Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET
Abstract
:1. Introduction
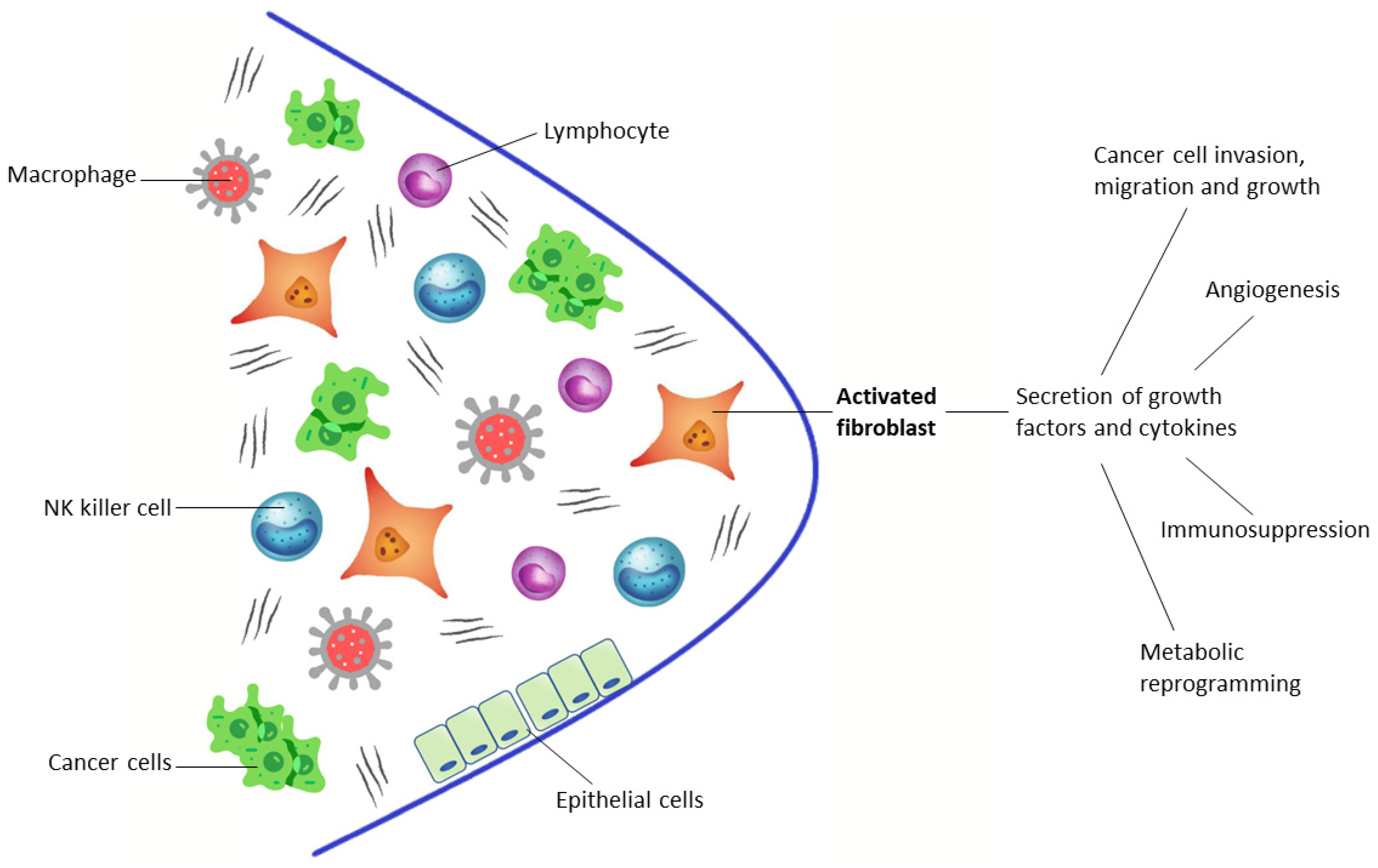
1.1. Targeting the Tumor Microenvironment
- (1)
- A small cluster of homogeneous cancer cells. These tumors evade immune surveillance as they are in a very early stage of development or because they are a newly metastasized colony.
- (2)
- Tumors with lymphocyte infiltration that release cytokines and directly engage with cancer cells, recruiting blood cells to the tumor. At the same time, nearby macrophages and fibroblasts are converted into tumor-associated macrophages (TAMs) and cancer-associated fibroblasts (CAFs).
- (3)
- Tumors without infiltrating lymphocytes, encapsulated by CAFs with ECMs. They are filled with many stromal cells, including TAMs, CAFs, and myeloid-derived suppressor cells (MDSCs) and do not release cancer cells into the blood circulation.
- (4)
- Tumors with a subgroup of cancer cells that undergo epithelial-mesenchymal transition, which downregulate some genes (such as E-cadherin, β-catenin, cytokeratin 5 and 6) and upregulate other genes (such as E-cadherin, vimentin, Snail, Slug, Twist, ZEB1 and 2, S100A4, MMP2 and 3, α-smooth muscle actin), so these tumors become metastasized, activating mobility-enhancing genes (such as S100 CBPP) and releasing cancer cells into the blood circulation that are often chaperoned by stromal cells.
1.2. Developing PET Radiopharmaceuticals
1.3. Article Selection and Data Extraction
2. Clinical Application of Fapi Pet in Oncology
2.1. Preclinical Studies
2.2. Pan-Tumoral Radiotracer
2.3. Gliomas, Primary Liver Cancer, and Gastro-Entero-Pancreatic Cancers
2.4. Head and Neck Cancers
| Authors | n of Patients | Tumor Type | Clinical Setting | Injected Activity | Acquisition Timing | Image Analyses | Reference Standard | 68Ga-FAPI Performance | Highest FAPi Uptake | Lowest FAPi Uptake |
|---|---|---|---|---|---|---|---|---|---|---|
| Mona CE et al. [22] | 141 | Various cancer (14 types) | Biodistribution and kinetics | 174–185 MBq | 54–96 min | S | HP | SE 80.9% | Bile duct, bladder, colon, esophagus, stomach, lung, oropharynx, ovary and pancreas cancer | Liver, prostate, and renal cell cancer |
| Kratoch wil C et al. [15] | 80 | Various cancer (28 types) | Staging, Restaging, RT planning | 122–312 MBq | 60 min | S | HP, imaging follow-up | Low uptake (≤6): 7/28 TT; Mean uptake (6 > SUV < 12): 14/28 TT; High Uptake (≥12): 7/28 TT | Lung, breast and esophageal cancer, cholangiocellular carcinoma and sarcoma (SUVmax ≥ 12) | Pheochromocytoma, renal cell, differentiated thyroid, adenoid cystic and gastric cancer (SUVmax ≤ 6) |
| Chen H et al. [32] | 68 | Various cancer (13 types) | Staging, Restaging | 1.8–2.2 MBq/Kg | 60 min | V, S | HP, imaging and clinical follow-up | T: SE 86.4% | T: liver, gastric, pancreatic and cervical cancer | T: oroesophageal and lung cancer |
| Chen H et al. [33] | 75 | Various cancer (12 types) | Staging, Restaging | 1.8–2.2 MBq/Kg | 60 min | V, S | HP | T: SE 98.2% | Pancreatic, liver and oroesophageal cancers, sarcoma and cholangiocarcinoma (SUVmax ≥ 12) | Brain cancer |
| N: SE 86.4%, SP 58.8% | ||||||||||
| M: SE 83.8%, SP 41.7% |
| Authors | n of Patients | Tumor Type | Clinical Setting | Injected Activity | Acquisition Timing | Image Analyses | Reference Standard | 68Ga-FAPI Performance | 18F-FDG Performance | MRI Performance |
|---|---|---|---|---|---|---|---|---|---|---|
| Röhrich M et al. [23] | 18 | Gliomas | Staging, Restaging | 150–250 MBq | 30 min (FA-Pi04); 10, 60 and 180 min (FAPi-02) | S | MRI | SE 83.3% | - | SE 100% |
| Windisch P et al. [24] | 13 | GBM | RT planning | 150–250 MBq | 30 min | S | MRI | SE 100% | - | SE 100% |
| Guo W et al. [26] | 34 | Hepatic nodules | Staging | 148–259 MBq | 60 min | V, S | HP, imaging follow-up | SE 87.4% | SE 64.9% | - |
| Şahin E et al. [27] | 31 | GEP | Staging and follow-up after treatment | 2–3 MBq/Kg | 45 min | V, S | Imaging follow-up, tumor biomarker findings, HP | SE 93.5% (patient based) | SE 71% (patient based) | - |
| SE 95.9% (lesion based) | SE 79.6% (lesion based) | - | ||||||||
| Pang Y et al. [28] | 35 | GI tract | Staging, Restaging | 1.8–2.2 MBq/Kg | 60 min | V, S | HP | SE 100% | SE 43.8% | - |
| T: SE 100% | T: SE 52.6% | - | ||||||||
| N: SE 78.6%, SP 82.1% | N: SE 53.6%, SP 89.3% | - | ||||||||
| M: SE 88.6%, SP 28.6% | M: SE 57.1%, SP 85.7% | - | ||||||||
| Jiang D et al. [29] | 38 | Gastric cancer | Staging | 111–185 MBq | 60 min | S | HP | T: SE 100% | T: SE 75% | - |
| N: SE 60%, SP 92.9% | N: SE 50%, SP 92.9% | - |
| Authors | n of Patients | Tumor Type | Clinical Setting | Injected Activity | Acquisition Timing | Image Analyses | Reference Standard | 68Ga-FAPI Performance | 18F-FDG Performance | MRI Performance |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhao L et al. [30] | 45 | Nasopharyngeal carcinoma | Staging, Restaging | 1.8–2.2 MBq/Kg | 40 min | V, S | HP, imaging follow-up | T: SE 86.7% | T: SE 84.4% | - |
| N: SE 95% | N: SE 75.2% | N: SE 97.5% | ||||||||
| Qin C et al. [31] | 15 | Nasopharyngeal carcinoma | Staging, Restaging | 1.85–3.7 MBq/Kg | 30–60 min | V, S | MRI | T: SE 100% | T: SE 100% | - |
| N: SE 48% | N: SE 100% | - | ||||||||
| M: SE 100% | M: SE 0% | - | ||||||||
| Chen H et al. [32] | 68 | Various cancer (13 types) | Staging, Restaging | 1.8–2.2 MBq/Kg | 60 min | V, S | HP, imaging and clinical follow-up | T: SE 86.4% | - | - |
| Chen H et al. [33] | 75 | Various cancer (12 types) | Staging, Restaging | 1.8–2.2 MBq/Kg | 60 min | V, S | HP | T: SE 98.2% | T: SE 82.1% | - |
| N: SE 86.4%, SP 58.8% | N: SE 45.5%, SP 76.5% | - | ||||||||
| M: SE 83.8%, SP 41.7% | M: SE 59.5%, SP 58.3% | - |
2.5. Breast Cancer
2.6. Peritoneal Carcinomatosis
3. Radioligand Therapy Targeting the Tumor Micro-Environment
4. Clinical Application of Fapi Pet in Non-Oncological Disease
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Perkins, G. Animal models for studying tumor microenvironment (TME) and resistance to lymphocytic infiltration. Cancer Biol. Ther. 2018, 18, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurose, K.; Gilley, K.; Matsumoto, S.; Watson, P.H.; Zhou, X.P.; Eng, C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat. Genet. 2002, 32, 355–357, Erratum in Nat. Genet. 2002, 32, 681. [Google Scholar] [CrossRef] [PubMed]
- Fukino, K.; Shen, L.; Patocs, A.; Mutter, G.L.; Eng, C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA 2007, 297, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Raj, B.K.; Calvo, B.; Garin-Chesa, P.; Sanz-Moncasi, M.P.; Healey, J.H.; Old, L.J.; Rettig, W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA 1994, 91, 5657–5661. [Google Scholar] [CrossRef] [Green Version]
- Busek, P.; Hrabal, P.; Fric, P.; Sedo, A. Co-expression of the homologous proteases fibroblast activation protein and dipeptidyl peptidase-IV in the adult human Langerhans islets. Histochem. Cell Biol. 2015, 143, 497–504. [Google Scholar] [CrossRef]
- Bae, S.; Park, C.W.; Son, H.K.; Ju, H.K.; Paik, D.; Jeon, C.J.; Koh, G.Y.; Kim, J.; Kim, H. Fibroblast activation protein alpha identifies mesenchymal stromal cells from human bone marrow. Br. J. Haematol. 2008, 142, 827–830. [Google Scholar] [CrossRef]
- Schuberth, P.C.; Hagedorn, C.; Jensen, S.M.; Gulati, P.; van den Broek, M.; Mischo, A.; Soltermann, A.; Jungel, A.; Belaunzaran, O.M.; Stahel, R.; et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J. Transl. Med. 2013, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Hamson, E.J.; Keane, F.M.; Tholen, S.; Schilling, O.; Gorrell, M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014, 8, 454–463. [Google Scholar] [CrossRef]
- Tillmanns, J.; Hoffmann, D.; Habbaba, Y.; Schmitto, J.D.; Sedding, D.; Fraccarollo, D.; Galuppo, P.; Bauersachs, J. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J. Mol. Cell. Cardiol. 2015, 87, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Waumans, Y.; Baerts, L.; Kehoe, K.; Lambeir, A.M.; De Meester, I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front. Immunol. 2015, 6, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A.; et al. 68Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [Green Version]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT using either 18F-AIF or xold-Kit68 Ga labeling: Biodistribution, radiation dosimetry, and tumor delineation in lung cancer patiens. J. Nucl. Med. 2021, 62, 201–207. [Google Scholar] [CrossRef]
- Sanchez-Crespo, A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl. Radiat. Isot. 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET imaging in cancer patients: Optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur. J. Nucl. Med. Mol. Imaging 2021. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Mona, C.E.; Benz, M.R.; Hikmat, F.; Grogan, T.R.; Lückerath, K.; Razmaria, A.; Riahi, R.; Slavik, R.; Girgis, M.D.; Carlucci, G.; et al. Correlation of 68Ga-FAPi-46 PET biodistribution with FAP expression by immunohistochemistry in patients with solid cancers: A prospective translational exploratory study. J. Nucl. Med. 2021, 121, 262426. [Google Scholar] [CrossRef]
- Röhrich, M.; Loktev, A.; Wefers, A.K.; Altmann, A.; Paech, D.; Adeberg, S.; Windisch, P.; Hielscher, T.; Flechsig, P.; Floca, R.; et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Windisch, P.; Röhrich, M.; Regnery, S.; Tonndorf-Martini, E.; Held, T.; Lang, K.; Bernhardt, D.; Rieken, S.; Giesel, F.; Haberkorn, U.; et al. Fibroblast Activation Protein (FAP) specific PET for advanced target volume delineation in Glioblastoma. Radiother. Oncol. 2020, 150, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Congwei, J.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Comparison of PET imaging of activated fibroblasts and 18F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1593–1603. [Google Scholar] [CrossRef]
- Guo, W.; Pang, Y.; Yao, L.; Zhao, L.; Fan, C.; Ke, J.; Guo, P.; Hao, B.; Fu, H.; Xie, C.; et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [68Ga]Ga-FAPI-04 PET/CT versus MRI and [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1604–1617. [Google Scholar] [CrossRef]
- Şahin, E.; Elboğa, U.; Çelen, Y.Z.; Sever, Ö.N.; Çayırlı, Y.B.; Çimen, U. Comparison of 68Ga-DOTA-FAPI and 18FDG PET/CT imaging modalities in the detection of liver metastases in patients with gastrointestinal system cancer. Eur. J. Radiol. 2021, 142, 109867. [Google Scholar] [CrossRef]
- Pang, Y.; Zhao, L.; Luo, Z.; Hao, B.; Wu, H.; Lin, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology 2021, 298, 393–402. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, X.; You, Z.; Wang, H.; Zhang, X.; Li, X.; Ren, S.; Huang, Q.; Hua, F.; Guan, Y.; et al. Comparison of [68Ga]Ga-FAPI-04 and [18F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: A bicentric retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 732–742. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, Y.; Zheng, H.; Han, C.; Gu, J.; Sun, L.; Wu, H.; Wu, S.; Lin, Q.; Chen, H. Clinical utility of [68Ga]Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography for primary staging and recurrence detection in nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3606–3617. [Google Scholar] [CrossRef]
- Qin, C.; Liu, F.; Huang, J.; Ruan, W.; Liu, Q.; Gai, Y.; Hu, F.; Jiang, D.; Hu, Y.; Yang, K.; et al. A head-to-head comparison of 68Ga-DOTA-FAPI-04 and 18F-FDG PET/MR in patients with nasopharyngeal carcinoma: A prospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Kömek, H.; Can, C.; Güzel, Y.; Oruç, Z.; Gündoğan, C.; Yildirim, Ö.A.; Kaplan, İ.; Erdur, E.; Yıldırım, M.S.; Çakabay, B. 68Ga-FAPI-04 PET/CT, a new step in breast cancer imaging: A comparative pilot study with the 18F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 744–752. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Boutitie, F.; Bereder, J.M.; Quenet, F.; Sideris, L.; Mansvelt, B.; Lorimier, G.; Msika, S.; Elias, D. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Cancer 2010, 116, 5608–5618. [Google Scholar] [CrossRef]
- Fagotti, A.; Fanfani, F.; Rossitto, C.; Lorusso, D.; De Gaetano, A.M.; Giordano, A.; Vizzielli, G.; Scambia, G. A treatment selection protocol for recurrent ovarian cancer patients: The role of FDG-PET/CT and staging laparoscopy. Oncology 2008, 75, 152–158. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.W. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br. J. Radiol. 2018, 91, 20170519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pang, Y.; Luo, Z.; Fu, K.; Yang, T.; Zhao, L.; Sun, L.; Wu, H.; Lin, Q.; Chen, H. Role of [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [18F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, H. 68Ga FAPI PET/CT Imaging in Peritoneal Carcinomatosis. Radiology 2020, 297, 521. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, L.; Chen, H. 68Ga-FAPI Outperforms 18F-FDG PET/CT in Identifying Bone Metastasis and Peritoneal Carcinomatosis in a Patient with Metastatic Breast Cancer. Clin. Nucl. Med. 2020, 45, 913–915. [Google Scholar] [CrossRef]
- Zhao, L.; Pang, Y.; Wei, J.; Hao, B.; Chen, H. Use of 68Ga-FAPI PET/CT for Evaluation of Peritoneal Carcinomatosis Before and After Cytoreductive Surgery. Clin. Nucl. Med. 2021, 46, 491–493. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.U.; et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: A case series of nine patients. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Assadi, M.; Rekabpour, S.J.; Jafari, E.; Divband, G.; Nikkholgh, B.; Amini, H.; Kamali, H.; Ebrahimi, S.; Shakibazad, N.; Jokar, N.; et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients with Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021, 46, e523–e530. [Google Scholar] [CrossRef]
- Baum, R.P.; Schuchardt, C.; Singh, A.; Chantadisai, M.; Robiller, F.C.; Zhang, J.; Mueller, D.; Eismant, A.; Almaguel, F.; Zboralski, D.; et al. Feasibility, Biodistribution and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy (PTRT) of Diverse Adenocarcinomas using 177Lu-FAP-2286: First-in-Human Results. J. Nucl. Med. 2021. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Heckmann, M.B.; Reinhardt, F.; Finke, D.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010628. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Zhang, W. IgG4-related disease revealed by 68Ga-FAPI and 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2625–2626. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, Y.; Zhang, W. Recurrent immunoglobulin G4-related disease shown on 18F-FDG and 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2020, 45, 312–313. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilardi, L.; Airò Farulla, L.S.; Demirci, E.; Clerici, I.; Omodeo Salè, E.; Ceci, F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines 2022, 10, 523. https://doi.org/10.3390/biomedicines10030523
Gilardi L, Airò Farulla LS, Demirci E, Clerici I, Omodeo Salè E, Ceci F. Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines. 2022; 10(3):523. https://doi.org/10.3390/biomedicines10030523
Chicago/Turabian StyleGilardi, Laura, Lighea Simona Airò Farulla, Emre Demirci, Ilaria Clerici, Emanuela Omodeo Salè, and Francesco Ceci. 2022. "Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET" Biomedicines 10, no. 3: 523. https://doi.org/10.3390/biomedicines10030523
APA StyleGilardi, L., Airò Farulla, L. S., Demirci, E., Clerici, I., Omodeo Salè, E., & Ceci, F. (2022). Imaging Cancer-Associated Fibroblasts (CAFs) with FAPi PET. Biomedicines, 10(3), 523. https://doi.org/10.3390/biomedicines10030523







