Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Estimation of Immune Cell Composition
2.3. Measurement of Cytokines in Plasma from Patients in Intensive Care Units
2.4. Statistical Analysis
3. Results
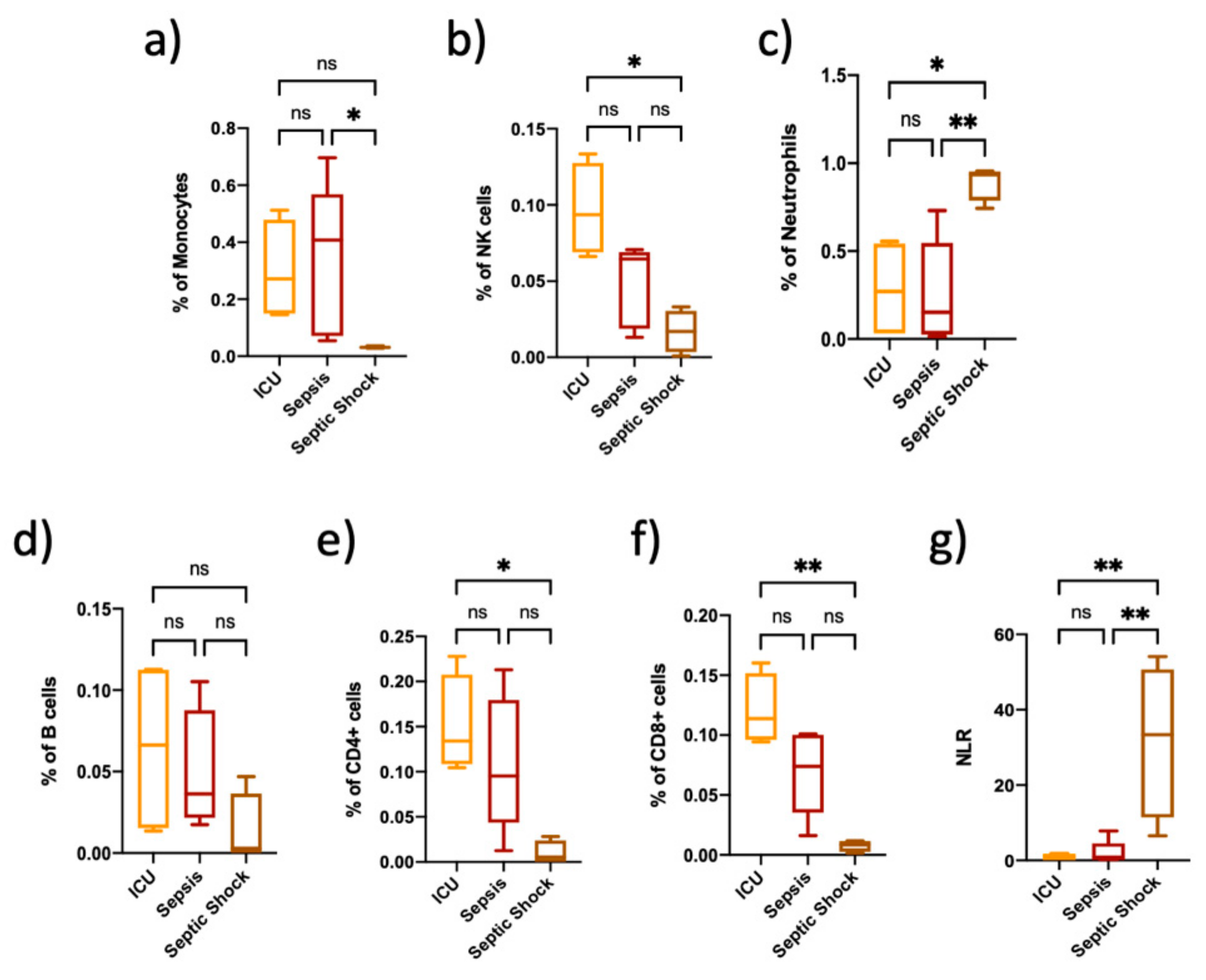
3.1. Immune Cell Proportions Are Different among Critically Ill Non-Septic Patients, Sepsis and Septic Shock Patients
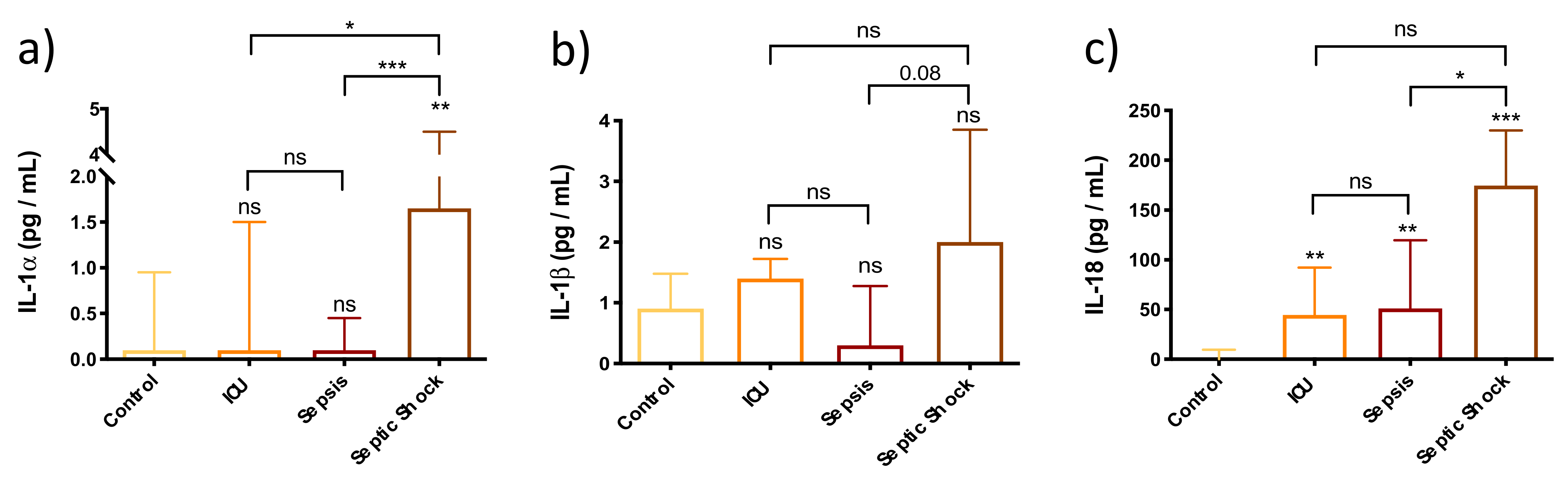
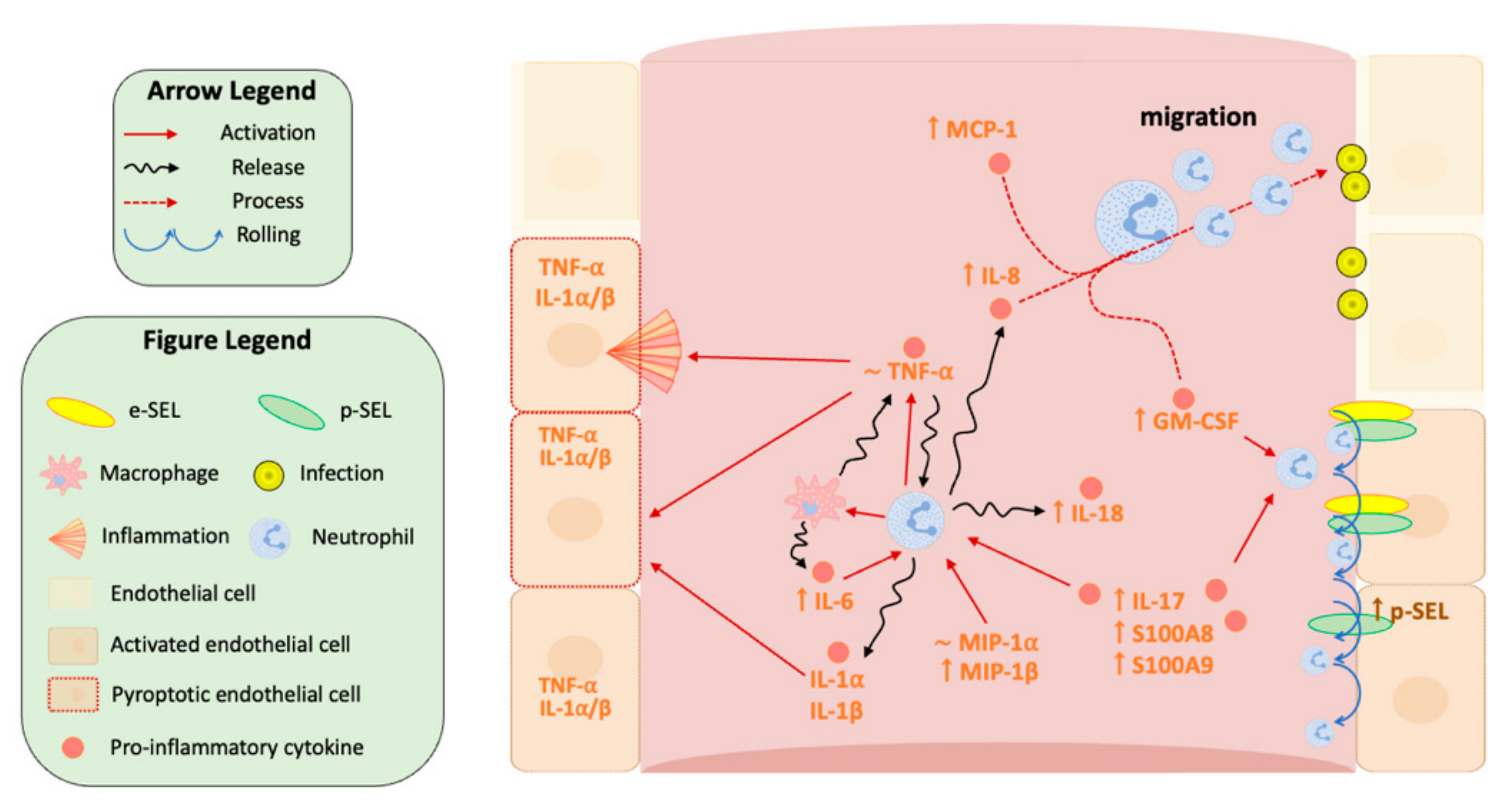
3.2. Enhancement of Pyroptosis-Related Interleukins in Sepsis and Septic Shock Patients
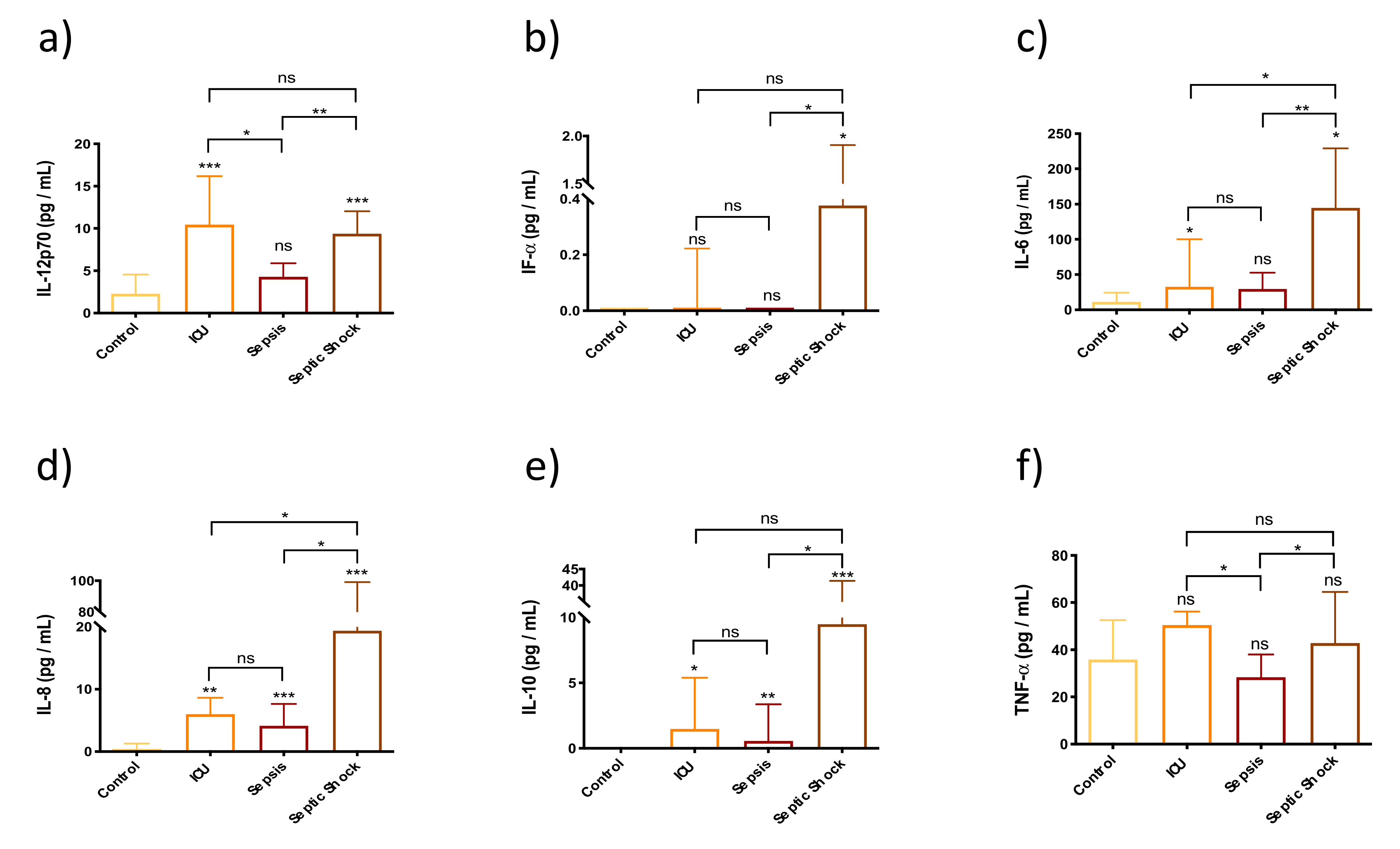
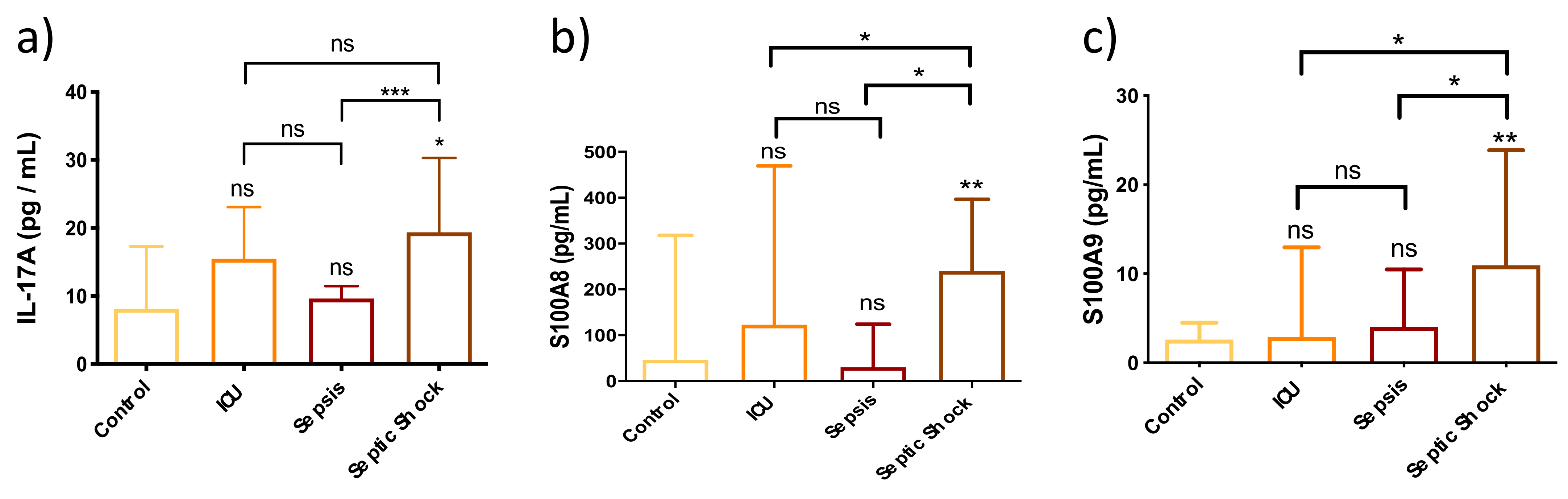
3.3. Innate Immunity Is Overactivated in Septic Shock Patients at Early Stages of the Septic Episode
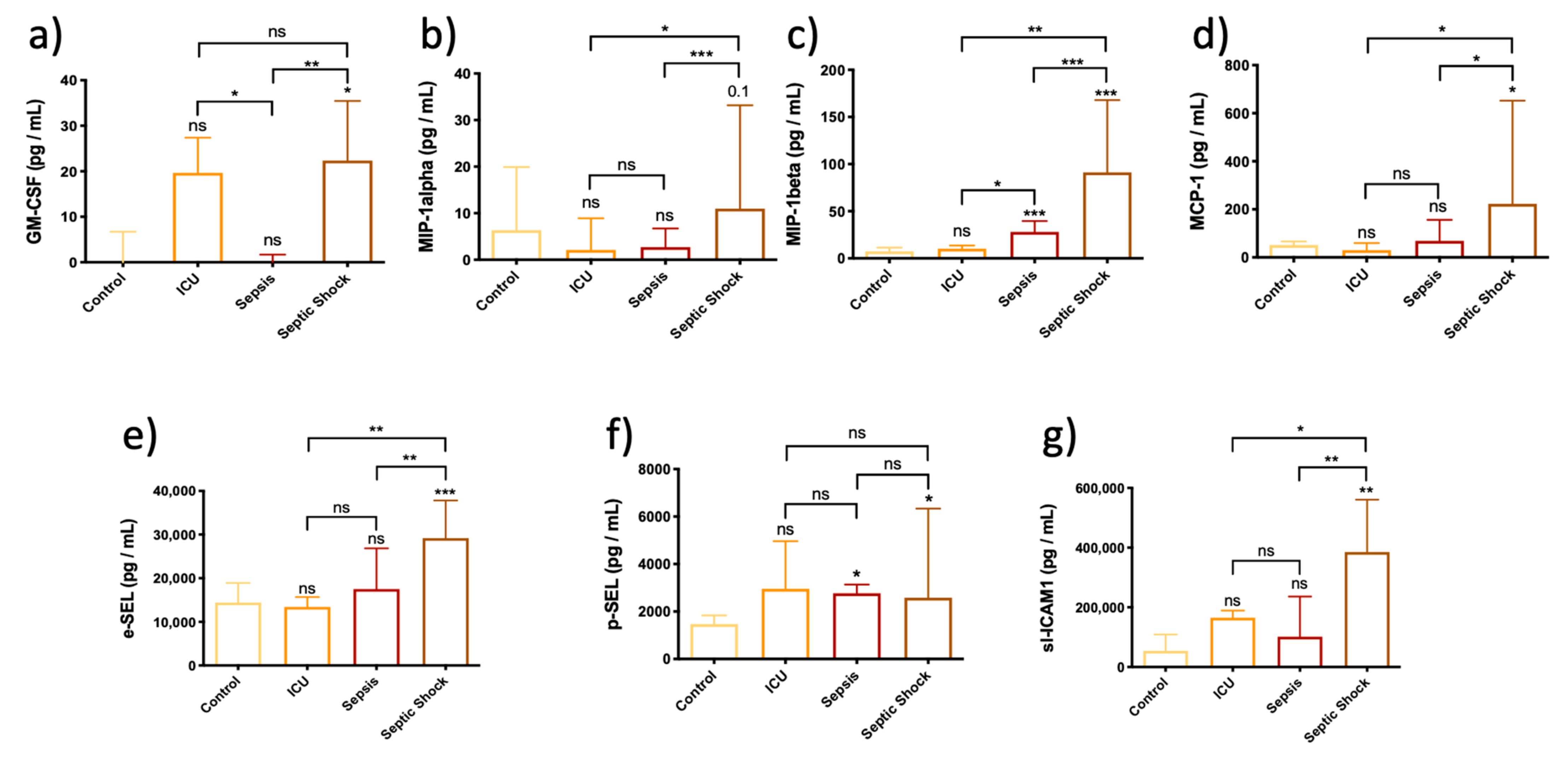
3.4. Chemokines Are Overexpressed in Plasma from Septic Shock Patients, Thereby Altering Endothelial-Mediated Immune Response
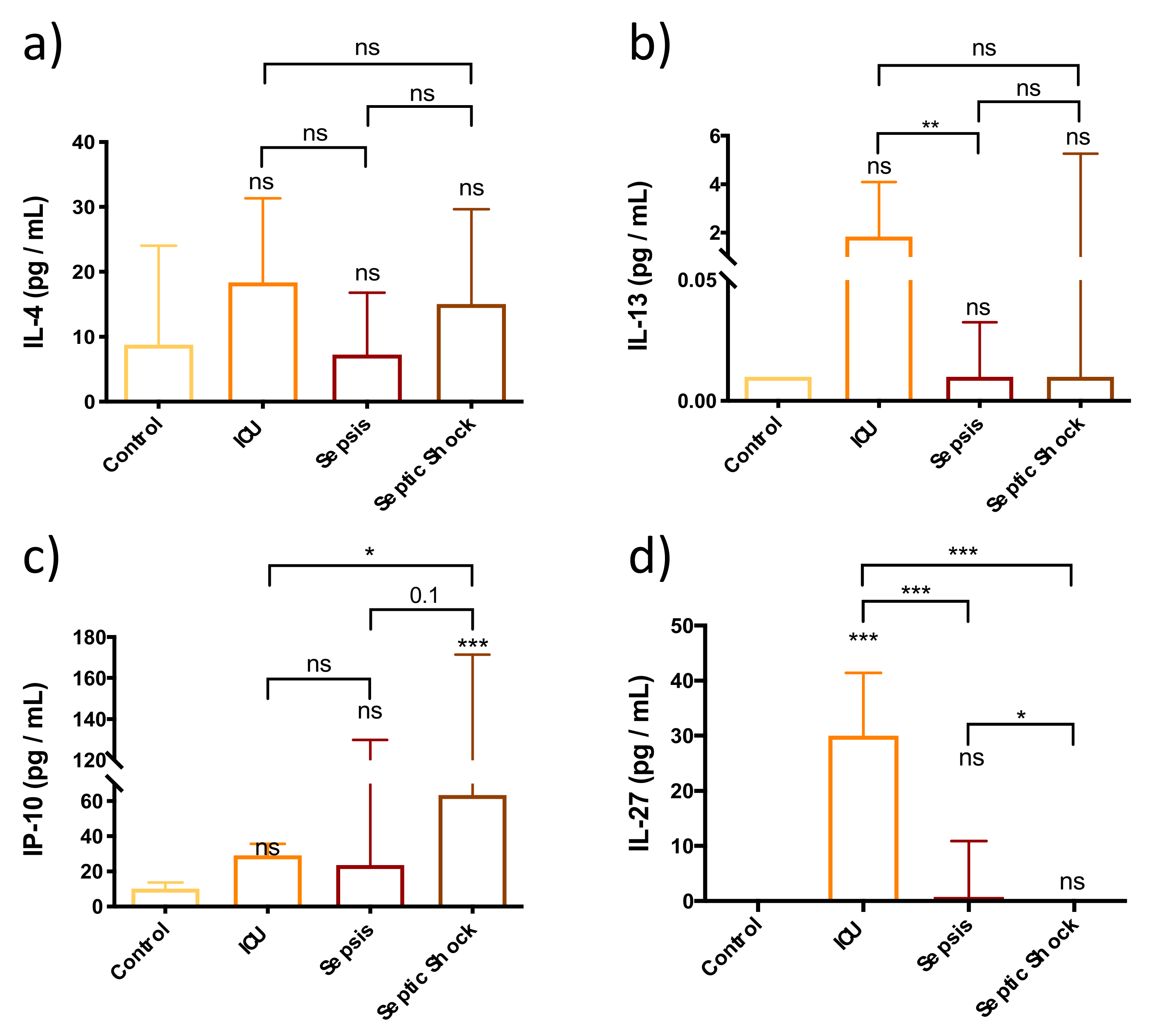
3.5. Adaptive Immune Response Is Not Fully Functional in the First Stages of Septic Shock Development
3.6. Immunomodulators Are Strongly Overexpressed in Septic Shock Patients
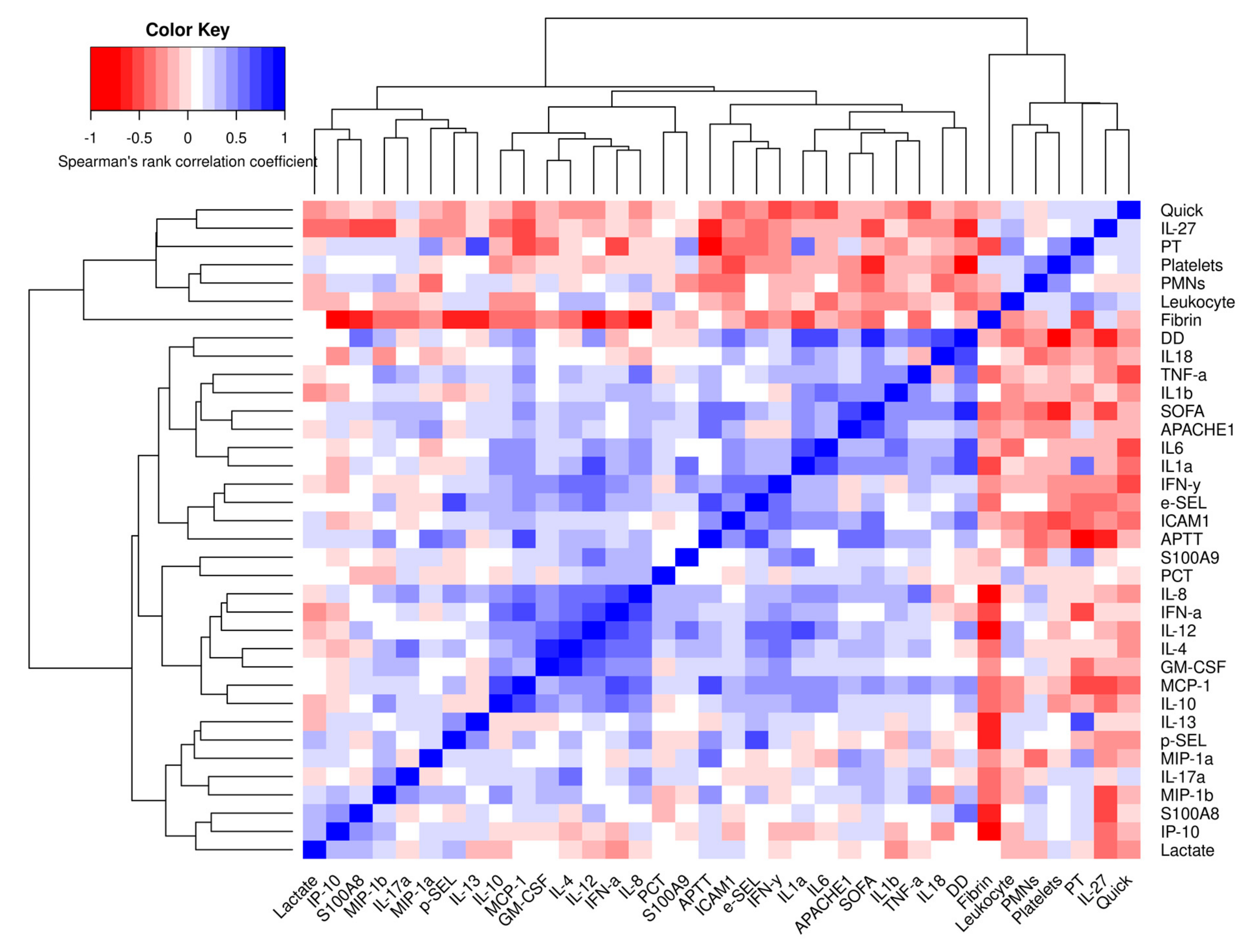
3.7. Correlations of Different Cytokines and Immune Mediators
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3). J. Am. Med. Assoc. 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. Increasing awareness of sepsis: World Sepsis Day. Crit. Care 2012, 16, 152. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Romá-Mateo, C.; Carbonell, N.; Ferreres, J.; Rodríguez, M.; Mulet, S.; García-López, E.; Pallardó, F.V.; García-Giménez, J.L. Epigenetic biomarkers for human sepsis and septic shock: Insights from immunosuppression. Epigenomics 2020, 12, 617–646. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating Cytokine/Inhibitor Profiles Reshape the Understanding of the SIRS/CARS Continuum in Sepsis and Predict Mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef]
- Xiao, W.; Mindrinos, M.N.; Seok, J.; Cuschieri, J.; Cuenca, A.G.; Gao, H.; Hayden, D.L.; Hennessy, L.; Moore, E.E.; Minei, J.P.; et al. A genomic storm in critically injured humans. J. Exp. Med. 2011, 208, 2581–2590. [Google Scholar] [CrossRef]
- Vught, L.A.; Klouwenberg, P.M.C.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nürnberg, P.; Bonten, M.J.M.; Cremer, O.L.; et al. Incidence, risk factors and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 2016, 315, 1469. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Coopersmith, C.M.; McDunn, J.E.; Ferguson, T.A. The sepsis seesaw: Tilting toward immunosuppression. Nat. Med. 2009, 15, 496–497. [Google Scholar] [CrossRef]
- Dalli, J.; Chiang, N.; Serhan, C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 2015, 21, 1071–1075. [Google Scholar] [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Tschaikowsky, K.; Hedwig-Geissing, M.; Schiele, A.; Bremer, F.; Schywalsky, M.; Schüttler, J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit. Care Med. 2002, 30, 1015–1023. [Google Scholar]
- Pinsky, M.R. Sepsis: A pro- and anti-inflammatory disequilibrium syndrome. Contrib. Nephrol. 2001, 132, 354–366. [Google Scholar]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Nelson, J.E.; Cox, C.E.; Hope, A.A.; Carson, S.S. Chronic Critical Illness. Am. J. Respir. Crit. Care Med. 2010, 182, 446–454. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent inflammatory, immunosuppressed, catabolic syndrome (PICS): A new phenotype of multiple organ failure. J. Adv. Nutr. Hum. Metab. 2015, 1, e784. [Google Scholar]
- Nakamura, K.; Ogura, K.; Nakano, H.; Naraba, H.; Takahashi, Y.; Sonoo, T.; Hashimoto, H.; Morimura, N. C-reactive protein clustering to clarify persistent inflammation, immunosuppression and catabolism syndrome. Intensive Care Med. 2020, 46, 437–443. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Vanzant, E.L.; Efron, P.A.; McKinley, B.; Moore, F.; Moldawer, L.L. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods 2013, 61, 3–9. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation Is a Necessary Evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Lorente-Pozo, S.; Navarrete, P.; Garzón, M.J.; Lara-Cantón, I.; Beltrán-García, J.; Osca-Verdegal, R.; Mena-Mollá, S.; García-López, E.; Vento, M.; Pallardó, F.V.; et al. DNA Methylation Analysis to Unravel Altered Genetic Pathways Underlying Early Onset and Late Onset Neonatal Sepsis. A Pilot Study. Front. Immunol. 2021, 12, 622599. [Google Scholar] [CrossRef]
- Fortin, J.P.; Labbe, A.; Lemire, M.; Zanke, B.W.; Hudson, T.J.; Fertig, E.J.; Greenwood, C.M.T.; Hansen, K.D. Functional normalization of 450k methylation array data improves replication in large cancer studies. Genome Biol. 2014, 15, 503. [Google Scholar] [CrossRef]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef]
- Salas, L.A.; Koestler, D.C.; Butler, R.A.; Hansen, H.M.; Wiencke, J.K.; Kelsey, K.T.; Christensen, B.C. An optimized library for reference-based deconvolution of whole-blood biospecimens assayed using the Illumina HumanMethylationEPIC BeadArray. Genome Biol. 2018, 19, 64. [Google Scholar] [CrossRef]
- Koestler, D.C.; Jones, M.J.; Usset, J.; Christensen, B.C.; Butler, R.A.; Kobor, M.S.; Wiencke, J.K.; Kelsey, K.T. Improving cell mixture deconvolution by identifying optimal DNA methylation libraries (IDOL). BMC Bioinform. 2016, 17, 120. [Google Scholar]
- Terradas, R.; Grau, S.; Blanch, J.; Riu, M.; Saballs, P.; Castells, X.; Horcajada, J.P.; Knobel, H. Eosinophil Count and Neutrophil-Lymphocyte Count Ratio as Prognostic Markers in Patients with Bacteremia: A Retrospective Cohort Study. PLoS ONE 2012, 7, e42860. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M.; Farber, J. Cytokines. In Rheumatology: Sixth Edition; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1–2, pp. 99–112. ISBN 9780323325851. [Google Scholar]
- Kwaa, A.K.R.; Talana, C.A.G.; Blankson, J.N. Interferon Alpha Enhances NK Cell Function and the Suppressive Capacity of HIV-Specific CD8 + T Cells. J. Virol. 2018, 93, e01541-18. [Google Scholar] [CrossRef] [PubMed]
- Bellora, F.; Castriconi, R.; Doni, A.; Cantoni, C.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur. J. Immunol. 2012, 42, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Bellora, F.; Castriconi, R.; Dondero, A.; Carrega, P.; Mantovani, A.; Ferlazzo, G.; Moretta, A.; Bottino, C. Human NK cells and NK receptors. Immunol. Lett. 2014, 161, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Wang, J.; Lao, X.; Wang, J.; Li, L.; Li, S.; Zhang, J.; Dong, Y.; Chang, A.E.; Li, Q.; et al. Interleukin-6 Inhibits Regulatory T Cells and Improves the Proliferation and Cytotoxic Activity of Cytokine-induced Killer Cells. J. Immunother. 2012, 35, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, B. Neutrophil pyroptosis: New perspectives on sepsis. Cell. Mol. Life Sci. 2019, 76, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H.; et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 778. [Google Scholar] [CrossRef]
- Bai, B.; Yang, Y.; Wang, Q.; Li, M.; Tian, C.; Liu, Y.; Aung, L.H.H.; Li, P.; Yu, T.; Chu, X. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 2020, 11, 776. [Google Scholar] [CrossRef]
- Wu, C.; Lu, W.; Zhang, Y.; Zhang, G.; Shi, X.; Hisada, Y.; Grover, S.P.; Zhang, X.; Li, L.; Xiang, B.; et al. Inflammasome Activation Triggers Blood Clotting and Host Death through Pyroptosis. Immunity 2019, 50, 1401–1411. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Jian, C.; Wu, C.; Mackman, N.; Smyth, S.; Wei, Y.; Li, Z. Inflammasome Activation Promotes Deep Vein Thrombosis through Pyroptosis. Blood 2019, 134, 3644. [Google Scholar] [CrossRef]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74, ftw111. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Chen, J.; Liao, M.Y.; Gao, X.L.; Zhong, Q.; Tang, T.T.; Yu, X.; Liao, Y.H.; Cheng, X. IL-17A induces pro-inflammatory cytokines production in macrophages via MAPKinases, NF-κB and AP-1. Cell. Physiol. Biochem. 2013, 32, 1265–1274. [Google Scholar] [CrossRef]
- Liu, R.; Lauridsen, H.M.; Amezquita, R.A.; Pierce, R.W.; Jane-wit, D.; Fang, C.; Pellowe, A.S.; Kirkiles-Smith, N.C.; Gonzalez, A.L.; Pober, J.S. IL-17 Promotes Neutrophil-Mediated Immunity by Activating Microvascular Pericytes and Not Endothelium. J. Immunol. 2016, 197, 2400–2408. [Google Scholar] [CrossRef]
- Granger, D.N.; Senchenkova, E. Leukocyte–Endothelial Cell Adhesion. In Inflammation and the Microcirculation; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Yoshida, H.; Hamano, S.; Senaldi, G.; Covey, T.; Faggioni, R.; Mu, S.; Xia, M.; Wakeham, A.C.; Nishina, H.; Potter, J.; et al. WSX-1 Is Required for the Initiation of Th1 Responses and Resistance to L. major Infection. Immunity 2001, 15, 569–578. [Google Scholar] [CrossRef]
- Chen, Q.; Ghilardi, N.; Wang, H.; Baker, T.; Xie, M.-H.; Gurney, A.; Grewal, I.S.; de Sauvage, F.J. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 2000, 407, 916–920. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a Heterodimeric Cytokine Composed of EBI3 and p28 Protein, Induces Proliferation of Naive CD4+ T Cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Laurence, A.; Wilson, E.H.; Huang, E.; Tato, C.M.; Johnson, L.M.; Villarino, A.V.; Huang, Q.; Yoshimura, A.; Sehy, D.; et al. Interleukin 27 negatively regulates the development of interleukin 17–producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006, 7, 937–945. [Google Scholar] [CrossRef]
- Batten, M.; Li, J.; Yi, S.; Kljavin, N.M.; Danilenko, D.M.; Lucas, S.; Lee, J.; de Sauvage, F.J.; Ghilardi, N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17–producing T cells. Nat. Immunol. 2006, 7, 929–936. [Google Scholar] [CrossRef]
- Colgan, J.; Rothman, P. All in the family: IL-27 suppression of TH-17 cells. Nat. Immunol. 2006, 7, 899–901. [Google Scholar] [CrossRef]
- Wirtz, S.; Tubbe, I.; Galle, P.R.; Schild, H.J.; Birkenbach, M.; Blumberg, R.S.; Neurath, M.F. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J. Exp. Med. 2006, 203, 1875–1881. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Yao, Y.; Zhao, D.; Liu, S. Interleukin-27 as a Diagnostic Biomarker for Patients with Sepsis: A Meta-Analysis. Biomed Res. Int. 2021, 2021, 5516940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ting, S.-M.; Liu, C.-H.; Sun, G.; Kruzel, M.; Roy-O’Reilly, M.; Aronowski, J. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat. Commun. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, S.; Lim, S.Y.; Muschel, R.J.; Brunner, T.B. IP-10/CXCL10 attracts regulatory T cells: Implication for pancreatic cancer. Oncoimmunology 2015, 4, e1027473. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent inflammation, immunosuppression, and catabolism: Evolution of multiple organ dysfunction. Surg. Infect. 2016, 17, 167–172. [Google Scholar] [CrossRef]
- Opp, M.R.; Smith, E.M.; Hughes, T.K. Interleukin-10 (cytokine synthesis inhibitory factor) acts in the central nervous system of rats to reduce sleep. J. Neuroimmunol. 1995, 60, 165–168. [Google Scholar] [CrossRef]
- Dai, J.; Kumbhare, A.; Youssef, D.; McCall, C.E.; Gazzar, M. El Intracellular S100A9 Promotes Myeloid-Derived Suppressor Cells during Late Sepsis. Front. Immunol. 2017, 8, 1565. [Google Scholar] [CrossRef]
- Muller, W.A. Getting Leukocytes to the Site of Inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef]
- Yanaba, K.; Kaburagi, Y.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. Relative Contributions of Selectins and Intercellular Adhesion Molecule-1 to Tissue Injury Induced by Immune Complex Deposition. Am. J. Pathol. 2003, 162, 1463–1473. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Naldini, A.; Aarden, L.; Pucci, A.; Bernini, C.; Carraro, F. Inhibition of interleukin-12 expression by α -thrombin in human peripheral blood mononuclear cells: A potential mechanism for modulating Th1/Th2 responses. Br. J. Pharmacol. 2003, 140, 980–986. [Google Scholar] [CrossRef][Green Version]
- Foley, J.H.; Conway, E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef]
- Evans, C.E.; Spier, A.B.; Zhao, Y.-Y. Sepsis-induced thrombus formation and cell-specific HIFs. Thromb. Res. 2018, 171, 187–189. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Lee, M.K.S.; Al-Sharea, A.; Dragoljevic, D.; Barrett, T.J.; Montenont, E.; Basu, D.; Heywood, S.; Kammoun, H.L.; Flynn, M.; et al. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Investig. 2017, 127, 2133–2147. [Google Scholar] [CrossRef]
- Deguchi, A.; Yamamoto, T.; Shibata, N.; Maru, Y. S100A8 may govern hyper-inflammation in severe COVID-19. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Silva-Filho, J.L.; Caruso-Neves, C.; Pinheiro, A.A.S. IL-4: An important cytokine in determining the fate of T cells. Biophys. Rev. 2014, 6, 111–118. [Google Scholar] [CrossRef]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, A.; Huang, S.; Ding, G.; Pan, X.; Chen, R. Interleukin-13 inhibits cytokines synthesis by blocking nuclear factor-κB and c-Jun N-terminal kinase in human mesangial cells. J. Biomed. Res. 2010, 24, 308–316. [Google Scholar] [CrossRef]
- McKenzie, A.N.; Culpepper, J.A.; de Waal Malefyt, R.; Briere, F.; Punnonen, J.; Aversa, G.; Sato, A.; Dang, W.; Cocks, B.G.; Menon, S. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc. Natl. Acad. Sci. USA 1993, 90, 3735–3739. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
| Participant Characteristics | Control ICU Non-Septic Patients (n = 5) | Sepsis (n = 10) | Septic Shock (n = 15) | p ≤ 0.05 |
|---|---|---|---|---|
| Age (years) | 68 ± 8 | 68 ± 11 | 65 ± 15 | n.s. |
| Male–female ratio | 3:2 | 6:4 | 11:4 | NA |
| APACHE II score | 15 ± 4 | 18 ± 7 | 23 ± 7 | n.s. |
| SOFA score 1st day | 5 ± 3 | 6 ± 2 | 9 ± 3 | 0.004 |
| CRP (mg/L) | 8.2 ± 8.7 | 225.3 ± 153.7 | 277.1 ± 130.5 | 0.003 |
| Procalcitonin (ng/mL) | 0.5 ± 0.8 | 7.4 ± 9.7 | 41.2 ± 32.7 | 0.005 |
| Lactate 1st h (mmol/L) | 1.9 ± 0.3 | 1.9 ± 1.2 | 5.9 ± 4.7 | 0.009 |
| Origin of infection | NA | Gram-positive = 22% Gram-negative = 11% Virus = 0% Others = 0% Not available: 67% | Gram-positive = 40% Gram-negative = 27% Virus = 6% Others = 0% Not available: 27% | NA |
| Body Mass Index | 26.2 ± 2.6 | 28.6 ± 3.9 | 26.1 ± 2.4 | n.s |
| ICU LOS (days) | 4 ± 2 | 10 ± 13 | 7 ± 6 | n.s |
| Hospital LOS (days) | 14 ± 9 | 18 ± 11 | 13 ± 11 | n.s |
| White blood cells | 11,056 ± 4288 | 14,751 ± 12,106 | 14,887 ± 12,042 | n.s |
| Glucose (mg/dL) | 149 ± 26 | 164 ± 58 | 158 ± 60 | n.s |
| Platelet’s count | 279,600 ± 103,919 | 221,500 ± 176,025 | 154866 ± 91357 | n.s |
| Antimicrobial first hour (%) | - | 4 (40%) | 11 (73%) | 0.006 |
| Vasopressor therapy (%) | 1 (20%) | 2 (20%) | 14 (93%) | 0.001 |
| Renal Replacement Therapy (%) | - | - | 4 (27%) | n.s |
| Mechanical Ventilation (%) | 2 (40%) | - | 3 (20%) | n.s |
| D-Dimer | NA | 1377 ± 481 | 5512.75 ± 8045.76 | 0.045 |
| Fibrin | NA | 7.48 ± 2.34 | 5.87 ± 1.71 | n.s |
| Direct WBC count | 12,421.67 ± 5089.37 | 13,566.67 ± 11,868.66 | 14,730.20 ± 12,216.10 | n.s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán-García, J.; Osca-Verdegal, R.; Jávega, B.; Herrera, G.; O’Connor, J.-E.; García-López, E.; Casabó-Vallés, G.; Rodriguez-Gimillo, M.; Ferreres, J.; Carbonell, N.; et al. Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock. Biomedicines 2022, 10, 525. https://doi.org/10.3390/biomedicines10030525
Beltrán-García J, Osca-Verdegal R, Jávega B, Herrera G, O’Connor J-E, García-López E, Casabó-Vallés G, Rodriguez-Gimillo M, Ferreres J, Carbonell N, et al. Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock. Biomedicines. 2022; 10(3):525. https://doi.org/10.3390/biomedicines10030525
Chicago/Turabian StyleBeltrán-García, Jesús, Rebeca Osca-Verdegal, Beatriz Jávega, Guadalupe Herrera, José-Enrique O’Connor, Eva García-López, Germán Casabó-Vallés, María Rodriguez-Gimillo, José Ferreres, Nieves Carbonell, and et al. 2022. "Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock" Biomedicines 10, no. 3: 525. https://doi.org/10.3390/biomedicines10030525
APA StyleBeltrán-García, J., Osca-Verdegal, R., Jávega, B., Herrera, G., O’Connor, J.-E., García-López, E., Casabó-Vallés, G., Rodriguez-Gimillo, M., Ferreres, J., Carbonell, N., Pallardó, F. V., & García-Giménez, J. L. (2022). Characterization of Early Peripheral Immune Responses in Patients with Sepsis and Septic Shock. Biomedicines, 10(3), 525. https://doi.org/10.3390/biomedicines10030525







