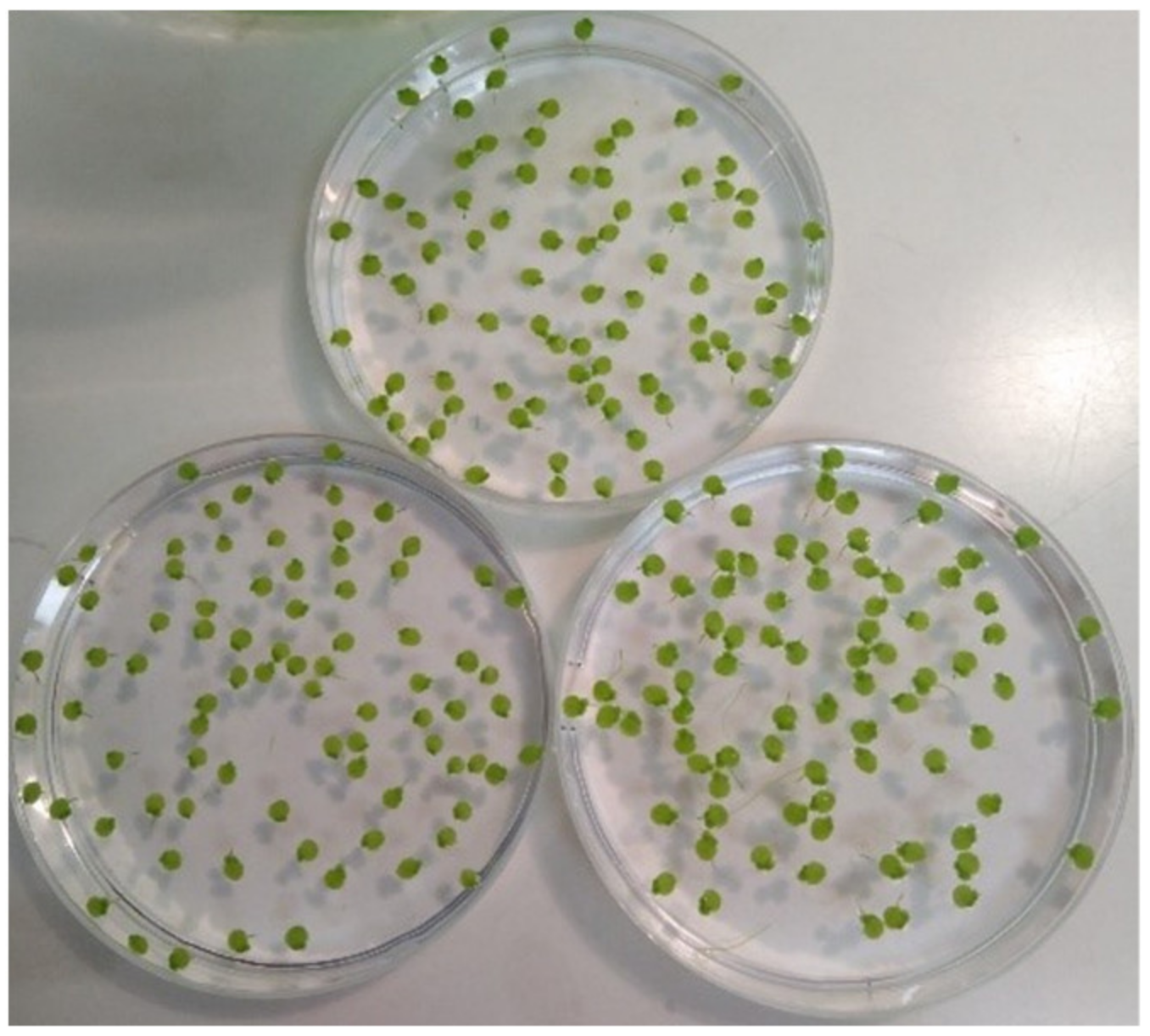
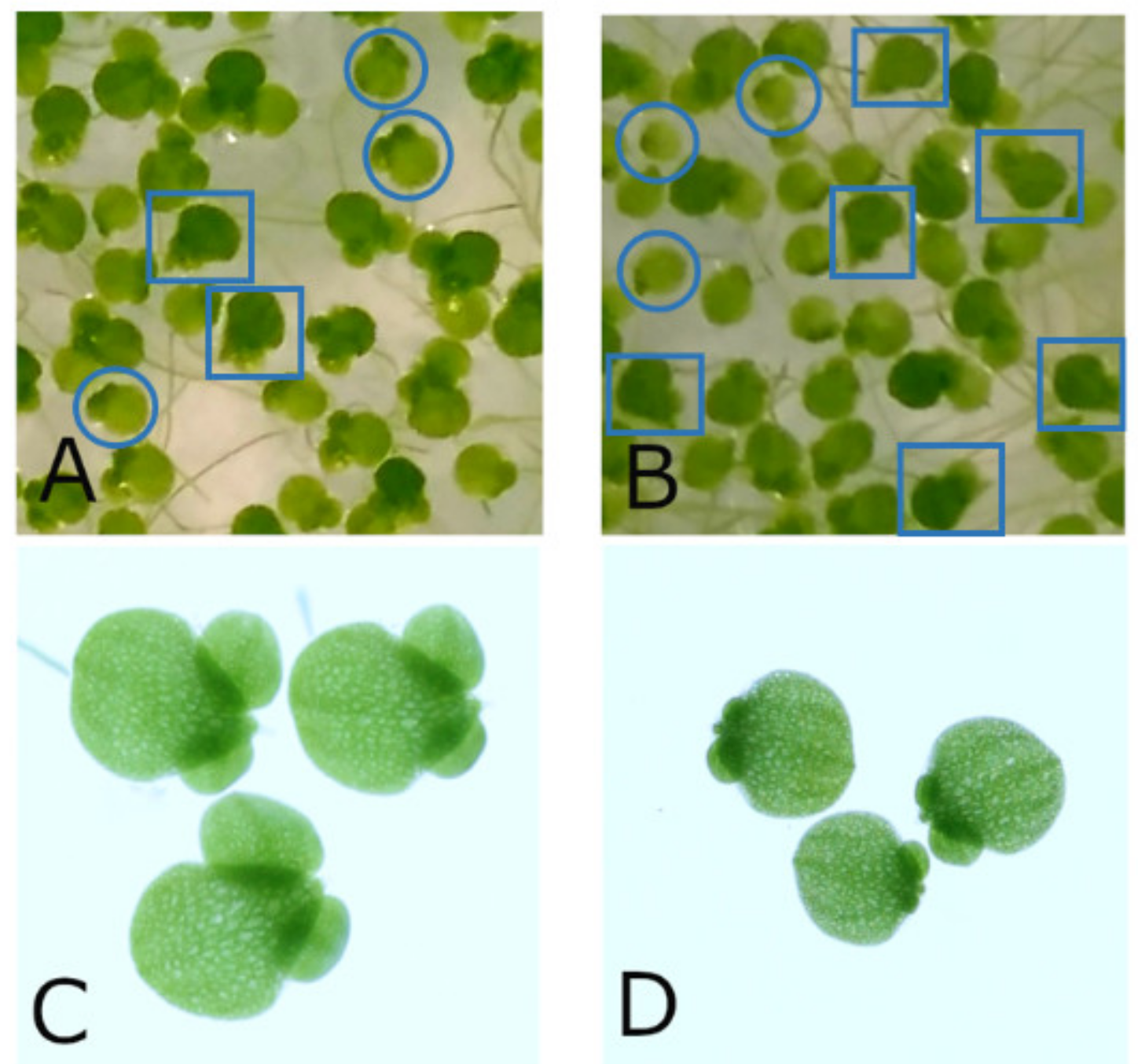


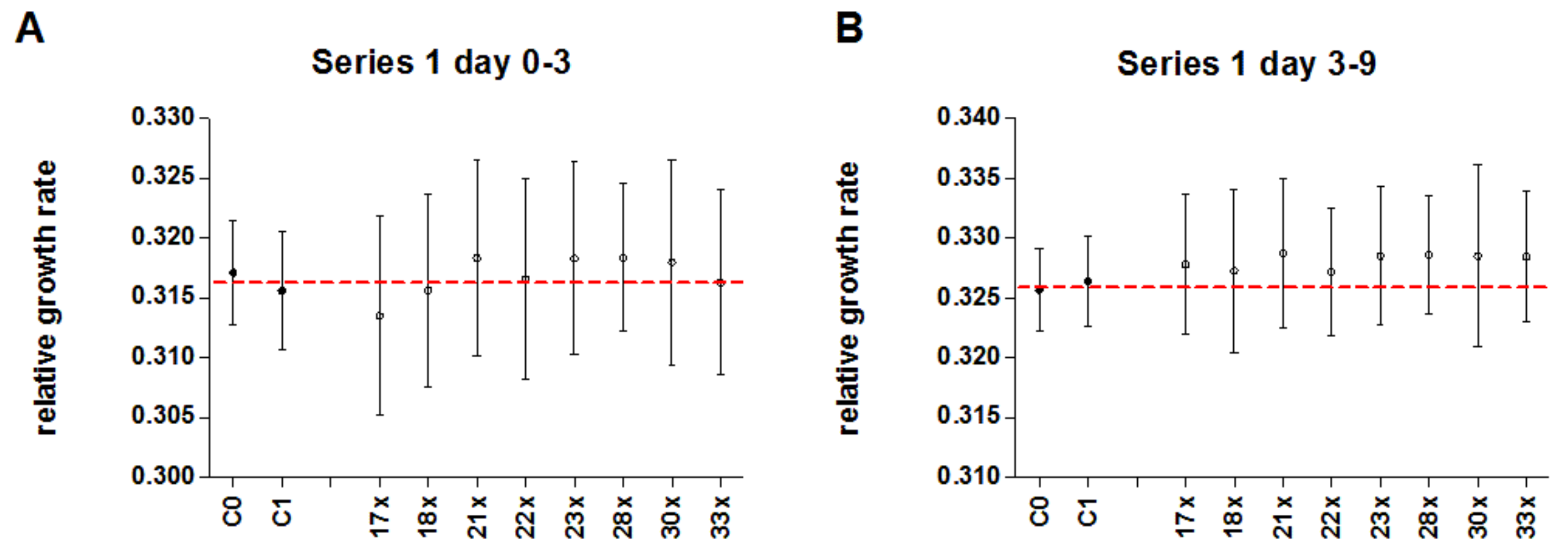

| Series | Arsenic Concentration [mg/L] | Experiment | Corresponding Verum Experiment | Light Cycle (h Light:h Darkness) | Location | Chamber | Start of Experiment | Early Time Period [d] | Late Time Period [d] | Sample Size Control/Treatment | No. Non Stressed | Image Analysis Software | Starting Ars alb Potency | Used Potency Levels |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 158 | Verum 6 | 16:08 | Freiburg | Chamber 1 | 11 March 2017 | 0–3 | 3–9 | 40/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |
| Verum 7 | 16:08 | Freiburg | Chamber 1 | 1 April 2017 | 0–3 | 3–9 | 40/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 8 | 16:08 | Arlesheim | Chamber 1 | 13 February 2018 | 0–3 | 3–9 | 30/40 | 3 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 9 | 16:08 | Arlesheim | Chamber 1 | 9 March 2018 | 0–3 | 3–9 | 30/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 10 | 16:08 | Arlesheim | Chamber 2 | 11 May 2018 | 0–3 | 3–9 | 30/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| SNC 6 | Verum 6 | 16:08 | Freiburg | Chamber 1 | 2 July 2016 | 0–3 | 3–9 | 40/40 | 2 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 7 | Verum 7 | 16:08 | Freiburg | Chamber 1 | 15 April 2017 | 0–3 | 3–9 | 40/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 8 * | Verum 8 | 16:08 | Arlesheim | Chamber 1 | 20 October 2017 | 0–3 | 3–9 | 30/40 | 5 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 9 * | Verum 9 | 16:08 | Arlesheim | Chamber 1 | 1 December 2017 | 0–3 | 3–9 | 30/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 10 | Verum 10 | 16:08 | Arlesheim | Chamber 1 | 12 January 2019 | 0–3 | 3–9 | 30/40 | 4 | imageJ A | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| 2 | 250 | Verum 11 | 16:08 | Arlesheim | Chamber 1 | 20 July 2018 | 0–3 | 3–9 | 30/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |
| Verum 12 | 16:08 | Arlesheim | Chamber 1 | 7 September 2018 | 0–3 | 3–9 | 30/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 13 | 16:08 | Arlesheim | Chamber 1 | 7 December 2018 | 0–3 | 3–9 | 30/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 14 | 16:08 | Arlesheim | Chamber 2 | 23 May 2019 | 0–3 | 3–9 | 40/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| Verum 15 | 16:08 | Arlesheim | Chamber 2 | 1 August 2019 | 0–3 | 3–9 | 40/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | |||
| SNC 11 * | Verum 11 | 16:08 | Arlesheim | Chamber 2 | 9 May 2019 | 0–3 | 3–9 | 30/40 | 4 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 12 * | Verum 12 | 16:08 | Arlesheim | Chamber 2 | 18 July 2019 | 0–3 | 3–9 | 30/40 | 4 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 13 * | Verum 13 | 16:08 | Arlesheim | Chamber 2 | 7 November 2019 | 0–3 | 3–9 | 30/40 | 4 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 14 | Verum 14 | 16:08 | Arlesheim | Chamber 2 | 21 Nevember 2019 | 0–3 | 3–9 | 40/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| SNC 15 | Verum 15 | 16:08 | Arlesheim | Chamber 2 | 5 December 2019 | 0–3 | 3–9 | 40/40 | 5 | imageJ B | 5x dilution | 17x, 18x, 20–23x, 28x, 30x, 33x | ||
| 0 | 158 | Verum 1 | 24:00 | Frick | Chamber 0 | 25 March 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | |
| (orig. | Verum 2 | 24:00 | Frick | Chamber 0 | 3 June 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| series) | Verum 3 | 24:00 | Frick | Chamber 0 | 29 July 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| Verum 4 | 24:00 | Frick | Chamber 0 | 12 August 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | |||
| Verum 5 | 24:00 | Frick | Chamber 0 | 28 August 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | |||
| SNC 1 | Verum 1 | 24:00 | Frick | Chamber 0 | 20 January 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| SNC 2 | Verum 2 | 24:00 | Frick | Chamber 0 | 22 April 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| SNC 3 | Verum 3 | 24:00 | Frick | Chamber 0 | 10 June 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| SNC 4 | Verum 4 | 24:00 | Frick | Chamber 0 | 8 July 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x | ||
| SNC 5 | Verum 5 | 24:00 | Frick | Chamber 0 | 2 September 2009 | 0–2 | 2–6 | 45/45 | 5 | Scanalyzer | 5x trituration | 17x, 18x, 20–24x, 28x, 30x, 33x |
| CV Series 1 | Early Time Period (Day 0–3) | Late Time Period (Day 3–9) | CV Series 2 | Early Time Period (Day 0–3) | Late Time Period (Day 3–9) |
|---|---|---|---|---|---|
| SNC 1 | 3.00 | 2.27 | SNC 1 | 1.20 | 1.38 |
| SNC 2 | 1.35 | 1.25 | SNC 2 | 1.66 | 1.24 |
| SNC 3 | 2.02 | 0.83 | SNC 3 | 1.69 | 0.97 |
| SNC 4 | 1.96 | 0.79 | SNC 4 | 1.65 | 0.86 |
| SNC 5 | 0.90 | 0.73 | SNC 5 | 2.59 | 1.04 |
| SNC 1–5 | 1.78 | 1.14 | SNC 1–5 | 1.75 | 1.10 |
| Series 1 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group | 1 | 0.00000023 | 0.0013 | 0.9712 |
| Experiment number | 4 | 0.08786236 | 125.4573 | <0.0001 |
| Interaction | 4 | 0.00027976 | 0.3995 | 0.809 |
| Series 1 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group | 1 | 0.00019524 | 2.7635 | 0.0973 |
| Experiment number | 4 | 0.06368114 | 225.3325 | <0.0001 |
| Interaction | 4 | 0.0004133 | 1.4624 | 0.2131 |
| Series 1 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group | 9 | 0.00057831 | 0.3431 | 0.9599 |
| Experiment number | 4 | 0.0612927 | 81.8193 | <0.0001 |
| Interaction | 36 | 0.00275917 | 0.4092 | 0.9991 |
| Series 1 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group | 9 | 0.00029427 | 0.4437 | 0.9106 |
| Experiment number | 4 | 0.04412396 | 149.7021 | <0.0001 |
| Interaction | 36 | 0.00217615 | 0.8204 | 0.7605 |
| Series 2 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group | 1 | 0.00000771 | 0.0705 | 0.7908 |
| Experiment number | 4 | 0.21284402 | 486.3185 | <0.0001 |
| Interaction | 4 | 0.0005176 | 1.1826 | 0.3181 |
| Series 2 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group | 1 | 0.00029398 | 4.0814 | 0.0441 |
| Experiment number | 4 | 0.03503934 | 121.617 | <0.0001 |
| Interaction | 4 | 0.0002879 | 0.9993 | 0.4079 |
| Series 2 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group | 9 | 0.00063312 | 0.6484 | 0.7553 |
| Experiment number | 4 | 0.16981641 | 391.2808 | <0.0001 |
| Interaction | 36 | 0.00454901 | 1.1646 | 0.245 |
| Series 2 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group | 9 | 0.00073737 | 1.1146 | 0.3517 |
| Experiment number | 4 | 0.02844692 | 96.7118 | <0.0001 |
| Interaction | 36 | 0.00223863 | 0.8456 | 0.723 |
| Pool Series 1 + 2 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group (tg) | 1 | 0.00000529 | 0.0372 | 0.8471 |
| Experiment number (en) | 4 | 0.14827807 | 260.594 | <0.0001 |
| Experimental series (es) | 1 | 0.01723593 | 121.1664 | <0.0001 |
| tg * en | 4 | 0.00063099 | 1.1089 | 0.3511 |
| tg * es | 1 | 0.00000264 | 0.0186 | 0.8916 |
| en * es | 4 | 0.13905187 | 244.3792 | <0.0001 |
| tg * en * es | 4 | 0.00015998 | 0.2812 | 0.8902 |
| Pool Series 1 + 2 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group (tg) | 1 | 0.00048419 | 6.787 | 0.0094 |
| Experiment number (en) | 4 | 0.05417827 | 189.8587 | <0.0001 |
| Experimental series (es) | 1 | 0.01532783 | 214.8553 | <0.0001 |
| tg * en | 4 | 0.00053627 | 1.8793 | 0.1122 |
| tg * es | 1 | 0.00000503 | 0.0706 | 0.7906 |
| en * es | 4 | 0.0399193 | 139.8905 | <0.0001 |
| tg * en * es | 4 | 0.00015089 | 0.5288 | 0.7146 |
| Pool Series 1 + 2 Early Time Period (Day 0–3) | df | Sum of Squares | F Ratio | p Value |
|---|---|---|---|---|
| Treatment group (tg) | 9 | 0.00029496 | 0.2216 | 0.9914 |
| Experiment number (en) | 4 | 0.11806456 | 199.581 | <0.0001 |
| Experimental series (es) | 1 | 0.01398425 | 94.5581 | <0.0001 |
| tg * en | 36 | 0.00418589 | 0.7862 | 0.8117 |
| tg * es | 9 | 0.00091647 | 0.6885 | 0.7197 |
| en * es | 4 | 0.11179632 | 188.9849 | <0.0001 |
| tg * en * es | 36 | 0.00316782 | 0.595 | 0.972 |
| Pool Series 1 + 2 Late Time Period (Day 3–9) | df | Sum of Squares | F Ratio | pValue |
| Treatment group (tg) | 9 | 0.00078236 | 1.1809 | 0.3042 |
| Experiment number (en) | 4 | 0.04162298 | 141.3617 | <0.0001 |
| Experimental series (es) | 1 | 0.01252126 | 170.1009 | <0.0001 |
| tg * en | 36 | 0.00246072 | 0.9286 | 0.5908 |
| tg * es | 9 | 0.00024958 | 0.3767 | 0.9463 |
| en * es | 4 | 0.03053381 | 103.7002 | <0.0001 |
| tg * en * es | 36 | 0.0019505 | 0.736 | 0.8718 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ücker, A.; Baumgartner, S.; Martin, D.; Jäger, T. Critical Evaluation of Specific Efficacy of Preparations Produced According to European Pharmacopeia Monograph 2371. Biomedicines 2022, 10, 552. https://doi.org/10.3390/biomedicines10030552
Ücker A, Baumgartner S, Martin D, Jäger T. Critical Evaluation of Specific Efficacy of Preparations Produced According to European Pharmacopeia Monograph 2371. Biomedicines. 2022; 10(3):552. https://doi.org/10.3390/biomedicines10030552
Chicago/Turabian StyleÜcker, Annekathrin, Stephan Baumgartner, David Martin, and Tim Jäger. 2022. "Critical Evaluation of Specific Efficacy of Preparations Produced According to European Pharmacopeia Monograph 2371" Biomedicines 10, no. 3: 552. https://doi.org/10.3390/biomedicines10030552
APA StyleÜcker, A., Baumgartner, S., Martin, D., & Jäger, T. (2022). Critical Evaluation of Specific Efficacy of Preparations Produced According to European Pharmacopeia Monograph 2371. Biomedicines, 10(3), 552. https://doi.org/10.3390/biomedicines10030552








