Screening and Identification of Putative Long Non-Coding RNA in Childhood Obesity: Evaluation of Their Transcriptional Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. RNA Extraction
2.3. miRNAs and LncRNAs Reverse Transcription and Real-Time PCR
2.4. Statistics
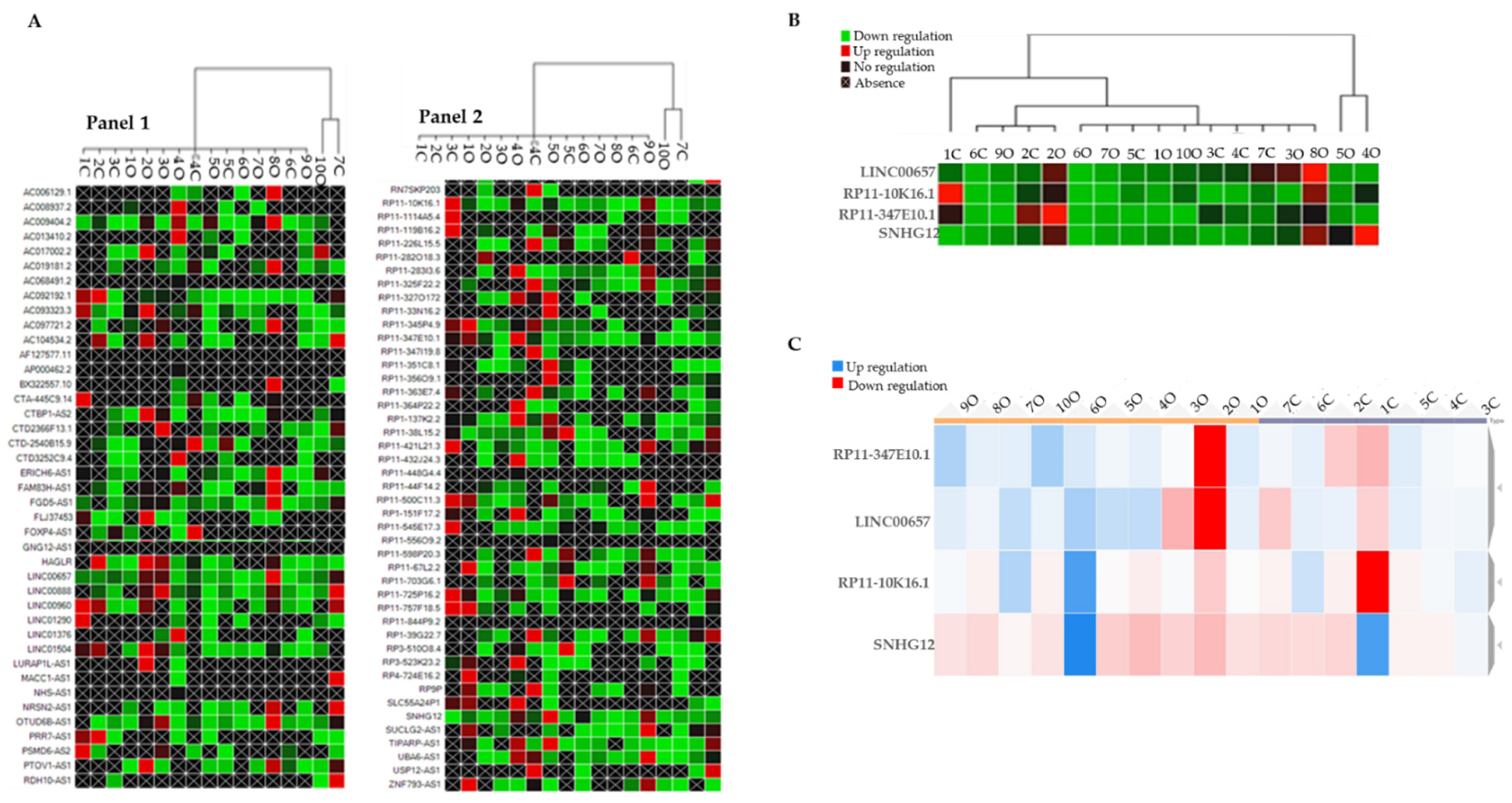
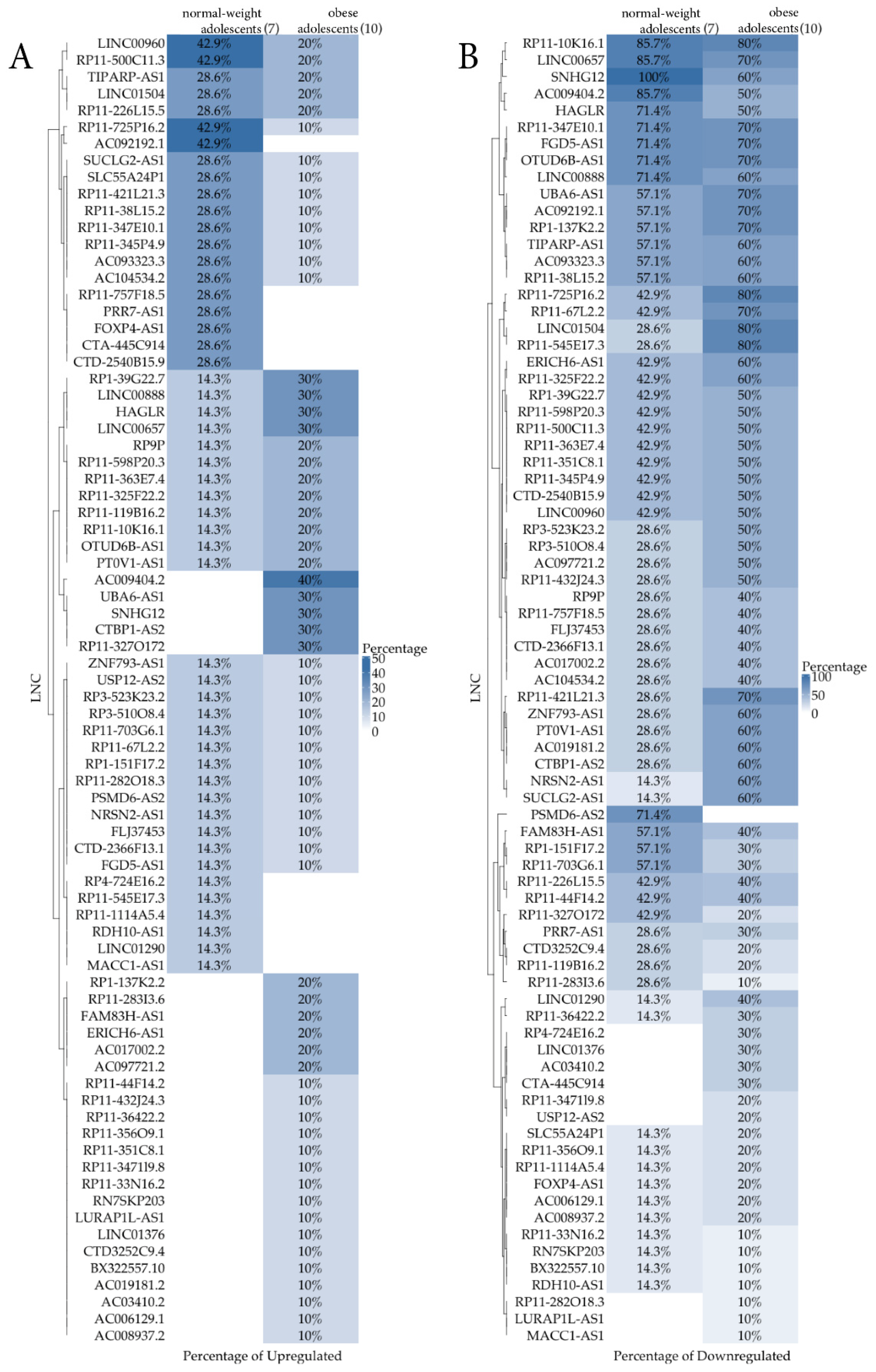
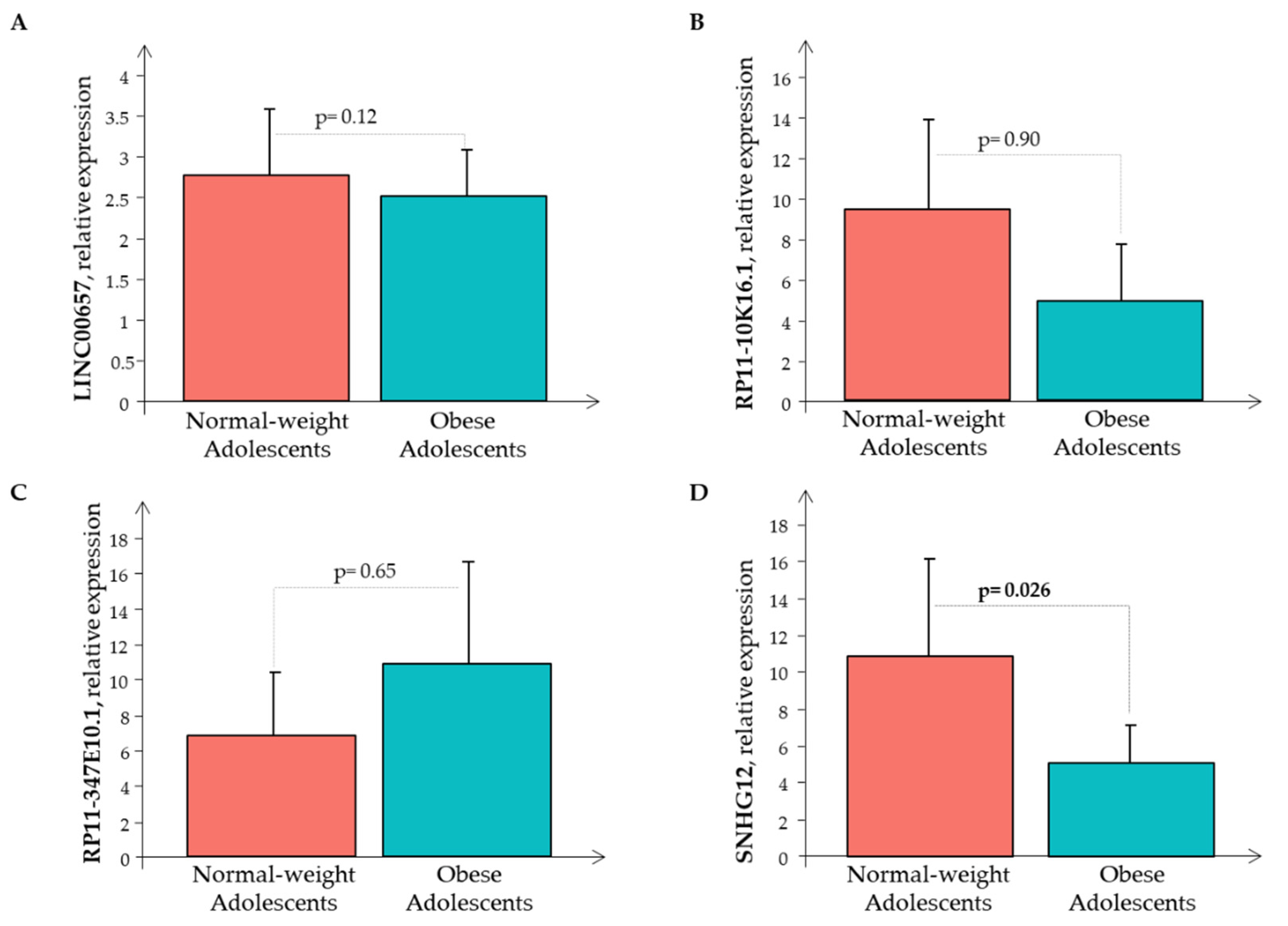
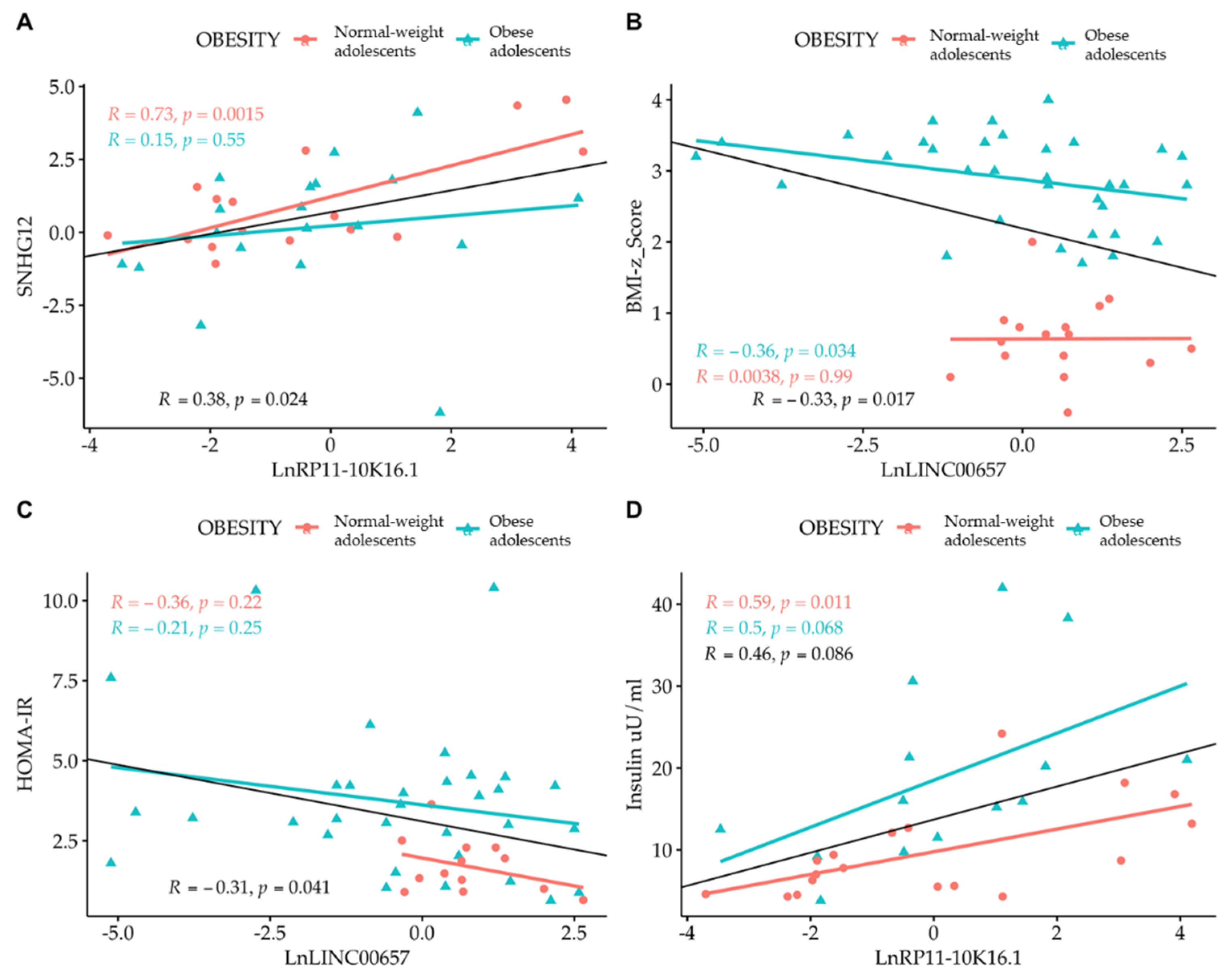
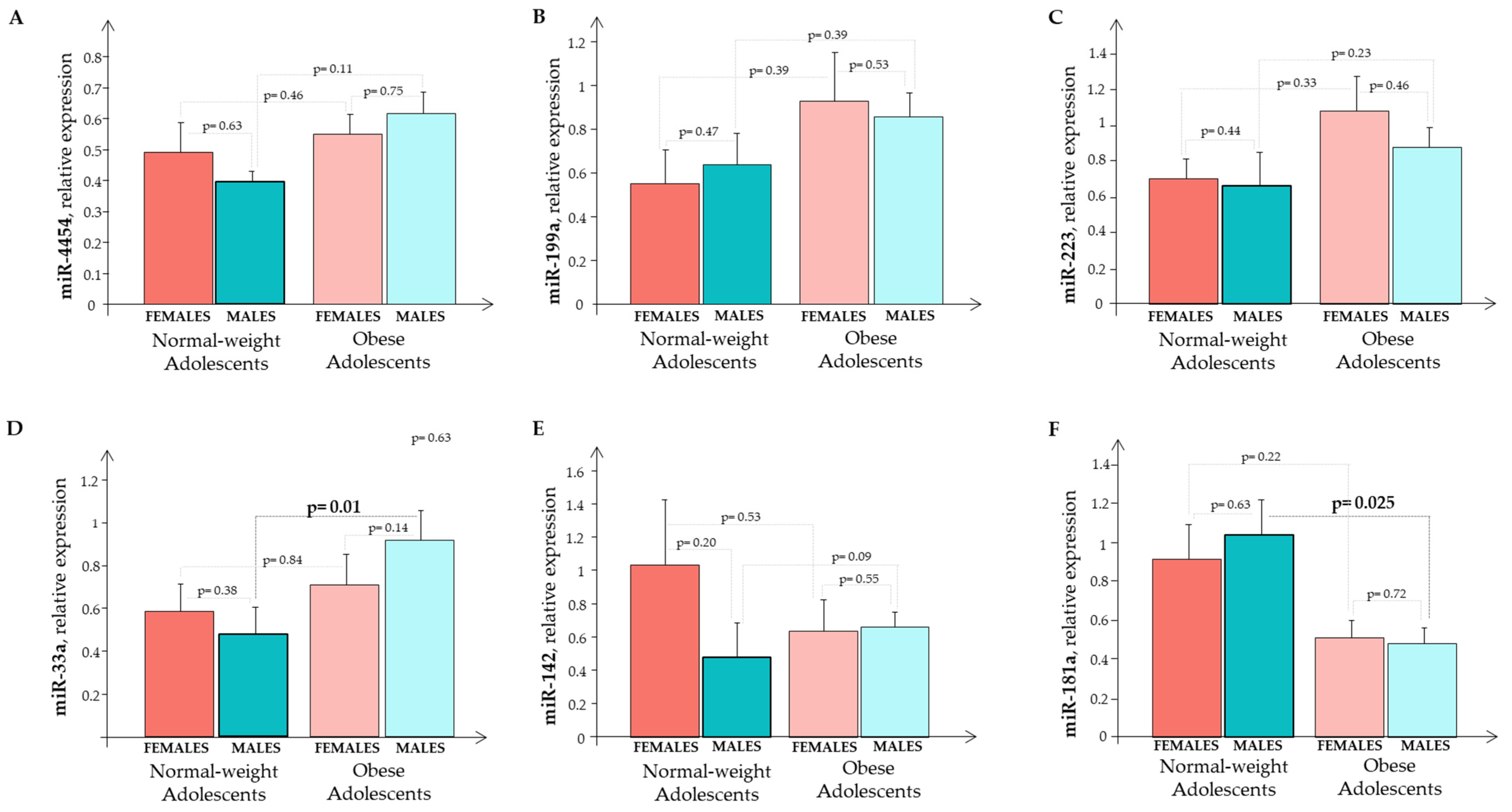
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lifshitz, F.; Lifshitz, J.Z. Globesity: The root causes of the obesity epidemic in the USA and now worldwide. Pediatr. Endocrinol. Rev. 2014, 12, 17–34. [Google Scholar] [PubMed]
- Kuchta, K.F. Pathophysiologic changes of obesity. Anesthesiol. Clin. N. Am. 2005, 23, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R. Complications of obesity in children and adolescents. Int. J. Obes. 2009, 33, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, F.X. Medical Hazards of Obesity. Ann. Intern. Med. 1993, 119, 655–660. [Google Scholar] [CrossRef]
- Scarpato, R.; Verola, C.; Fabiani, B.; Bianchi, V.; Saggese, G.; Federico, G. Nuclear damage in peripheral lymphocytes of obese and overweight Italian children as evaluated by the gamma-H2AX focus assay and micronucleus test. FASEB J. 2011, 25, 685–693. [Google Scholar] [CrossRef]
- Romacho, T.; Elsen, M.; Röhrborn, D.; Eckel, J. Adipose tissue and its role in organ crosstalk. Acta Physiol. 2014, 210, 733–753. [Google Scholar] [CrossRef]
- Ji, Z.Z.; Dai, Z.; Xu, Y.C. A new tumor necrosis factor (TNF)-α regulator, lipopolysaccharides-induced TNF-α factor, is associated with obesity and insulin resistance. Chin. Med. J. 2011, 124, 177–182. [Google Scholar]
- Rauschert, S.; Uhl, O.; Be, K.; Hellmuth, C. Metabolomic Biomarkers for Obesity in Humans: A Short Review. Ann. Nutr. Metab. 2014, 64, 314–324. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Bianchi, V.; Caponi, L.; Maltinti, M.; Caselli, C.; Kozakova, M.; Palombo, C.; Morizzo, C.; Marchetti, S.; et al. C-type natriuretic peptide is closely associated to obesity in Caucasian adolescents. Clin. Chim. Acta 2016, 460, 172–177. [Google Scholar] [CrossRef]
- Iacomino, G.; Russo, P.; Marena, P.; Lauria, F.; Venezia, A.; Ahrens, W.; De Henauw, S.; De Luca, P.; Foraita, R.; Günther, K.; et al. Circulating microRNAs are associated with early childhood obesity: Results of the I. Family Study. Genes Nutr. 2019, 14, 2. [Google Scholar] [CrossRef]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Salviano-Silva, A.; Lobo-Alves, S.C.; De Almeida, R.C.; Malheiros, D.; Petzl-Erler, M.L. Besides pathology: Long non-coding RNA in cell and tissue homeostasis. Non-Coding RNA 2018, 4, 3. [Google Scholar] [CrossRef]
- Riva, P.; Ratti, A.; Venturin, M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Curr. Alzheimer Res. 2016, 13, 1219–1231. [Google Scholar] [CrossRef]
- Nuermaimaiti, N.; Liu, J.; Liang, X.; Jiao, Y.; Zhang, D.; Liu, L.; Meng, X.; Guan, Y. Effect of lncRNA HOXA11-AS1 on adipocyte differentiation in human adipose-derived stem cells. Bioch. Biophy. Res. Commun. 2018, 495, 1878–1884. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, C.; Lin, J.; Ferguson, J.F.; Weiner, A.; Liu, W.; Han, Y.; Hinkle, C.; Li, W.; Jiang, H.; et al. Interrogation of non-conserved human adipose lincRNAs identifies a regulatory role of linc-ADAL in adipocyte metabolism. Sci. Transl. Med. 2018, 10, eaar5987. [Google Scholar] [CrossRef]
- Wijesinghe, S.N.; Nicholson, T.; Tsintzas, K.; Jones, S.W. Involvements of long noncoding RNAs in obesity-associated inflammatory diseases. Obes. Rev. 2021, 22, e13156. [Google Scholar] [CrossRef]
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III-27. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Diettz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. Br. Med. J. 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The LMS method and penalized likehood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef]
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Haller, M.J.; Stein, J.; Shuster, J.; Theriaque, D.; Silverstein, J.; Schatz, D.A.; Earing, M.G.; Lerman, A.; Mahmud, F.H. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediat. Diabet. 2007, 8, 193–198. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M.; Morizzo, C.; Gnesi, L.; Barsotti, M.C.; Spontoni, P.; Massart, F.; Salvi, P.; Balbarini, A.; Saggese, G.; et al. Circulating endothelial progenitor cells and large artery structure and function in young subjects with uncomplicated type 1 diabetes. Cardiovasc. Diabetol. 2011, 10, 88. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J. Obesity and cancer. Oncogene 2004, 23, 6365–6378. [Google Scholar] [CrossRef]
- Van Putte-Katier, N.; Rooman, R.P.; Haas, L.; Verhulst, S.L.; Desager, K.N.; Ramet, J.; Suys, B.E. Early cardiac abnormalities in obese children: Importance of obesity per se versus associated cardiovascular risk factors. Pediatr. Res. 2008, 64, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Francès, Y.; Kingwell, B.A.; Ahimastos, A.A. Gender differences in artery wall biomechanical properties throughout life. J. Hypertens. 2011, 29, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, M.; Morizzo, C.; Bianchi, V.; Marchetti, S.; Federico, G.; Palombo, C. Hemodynamic overload and intra-abdominal adiposity in obese children: Relationships with cardiovascular structure and function. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Raitakari, M.; Viikari, J.S.; Raitakari, O.T. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2006, 185, 388–393. [Google Scholar] [CrossRef]
- Katsareli, E.A.; Dedoussis, G.V. Biomarkers in the field of obesity and its related comorbidities. Expert Opin. Ther. Targets 2014, 18, 385–401. [Google Scholar] [CrossRef]
- Del Ry, S.; Cabiati, M.; Bianchi, V.; Storti, S.; Caselli, C.; Prescimone, T.; Clerico, A.; Saggese, G.; Giannessi, D.; Federico, G. C-type natriuretic peptide plasma levels are reduced in obese adolescents. Peptides 2013, 50, 50–54. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, H.T.; Yu, J.P.; Han, X.P.; Xu, Z.P.; Li, Y.M.; Jiao, Z.Y.; Liu, H.B. A competing endogenous RNA network reveals novel potential lncRNA, miRNA, and mRNA biomarkers in the prognosis of human colon adenocarcinoma. J. Surg. Res. 2019, 235, 22–33. [Google Scholar] [CrossRef]
- Cabiati, M.; Randazzo, E.; Salvadori, C.; Peroni, D.; Federico, G.; Del Ry, S. Circulating microRNAs associated with C-type natriuretic peptide in childhood obesity. Peptides 2020, 133, 170387. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Bao, M.H.; Li, G.Y.; Huang, X.S.; Tang, L.; Dong, L.P.; Li, J.M. Long Noncoding RNA LINC00657 acting as a mir-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein induced angiogenesis. Molec. Pharmacol. 2018, 93, 368–375. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Pan, S.; Yang, T.; Sun, X.; Wang, Y.; Shi, X.; Zhao, X.; Guo, J.; Zhang, X. LINC00657 played oncogenic roles in esophageal squamous cell carcinoma by targeting miR-615-3p and JunB. Biomed. Pharmacother. 2018, 108, 316–324. [Google Scholar] [CrossRef]
- He, H.; Chen, D.; Cui, S.; Piao, H.; Tang, H.; Wang, X.; Ye, P.; Jin, S. Identification of a long non-coding RNA mediated competitive endogenous RNA network in hepatocellular carcinoma. Oncol. Rep. 2019, 42, 745–752. [Google Scholar] [CrossRef]
- Zhang, R.; Niu, Z.; Pei, H.; Peng, Z. Long noncoding RNA LINC00657 induced by SP1 contributes to the non-small cell lung cancer progression through targeting miR-26b-5p/COMMD8 axis. J. Cell. Physiol. 2020, 235, 3340–3349. [Google Scholar] [CrossRef]
- Tamang, S.; Acharya, V.; Roy, D.; Sharma, R.; Aryaa, A.; Sharma, U.; Khandelwal, A.; Prakash, H.; Vasquez, K.M.; Jain, A. SNHG12: A LncRNA as a potential therapeutic target and biomarker for human cancer. Front. Oncol. 2019, 9, 901. [Google Scholar] [CrossRef]
- Ruan, W.; Wang, P.; Feng, S.; Xue, Y.; Li, Y. Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12) promotes cell proliferation and migration by upregulating angiomotin gene expression in human osteosarcoma cells. Tumor. Biol. 2016, 37, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.F.; Cai, W.; Chen, B. LncRNA SNHG12 regulated the proliferation of gastric carcinoma cell BGC-823 by targeting microRNA-199a/b-5p. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1297–1306. [Google Scholar] [PubMed]
- Liu, Y.; Ji, Y.; Li, M.; Wang, M.; Yi, X.; Yin, C.; Wang, S.; Zhang, M.; Zhao, Z.; Xiao, Y. Integrated analysis of long noncoding RNA and mRNA expression profile in children with obesity by microarray analysis. Sci. Rep. 2018, 8, 8750. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; You, L.; Zhu, L.; Wang, X.; Zhou, Y.; Li, Y.; Wen, J.; Xia, Y.; Wang, X.; Ji, C.; et al. Change in circulating microRNA profile of obese children indicates future risk of adult diabetes. Metabolism 2018, 78, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Toolabi, K.; Jannat Ali Pour, N.; Mohassel Azadi, S.; Bahiraee, A.; Zamani-Garmsiri, F.; Emamgholipour, S. Adipose tissue gene expression of long non-coding RNAs; MALAT1, TUG1 in obesity: Is it associated with metabolic profile and lipid homeostasis-related genes expression? Diabetol. Metab. Syndr. 2020, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Taheri, M. The expression profile and role of non-coding RNAs in obesity. Eur. J. Pharmacol. 2021, 892, 173809. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Urrata, V.; Gilardini, L.; Bertoli, S.; Calcaterra, V.; Zuccotti, G.V.; Cancello, R.; Carelli, S. Role of long non-coding RNAs in adipogenesis: State of the art and implications in obesity and obesity-associated diseases. Obes. Rev. 2021, 22, e13203. [Google Scholar] [CrossRef]
- Wu, T.; Du, Y. LncRNAs: From basic research to medical application. Int. J. Biol. Sci. 2017, 13, 295–307. [Google Scholar] [CrossRef]
- Sun, J.; Ruan, Y.; Wang, M.; Chen, R.; Yu, N.; Sun, L.; Liu, T.; Chen, H. Differentially expressed circulating LncRNAs and mRNA identified by microarray analysis in obese patients. Sci. Rep. 2016, 6, 35421. [Google Scholar] [CrossRef]
- Ouyang, S.; Tang, R.; Liu, Z.; Ma, F.; Li, Y.; Wu, J. Characterization and predicted role of microRNA expression profiles associated with early childhood obesity. Mol. Med. Rep. 2017, 16, 3799–8306. [Google Scholar] [CrossRef][Green Version]
- Zhuang, G.; Meng, C.; Guo, X.; Cheruku, P.S.; Shi, L.; Xu, H.; Li, H.; Wang, G.; Evans, A.R.; Safe, S.; et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation 2012, 125, 2892–2903. [Google Scholar] [CrossRef]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–1129. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O'Brien, P.E.; Harrison, L.C. Pro-inflammatory CD11c+CD206+adiposetissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sinnaeve, P.; Van der Schueren, B.; Mathieu, C.; Janssens, S.; Holvoet, P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J. Clin. Endocrinol. Metab. 2012, 97, E1213–E1218. [Google Scholar] [CrossRef]

| Gene | Forward Primer Sequence (5′-3′) | Genbank Accession Number | Location | Temperature °C |
|---|---|---|---|---|
| hsa-miR-33a-3p | CAATGTTTCCACAGTGCATCAC | NR_029507 | chr 22 q13.2 | 55 |
| hsa-miR-223-5p | CGTGTATTTGACAAGCTGAGTT | LM608368 | chr X q12 | 55 |
| hsa-miR-142-5p | CATAAAGTAGAAAGCACTACT | NR_029683 | chr 17 q22 | 55 |
| hsa-miR-199a-5p | CCCAGTGTTCAGACTACCTGTTC | NR_029586 | chr 19 p13.2 | 55 |
| hsa-miR-4454 | GGATCCGAGTCACGGCACCA | NR_039659 | chr 4 q32.2 | 55 |
| hsa-miR-181a-5p | AACATTCAACGCTGTCGGTGAGT | NC_000009.12 | chr 22 1q32.1 | 55 |
| (A) | |||
|---|---|---|---|
| Normal-Weight Subjects (n = 26) | Obese Subjects (n = 54) | p | |
| Clinical parameters | |||
| Age (years) | 12.8 ± 0.2 | 12.5 ± 0.41 | n.s. |
| Male: Female | 14:12 | 34:20 | n.s. |
| Pubertal Stage | 3.2 ± 0.2 | 3.7 ± 0.1 | n.s. |
| Height z-score | 0.3 ± 0.15 | 0.9 ± 0.1 | <0.001 |
| Weight (Kg) | 52.6 ± 2.4 | 74.3 ± 2.1 | <0.0001 |
| BMI | 21.1 ±0.54 | 29.4 ± 0.65 | <0.0001 |
| BMI z-score | 0.7 ± 0.05 | 2.8 ± 0.06 | <0.0001 |
| FM (%) | 18 ± 1.0 | 36 ± 1.0 | <0.0001 |
| WC (cm) | 76.0 ± 0.4 | 94.8 ± 2.0 | <0.0001 |
| WC/H | 0.48 ± 0.004 | 0.61 ± 0.001 | <0.0001 |
| SBP (mmHg) females | 106 ± 1.8 | 113 ± 2.3 | <0.03 |
| DBP (mmHg) females | 61 ± 1.7 | 67 ± 1.4 | <0.01 |
| SBP (mmHg) males | 116 ± 0.9 | 111 ± 2.0 | n.s. |
| DBP (mmHg) males | 62 ± 1.9 | 63 ± 1.3 | n.s. |
| Hematochemical parameters | |||
| Glycemia (mg/dL) | 77 ± 1.5 | 87 ± 1.6 | <0.0001 |
| Insulin (uU/mL) | 7.3 ± 0.4 | 19.2 ± 1.5 | <0.0001 |
| Homa-IR | 1.5 ± 0.1 | 3.7 ± 0.3 | <0.0001 |
| Cholesterol (mg/dL) | 133 ± 8.8 | 169 ± 4.1 | <0.0001 |
| HDL (mg/dL) | 45 ± 3.0 | 48 ± 1.4 | n.s. |
| LDL (mg/dL) | 73 ± 6.3 | 104 ± 3.8 | <0.0001 |
| Triglycerides (mg/dL) | 69 ± 15 | 86 ± 6.7 | n.s. |
| CRP (mg/dL) | 0.04 ± 0.02 | 0.33 ± 0.01 | <0.0001 |
| RHI | 2.1 ± 0.05 | 1.4 ± 0.05 | <0.0001 |
| (B) | |||
| Normal-Weight Subjects (n = 7) | Obese Subjects (n = 10) | p | |
| Clinical parameters | |||
| Age (years) | 12.9 ± 0.2 | 12.2 ± 0.3 | n.s. |
| Male: Female | 5:4 | 3:5 | n.s. |
| Pubertal Stage | 3.3 ± 0.3 | 3.7 ± 0.4 | n.s. |
| Height z-score | 0.2 ± 0.1 | 1.0 ± 0.2 | 0.03 |
| Weight (Kg) | 50.4 ± 1.5 | 65.3 ± 4.2 | <0.001 |
| BMI | 20.2 ±0.4 | 29.1 ± 1.2 | <0.0001 |
| BMI z-score | 0.6 ± 0.1 | 2.8 ± 0.2 | <0.0001 |
| FM (%) | 18.3 ± 1.8 | 38.2 ± 2.4 | <0.0001 |
| WC (cm) | 76.4 ± 0.5 | 91.5 ± 3.3 | <0.002 |
| WC/H | 0.48 ± 0.006 | 0.63 ± 0.01 | <0.0001 |
| SBP (mmHg) females | 107 ± 2.2 | 114 ± 1.1 | <0.03 |
| DBP (mmHg) females | 60 ± 1.3 | 66 ± 0.6 | <0.007 |
| SBP (mmHg) males | 115 ± 1.4 | 112 ± 0.8 | n.s. |
| DBP (mmHg) males | 61 ± 1.3 | 62 ± 1.5 | n.s. |
| miR-33a miR-33a N/O | miR-223 miR-223 N/O | miR-142 miR-142 N/O | miR-199a miR-199a N/O | miR-4454 miR-4454 N/O | miR-181a miR-181a N/O | |
|---|---|---|---|---|---|---|
| miR-33a miR-33aN miR-33aO | - | R = 0.36; p = 0.002 R = 0.17; p = 0.44 R = 0.38; p = 0.006 | R = 0.40; p = 0.0008 R = 0.30; p = ns R = 0.45; p = 0.001 | R = 0.34; p = 0.005 R = 0.08; p = ns R = 0.46; p = 0.001 | R = 0.30; p = 0.01 R = 0.22; p = ns R = 0.29; p = 0.037 | - |
| miR-223 miR-223N miR-223O | R = 0.36; p = 0.002 R = 0.17; p = 0.04 R = 0.38; p = 0.006 | - | R = 0.53; p< 0.0001 R = 0.64; p = 0.002 R = 0.49; p = 0.0003 | R = 0.27; p = 0.03 R = 0.02; p = ns R = 0.30; p = 0.04 | R = 0.53; p < 0.0001 R = 0.21; p = ns R = 0.60; p < 0.0001 | - |
| miR-142 miR-142N miR-142O | R = 0.40; p = 0.0008 R = 0.30; p = ns R = 0.45; p = 0.001 | R = 0.53; p< 0.0001 R = 0.64; p = 0.002 R = 0.49; p = 0.0003 | - | R = 0.61; p< 0.0001 R = 0.62; p = 0.004 R = 0.59; p < 0.0001 | R = 0.58; p< 0.0001 R = 0.54; p = 0.012 R = 0.60; p < 0.0001 | - |
| miR-199a miR-199aN miR-199aO | R = 0.34; p = 0.005 R = 0.08; p = ns R = 0.46; p = 0.001 | R = 0.27; p = 0.03 R = 0.02; p = ns R = 0.30; p = 0.04 | R = 0.61; p < 0.0001 R = 0.62; p = 0.004 R = 0.59; p < 0.0001 | - | R = 0.45; p = 0.0001 R = 0.50; p = 0.022 R = 0.41; p = 0.004 | R = 0.30; p = 0.02 R = 0.70; p < 0.0001 R = 0.23; p = ns |
| miR-4454 miR-4454N miR-4454O | R = 0.30; p = 0.01 R = 0.22; p = ns R = 0.29; p = 0.037 | R = 0.53; p < 0.0001 R = 0.21; p = ns R = 0.60; p < 0.0001 | R = 0.58; p< 0.0001 R = 0.54; p = 0.012 R = 0.60; p < 0.0001 | R = 0.45; p = 0.0001 R = 0.50; p = 0.022 R = 0.41; p = 0.004 | - | R = 0.24; p = 0.05 R = 0.60; p = 0.03 R = 0.18; p = ns |
| miR-181a miR-181aN miR-181aO | - | - | - | R = 0.30; p = 0.02 R = 0.70; p = <0.0001 R = 0.23; p = ns | R = 0.24; p = 0.05 R = 0.60; p = 0.03 R = 0.18; p = ns | - |
| Weight (Kg) N/O | BMI z-Score N/O | BMI N/O | WC (cm) N/O | Insulin (mU/mL) N/O | CRP (mg/dL) N/O | |
|---|---|---|---|---|---|---|
| miR-4454 miR-4454 N miR-4454O | R = 0.36; p = 0.02 R = 0.38; p = ns R = 0.37; p = ns | R = 0.40; p = 0.0006 R = 0.03; p = ns R = 0.48; p < 0.001 | R = 0.38; p = 0.02 R = 0.37; p = ns R = 0.40; p = 0.05 | R = 0.33; p = 0.04 R = 0.23; p = ns R = 0.39; p = 0.05 | - | - |
| miR-199a miR-199a N miR-199a O | - | R = 0.26; p = 0.03 R = 0.03; p = ns R = 0.29; p = 0.05 | - | - | - | - |
| miR-223 miR-223 N miR-223 O | - | R = 0.40; p = 0.0005 R = 0.49; p = 0.02 R = 0.42; p = 0.003 | - | - | - | R = 0.30; p = 0.01 R = 0.51; p = 0.014 R = 0.14; p = ns |
| miR-33a miR-33a N miR-33a O | - | R = 0.35; p = 0.003 R = 0.02; p = ns R = 0.34; p = 0.02 | - | - | R = 0.33; p = 0.02 R = 0.37; p = ns R = 0.11; p = ns | - |
| miR-181a miR-181a N miR-181a O | - | - | - | R = −0.33; p = 0.008 R = −0.26; p = ns R = 0.22; p = ns | - | R = −0.23; p = 0.05 R = 0.08; p = ns R = 0.009; p = ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabiati, M.; Fontanini, M.; Giacomarra, M.; Politano, G.; Randazzo, E.; Peroni, D.; Federico, G.; Del Ry, S. Screening and Identification of Putative Long Non-Coding RNA in Childhood Obesity: Evaluation of Their Transcriptional Levels. Biomedicines 2022, 10, 529. https://doi.org/10.3390/biomedicines10030529
Cabiati M, Fontanini M, Giacomarra M, Politano G, Randazzo E, Peroni D, Federico G, Del Ry S. Screening and Identification of Putative Long Non-Coding RNA in Childhood Obesity: Evaluation of Their Transcriptional Levels. Biomedicines. 2022; 10(3):529. https://doi.org/10.3390/biomedicines10030529
Chicago/Turabian StyleCabiati, Manuela, Martina Fontanini, Manuel Giacomarra, Gianfranco Politano, Emioli Randazzo, Diego Peroni, Giovanni Federico, and Silvia Del Ry. 2022. "Screening and Identification of Putative Long Non-Coding RNA in Childhood Obesity: Evaluation of Their Transcriptional Levels" Biomedicines 10, no. 3: 529. https://doi.org/10.3390/biomedicines10030529
APA StyleCabiati, M., Fontanini, M., Giacomarra, M., Politano, G., Randazzo, E., Peroni, D., Federico, G., & Del Ry, S. (2022). Screening and Identification of Putative Long Non-Coding RNA in Childhood Obesity: Evaluation of Their Transcriptional Levels. Biomedicines, 10(3), 529. https://doi.org/10.3390/biomedicines10030529