FCGR2A-HH Gene Variants Encoding the Fc Gamma Receptor for the C-Reactive Protein Are Associated with Enhanced Monocyte CD32 Expression and Cardiovascular Events’ Recurrence after Primary Acute Coronary Syndrome
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Follow-Up and Clinical Endpoint
2.3. Percutaneous Coronary Intervention
2.4. Medication
2.5. Data Collection
2.6. Blood Samples
2.7. Analysis of Soluble hsCRP Circulating Levels
2.8. Flow Cytometry
2.9. Genotyping
2.10. Reporter Analysis of Serum-Mediated Activation of CD32-HH
2.11. Statistical Analysis
3. RESULTS
3.1. Patients’ Characteristics
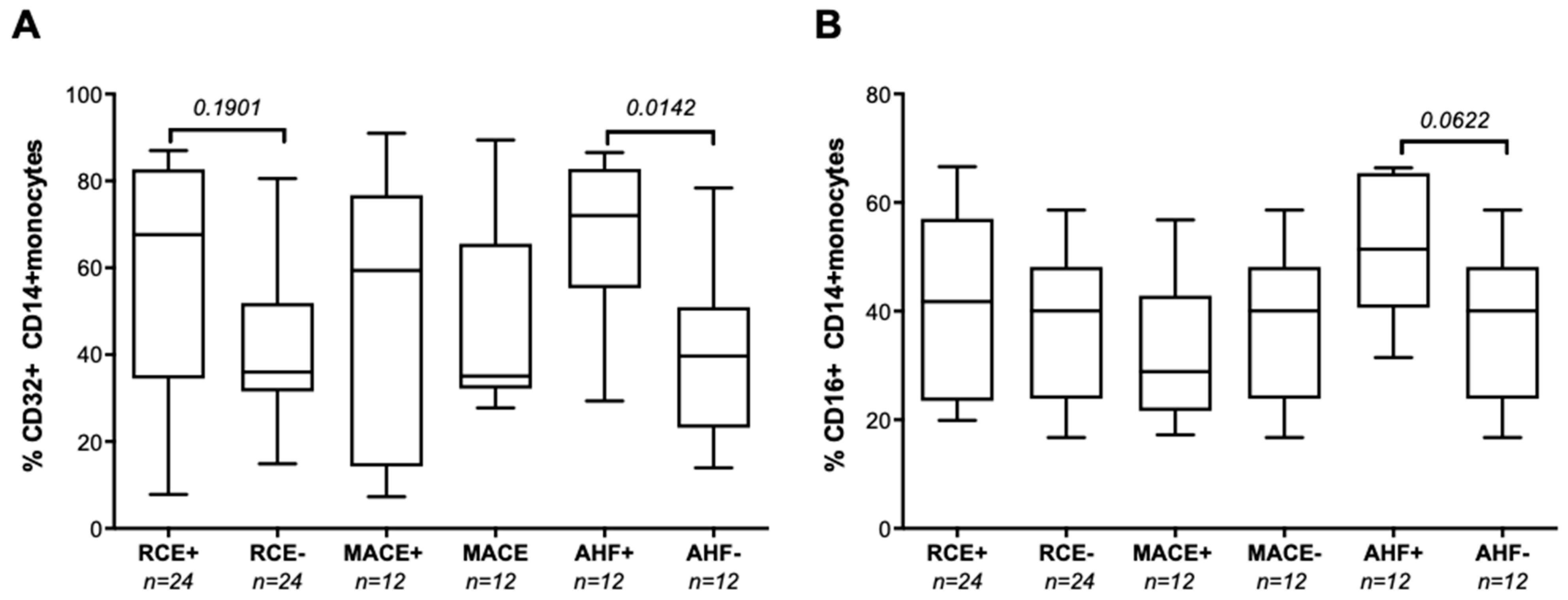
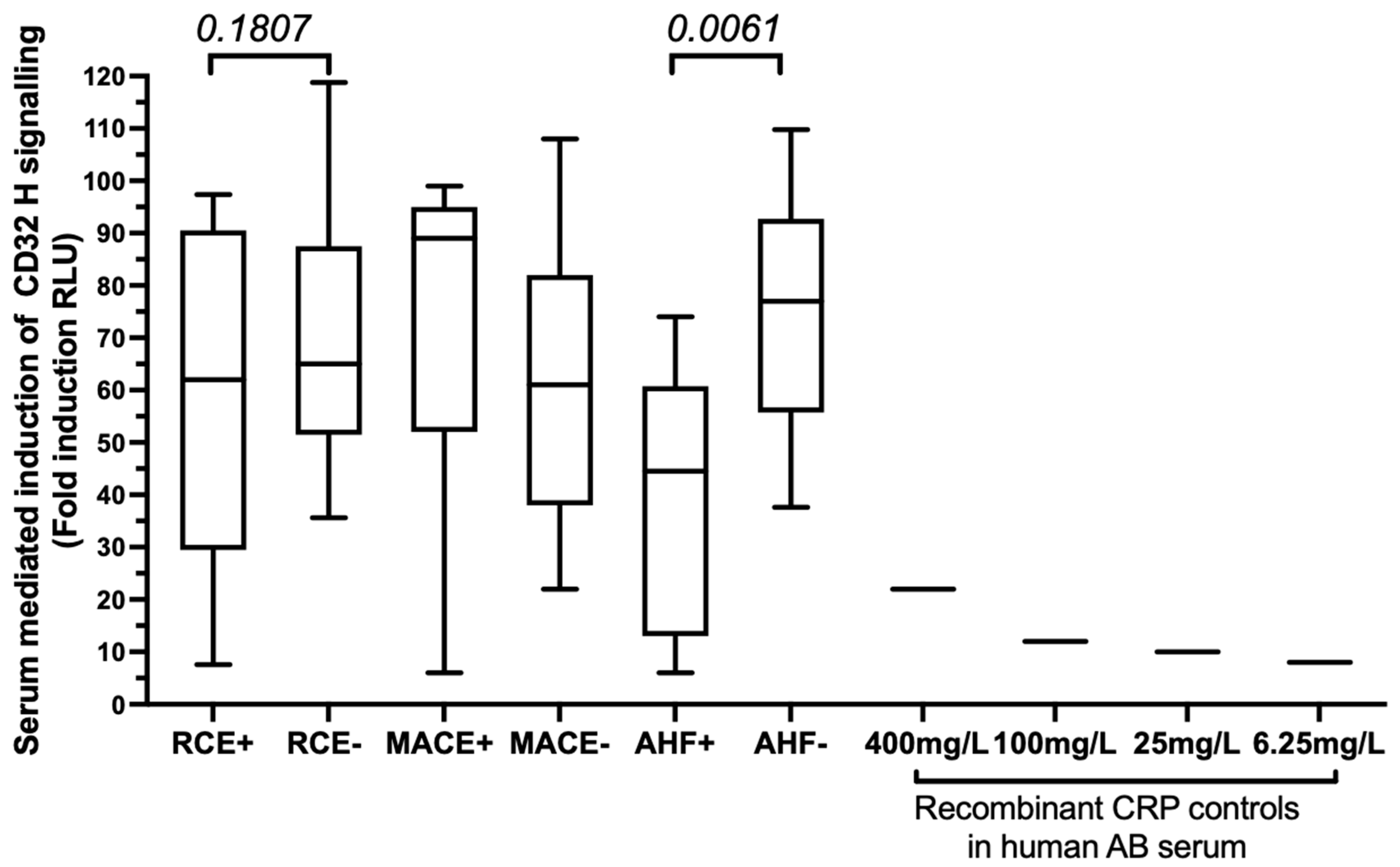
3.2. Baseline hsCRP Circulating Levels Are Associated with Subsequent AHF after PCI
3.3. The FCGR2A rs1801274 HH Genotype Associates with the Subsequent Risk of Developing MACEs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, R.Y.; Zheng, H.; Guo, J.; Redfearn, D.P. Prognostic Value of Plasma Biomarkers in Patients with Acute Coronary Syndrome: A Review of Advances in the Past Decade. Biomark. Med. 2016, 10, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindholm, D.; James, S.K.; Gabrysch, K.; Storey, R.F.; Himmelmann, A.; Cannon, C.P.; Mahaffey, K.W.; Steg, P.G.; Held, C.; Siegbahn, A.; et al. Association of Multiple Biomarkers With Risk of All-Cause and Cause-Specific Mortality After Acute Coronary Syndromes: A Secondary Analysis of the PLATO Biomarker Study. JAMA Cardiol. 2018, 3, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporali, A.; Bäck, M.; Daemen, M.J.; Hoefer, I.E.; Jones, E.A.; Lutgens, E.; Matter, C.M.; Bochaton-Piallat, M.-L.; Siekmann, A.F.; Sluimer, J.C.; et al. Future Directions for Therapeutic Strategies in Post-Ischaemic Vascularization: A Position Paper from European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology. Cardiovasc. Res. 2018, 114, 1411–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qamar, A.; Giugliano, R.P.; Bohula, E.A.; Park, J.-G.; Jarolim, P.; Murphy, S.A.; Blazing, M.A.; Califf, R.M.; Cannon, C.P.; Braunwald, E.; et al. Biomarkers and Clinical Cardiovascular Outcomes With Ezetimibe in the IMPROVE-IT Trial. J. Am. Coll. Cardiol. 2019, 74, 1057–1068. [Google Scholar] [CrossRef]
- Carrero, J.J.; Andersson Franko, M.; Obergfell, A.; Gabrielsen, A.; Jernberg, T. HsCRP Level and the Risk of Death or Recurrent Cardiovascular Events in Patients With Myocardial Infarction: A Healthcare-Based Study. J. Am. Heart Assoc. 2019, 8, e012638. [Google Scholar] [CrossRef]
- Tanigaki, K.; Sundgren, N.; Khera, A.; Vongpatanasin, W.; Mineo, C.; Shaul, P.W. Fcγ Receptors and Ligands and Cardiovascular Disease. Circ. Res. 2015, 116, 368–384. [Google Scholar] [CrossRef] [Green Version]
- Hussain, K.; Hargreaves, C.E.; Rowley, T.F.; Sopp, J.M.; Latham, K.V.; Bhatta, P.; Sherington, J.; Cutler, R.M.; Humphreys, D.P.; Glennie, M.J.; et al. Impact of Human FcγR Gene Polymorphisms on IgG-Triggered Cytokine Release: Critical Importance of Cell Assay Format. Front. Immunol. 2019, 10, 390. [Google Scholar] [CrossRef]
- Clancy, R.; El Bannoudi, H.; Rasmussen, S.E.; Bornkamp, N.; Allen, N.; Dann, R.; Reynolds, H.; Buyon, J.P.; Berger, J.S. Human Low-Affinity IgG Receptor FcγRIIA Polymorphism H131R Associates with Subclinical Atherosclerosis and Increased Platelet Activity in Systemic Lupus Erythematosus. J. Thromb. Haemost. 2019, 17, 532–537. [Google Scholar] [CrossRef]
- Bian, F.; Yang, X.-Y.; Xu, G.; Zheng, T.; Jin, S. CRP-Induced NLRP3 Inflammasome Activation Increases LDL Transcytosis Across Endothelial Cells. Front. Pharmacol. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Kumaresan, P.R.; Jialal, I. C-Reactive Protein Induces Release of Both Endothelial Microparticles and Circulating Endothelial Cells in Vitro and in Vivo: Further Evidence of Endothelial Dysfunction. Clin. Chem. 2011, 57, 1757–1761. [Google Scholar] [CrossRef] [PubMed]
- Staudt, A.; Eichler, P.; Trimpert, C.; Felix, S.B.; Greinacher, A. Fc(Gamma) Receptors IIa on Cardiomyocytes and Their Potential Functional Relevance in Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2007, 49, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Anania, J.C.; Chenoweth, A.M.; Wines, B.D.; Hogarth, P.M. The Human FcγRII (CD32) Family of Leukocyte FcR in Health and Disease. Front. Immunol. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Pedini, P.; Lyonnet, L.; Di Cristofaro, J.; Loundou, A.; Pelardy, M.; Basire, A.; Dignat-George, F.; Chiaroni, J.; Thomas, P.; et al. FCGR3A and FCGR2A Genotypes Differentially Impact Allograft Rejection and Patients’ Survival After Lung Transplant. Front. Immunol. 2019, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Picard, C.; Sampol, E.; Lyonnet, L.; Di Cristofaro, J.; Paul-Delvaux, L.; Lano, G.; Nicolino-Brunet, C.; Ravis, E.; Collart, F.; et al. Genetic and Functional Profiling of CD16-Dependent Natural Killer Activation Identifies Patients at Higher Risk of Cardiac Allograft Vasculopathy. Circulation 2018, 137, 1049–1059. [Google Scholar] [CrossRef]
- Gavasso, S.; Nygård, O.; Pedersen, E.R.; Aarseth, J.H.; Bleie, O.; Myhr, K.-M.; Vedeler, C.A. Fcgamma Receptor IIIA Polymorphism as a Risk-Factor for Coronary Artery Disease. Atherosclerosis 2005, 180, 277–282. [Google Scholar] [CrossRef]
- Li, G.; Gao, L.; Ma, R.; Tian, W.; Mingzhi, L. Associations between FCGR Polymorphisms and Immune Thrombocytopenia: A Meta-Analysis. Scand. J. Immunol. 2019, 89, e12758. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Legris, T.; Picard, C.; Todorova, D.; Lyonnet, L.; Laporte, C.; Dumoulin, C.; Nicolino-Brunet, C.; Daniel, L.; Loundou, A.; Morange, S.; et al. Antibody-Dependent NK Cell Activation Is Associated with Late Kidney Allograft Dysfunction and the Complement-Independent Alloreactive Potential of Donor-Specific Antibodies. Front. Immunol. 2016, 7, 288. [Google Scholar] [CrossRef] [Green Version]
- Garvin, D.; Stecha, P.; Gilden, J.; Wang, J.; Grailer, J.; Hartnett, J.; Fan, F.; Cong, M.; Cheng, Z.J. Determining ADCC Activity of Antibody-Based Therapeutic Molecules Using Two Bioluminescent Reporter-Based Bioassays. Curr. Protoc. 2021, 1, e296. [Google Scholar] [CrossRef] [PubMed]
- Perić, V.S.; Golubović, M.D.; Lazarević, M.V.; Kostić, T.L.; Stokanović, D.S.; Đorđević, M.N.; Marjanović, V.G.; Stošić, M.D.; Milić, D.J. Predictive Potential of Biomarkers and Risk Scores for Major Adverse Cardiac Events in Elderly Patients Undergoing Major Elective Vascular Surgery. Rev. Cardiovasc. Med. 2021, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Kažukauskienė, I.; Baltrūnienė, V.; Rinkūnaitė, I.; Žurauskas, E.; Vitkus, D.; Maneikienė, V.V.; Ručinskas, K.; Grabauskienė, V. Inflammation-Related Biomarkers Are Associated with Heart Failure Severity and Poor Clinical Outcomes in Patients with Non-Ischemic Dilated Cardiomyopathy. Life 2021, 11, 1006. [Google Scholar] [CrossRef]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front. Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef] [PubMed]
- Verwijmeren, L.; Bosma, M.; Vernooij, L.M.; Linde, E.M.; Dijkstra, I.M.; Daeter, E.J.; Van Dongen, E.P.A.; Van Klei, W.A.; Noordzij, P.G. Associations Between Preoperative Biomarkers and Cardiac Surgery-Associated Acute Kidney Injury in Elderly Patients: A Cohort Study. Anesth. Analg. 2021, 133, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramie, J.J.; Barber, J.L.; Lloyd-Jones, D.M.; Gross, M.D.; Rana, J.S.; Sidney, S.; Jacobs, D.R.; Lane-Cordova, A.D.; Sarzynski, M.A. Cardiovascular Health Trajectories and Elevated C-Reactive Protein: The CARDIA Study. J. Am. Heart Assoc. 2021, 10, e019725. [Google Scholar] [CrossRef]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.-Y. C-Reactive Protein and Ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. 1), 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Avan, A.; Tavakoly Sany, S.B.; Ghayour-Mobarhan, M.; Rahimi, H.R.; Tajfard, M.; Ferns, G. Serum C-Reactive Protein in the Prediction of Cardiovascular Diseases: Overview of the Latest Clinical Studies and Public Health Practice. J. Cell Physiol. 2018, 233, 8508–8525. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; Roubenoff, R.; Dinarello, C.A.; Harris, T.; Benjamin, E.J.; Sawyer, D.B.; Levy, D.; Wilson, P.W.F.; D’Agostino, R.B.; et al. Inflammatory Markers and Risk of Heart Failure in Elderly Subjects without Prior Myocardial Infarction: The Framingham Heart Study. Circulation 2003, 107, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, A.; Kayser, S.; Brunner, P.; Vogt, B. C-Reactive Protein Triggers Cell Death in Ischemic Cells. Front. Immunol. 2021, 12, 630430. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Yun, J.-M.; Duncan-Staley, C.; Jialal, I. C-Reactive Protein Induces M-CSF Release and Macrophage Proliferation. J. Leukoc. Biol. 2009, 85, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. C-Reactive Protein Polarizes Human Macrophages to an M1 Phenotype and Inhibits Transformation to the M2 Phenotype. Arter. Thromb. Vasc. Biol. 2011, 31, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, S.; Chen, X.; Adams-Huet, B.; Jialal, I. Increased Expression of Fc-γ Receptors on Monocytes in Patients with Nascent Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E1510–E1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Lv, J.; Wang, J.; Qin, Q.; He, J.; Wang, M.; Zhou, G.; Liu, G.; Zhong, F.; Zheng, Y.; et al. C-Reactive Protein Promotes the Activation of Fibroblast-Like Synoviocytes From Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gu, X.; Li, X.; Li, Y.; Zhou, H.; Lu, G.; Wu, Z.; Huang, H.; Tang, L.; Zeng, J. C-Reactive Protein and Inflammatory Cytokines during Percutaneous Coronary Intervention. J. Vasc. Res. 2016, 53, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Steffens, S.; Burger, F.; Pelli, G.; Monaco, C.; Mach, F. C-Reactive Protein (CRP) Induces Chemokine Secretion via CD11b/ICAM-1 Interaction in Human Adherent Monocytes. J. Leukoc. Biol. 2008, 84, 1109–1119. [Google Scholar] [CrossRef]
- Li, J.-J.; Ren, Y.; Chen, K.-J.; Yeung, A.C.; Xu, B.; Ruan, X.-M.; Yang, Y.-J.; Chen, J.-L.; Gao, R.-L. Impact of C-Reactive Protein on in-Stent Restenosis: A Meta-Analysis. Tex. Heart Inst. J. 2010, 37, 49–57. [Google Scholar]
- Aksu, U.; Gulcu, O.; Aksakal, E.; Kalkan, K.; Öztürk, M.; Korkmaz, A.F.; Uslu, A.; Demirelli, S. The Association between CRP/Albumin Ratio and in-Stent Restenosis Development in Patients with ST-Segment Elevation Myocardial Infarction. J. Clin. Lab. Anal. 2019, 33, e22848. [Google Scholar] [CrossRef]
- Kwon, Y.-C.; Kim, J.-J.; Yun, S.W.; Yu, J.J.; Yoon, K.L.; Lee, K.-Y.; Kil, H.-R.; Kim, G.B.; Han, M.-K.; Song, M.S.; et al. Male-Specific Association of the FCGR2A His167Arg Polymorphism with Kawasaki Disease. PLoS ONE 2017, 12, e0184248. [Google Scholar] [CrossRef] [Green Version]
- Khor, C.C.; Davila, S.; Breunis, W.B.; Lee, Y.-C.; Shimizu, C.; Wright, V.J.; Yeung, R.S.M.; Tan, D.E.K.; Sim, K.S.; Wang, J.J.; et al. Genome-Wide Association Study Identifies FCGR2A as a Susceptibility Locus for Kawasaki Disease. Nat. Genet. 2011, 43, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, S.Q.; Tacke, C.E.; Breunis, W.B.; Tanck, M.W.T.; Geissler, J.; Png, E.; Hoang, L.T.; van der Heijden, J.; Naim, A.N.M.; Yeung, R.S.M.; et al. Extensive Ethnic Variation and Linkage Disequilibrium at the FCGR2/3 Locus: Different Genetic Associations Revealed in Kawasaki Disease. Front. Immunol. 2019, 10, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuldt, K.; Esser, C.; Evans, J.; May, J.; Timmann, C.; Ehmen, C.; Loag, W.; Ansong, D.; Ziegler, A.; Agbenyega, T.; et al. FCGR2A Functional Genetic Variant Associated with Susceptibility to Severe Malarial Anaemia in Ghanaian Children. J. Med. Genet. 2010, 47, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The Role of IgG Fc Receptors in Antibody-Dependent Enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Stein, M.P.; Edberg, J.C.; Kimberly, R.P.; Mangan, E.K.; Bharadwaj, D.; Mold, C.; Du Clos, T.W. C-Reactive Protein Binding to FcgammaRIIa on Human Monocytes and Neutrophils Is Allele-Specific. J. Clin. Investig. 2000, 105, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Parren, P.W.; Warmerdam, P.A.; Boeije, L.C.; Arts, J.; Westerdaal, N.A.; Vlug, A.; Capel, P.J.; Aarden, L.A.; van de Winkel, J.G. On the Interaction of IgG Subclasses with the Low Affinity Fc Gamma RIIa (CD32) on Human Monocytes, Neutrophils, and Platelets. Analysis of a Functional Polymorphism to Human IgG2. J. Clin. Investig. 1992, 90, 1537–1546. [Google Scholar] [CrossRef] [Green Version]



| Variables | All Patients n = 145 | RCE+ n = 27 | RCE− n = 118 | p Value RCE vs. RCE− |
|---|---|---|---|---|
| Procedural characteristics | ||||
| Number of treated lesions | 1 (1–2) | 1 (1–2) | 1 (1–1.75) | ns |
| Radial access, % (n) | 96% (138) | 93% (25) | 97% (113) | ns |
| Number of diseased vessels | 3 (2–4) | 3 (2–4) | 3 (2–4) | ns |
| Monotroncular, % (n) | 34% (49) | 34% (9) | 33% (40) | ns |
| Bitroncular, % (n) | 30% (44) | 31% (8) | 30% (36) | ns |
| Tritroncular, % (n) | 36% (52) | 36% (10) | 37% (42) | ns |
| Mean Stent diameter (mm) | 3 (2.75–3.5) | 2.95 (2.71–3) | 3 (2.75–3.5) | ns |
| Number of stents | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.0947 t |
| Total stent length (mm) | 24 (17.5–32.2) | 26.5 (20.5–41.5) | 23 (16–32) | 0.0558 t |
| Diseased vessels | ||||
| Left anterior descending coronary artery | 85.5 % (124) | 85% (23) | 86% (101) | ns |
| Circumflex artery | 50% (73) | 63% (17) | 47% (56) | ns |
| Right coronary artery | 60% (87) | 52% (14) | 62% (73) | ns |
| Left main coronary artery | 8% (12) | 7.4% (2) | 8.5% (10) | ns |
| Left ventricular ejection fraction | 60 (45.7–64) | 47 (36–55) | 60 (50–65) | 0.0003 |
| All Patients n = 145 | RCE+ n = 27 | RCE− n = 118 | p Value RCE+ vs. RCE− | |
|---|---|---|---|---|
| Age (years) | 61 (55–71) | 65 (57–71) | 60 (54–71) | ns |
| Male gender % | 77.24 | 66.67 | 79.66 | ns |
| Body mass index (BMI), kg/m2 | 27.18 (24–31) | 27 (25–31) | 27 (24–31) | ns |
| Clinical presentation | ||||
| Non STEMI % | 73.79 | 88.89 | 70.34 | ns |
| STEMI % | 20 | 11.11 | 22.03 | ns |
| Unstable angina % | 6.21 | 0 | 7.63 | ns |
| Cardiovascular risk factors | ||||
| Active smoker | 36.55 | 25.93 | 38.98 | ns |
| Hypercholesterolemia | 37.24 | 59.26 | 32.2 | 0.0087 ** |
| Hypertension | 58.62 | 55.56 | 59.32 | ns |
| Obesity | 28.3 | 25.9 | 28.8 | ns |
| Heredity | 8.97 | 14.81 | 7.63 | ns |
| Diabetes Mellitus | 43.45 | 62.96 | 38.98 | 0.0234 * |
| Medical history | ||||
| Myocardial infraction (MI) | 27.08 | 37.04 | 24.79 | ns |
| Percutaneous coronary intervention | 37.93 | 48.15 | 35.59 | ns |
| Coronary artery bypass graft | 5.52 | 7.41 | 5.08 | ns |
| Stroke % | 5.5 | 7.4 | 5 | ns |
| Chronic kidney disease % | 5.52 | 14.8 | 3.4 | 0.0398 * |
| Peripheral arterial disease % | 8.97 | 22.2 | 6 | 0.0164 * |
| Clinico-biological parameters at time of enrolment (T0) | ||||
| White blood cell count (Tera/L) | 7.45 (6.42–10.19) | 7.94 (6.6–10.8) | 7.39 (6.4–10.1) | ns |
| Hemoglobin (g/L) | 133 (121–141) | 124 (112–138) | 135 (123–141) | 0.0285 * |
| Hematocrite (%) | 39 (36–42) | 37 (33–41) | 39 (36–42) | 0.0889 t |
| Platelets (Giga/L) | 234 (191–285) | 270 (210–332) | 226 (186–272) | 0.0263 * |
| Creatinine (umol/L) | 72 (59–87) | 79 (63–113) | 70 (59–85) | 0.0742 t |
| eGFR (mL/min/1.73 m2) | 92 (78–103) | 80 (51–96) | 95 (81–105) | 0.0065 ** |
| CRP (mg/mL) | 5 (3–10.7) | 5.5 (3.2–15) | 4.7 (3–10.6) | 0.0066 ** |
| hs CRP | 3.97 (1.46–9.84) | 4.79 (2.4–12.5) | 3.78–1.36–9.53) | 0.0067 ** |
| BNP (ng/L) | 59.65 (18–138) | 190 (94–460) | 50 (16–95) | 0.0068 ** |
| Troponin | 0.12 (0.01–1.8) | 0.2 (0.03–2.03) | 0.1 (0.01–1.76) | ns |
| Medications at discharge | ||||
| Aspirine | 95.86% | 88.89% | 97.46% | 0.0784 t |
| Beta blockers | 70.34% | 85.19% | 66.95% | 0.0613 t |
| Calcium channel blockers | 9.66% | 11.11% | 9.32% | ns |
| P2Y12 ADP receptor inhibitors | 93.8 | 92.6 | 94 | ns |
| ACE inhibitors/ARBs | 61.4 | 74.10 | 58.5 | ns |
| insulin | 17.2% | 29.63% | 14.41% | 0.0866 t |
| Biguanides | 6.9% | 85.19% | 94.92% | 0.0904 t |
| Proton pump inhibitors | 82.76% | 81.48% | 83.05% | ns |
| Statins | 90.34% | 96.3% | 88.98 | ns |
| Recurrent cardiovascular events during the first year (RCE) | ||||
| Cardiovascular death | 1.4% | 7.41% | na | na |
| Acute myocardial infarction | 6.2% | 33% | na | na |
| Urgent revascularization | 6.9% | 37% | na | na |
| Stroke | 0% | 0% | na | na |
| Acute Heart Failure (AHF) | 9% | 48% | na | na |
| RCE | Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | ||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio | p Value | 95% CI | Odds Ratio | p Value | 95% CI |
| FCgR2A-HH | 2.70 | 0.0228 | 1.15–6.35 | 2.71 | 0.0480 | 1.01–7.44 |
| FEVG < 40% | 4.46 | 0.0060 | 1.56–12.69 | 4.70 | 0.0124 | 1.40–16.2 |
| Hypercholesterolemia | 3.00 | 0.01 | 1.3–7.14 | 4.37 | 0.0024 | 1.67–12.42 |
| hs CRP ≥ 3 mg/L | 1.91 | 0.1419 | 0.81–4.87 | 2.08 | 0.1514 | 0.77–6.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, P.; Picard, C.; Lyonnet, L.; Resseguier, N.; Hubert, L.; Arnaud, L.; Di Cristofaro, J.; Laine, M.; Paganelli, F.; Dignat-George, F.; et al. FCGR2A-HH Gene Variants Encoding the Fc Gamma Receptor for the C-Reactive Protein Are Associated with Enhanced Monocyte CD32 Expression and Cardiovascular Events’ Recurrence after Primary Acute Coronary Syndrome. Biomedicines 2022, 10, 495. https://doi.org/10.3390/biomedicines10020495
Paul P, Picard C, Lyonnet L, Resseguier N, Hubert L, Arnaud L, Di Cristofaro J, Laine M, Paganelli F, Dignat-George F, et al. FCGR2A-HH Gene Variants Encoding the Fc Gamma Receptor for the C-Reactive Protein Are Associated with Enhanced Monocyte CD32 Expression and Cardiovascular Events’ Recurrence after Primary Acute Coronary Syndrome. Biomedicines. 2022; 10(2):495. https://doi.org/10.3390/biomedicines10020495
Chicago/Turabian StylePaul, Pascale, Christophe Picard, Luc Lyonnet, Noémie Resseguier, Lucas Hubert, Laurent Arnaud, Julie Di Cristofaro, Marc Laine, Franck Paganelli, Françoise Dignat-George, and et al. 2022. "FCGR2A-HH Gene Variants Encoding the Fc Gamma Receptor for the C-Reactive Protein Are Associated with Enhanced Monocyte CD32 Expression and Cardiovascular Events’ Recurrence after Primary Acute Coronary Syndrome" Biomedicines 10, no. 2: 495. https://doi.org/10.3390/biomedicines10020495
APA StylePaul, P., Picard, C., Lyonnet, L., Resseguier, N., Hubert, L., Arnaud, L., Di Cristofaro, J., Laine, M., Paganelli, F., Dignat-George, F., Frère, C., Sabatier, F., Guieu, R., & Bonello, L. (2022). FCGR2A-HH Gene Variants Encoding the Fc Gamma Receptor for the C-Reactive Protein Are Associated with Enhanced Monocyte CD32 Expression and Cardiovascular Events’ Recurrence after Primary Acute Coronary Syndrome. Biomedicines, 10(2), 495. https://doi.org/10.3390/biomedicines10020495






