Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. GDM Overview and Clinical Associations
3. Inconsistencies in the Diagnostic Assessment
| Guideline | Fasting Plasma Glucose (FPG) Cut-Off | Oral Glucose Tolerance Test (OGTT) (or Glucose Challenge Test (GCT)) | 1-h Threshold | 2-h Threshold | Observation | Ref. |
|---|---|---|---|---|---|---|
| International Association of Diabetes and Pregnancy Study Groups (IADPSG) | 5.1 mM (92 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5 mM (153 mg/dL) | [30] | |
| American Diabetic Association (ADA) | 5.1 mM (92 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5 mM (153 mg/dL) | [29,31] | |
| 5.3 mM (95 mg/dL) | 50 g | 10.0 mM (180 mg/dL) | 8.6 mM (155 mg/dL) | 3 h: 7.8 mM (140 mg/dL) At least two measures above limit. | ||
| Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) | 5.1 mM (92 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5 mM (153 mg/dL) | Initial 1-h non-fasting oral Glucose Challenge Test (GCT) is no longer recommended | [32] |
| New Zealand Society for the Study of Diabetes (NZSSD) | 5.5 mM (99 mg/dL) | 75 g | - | 9.0 mM (162 mg/dL) | Glycated haemoglobin (HbA1c) (week 20) | [33] |
| Royal Australian College of General Practitioners (RACGP) | 5.5 mM (99 mg/dL) | 75 g | - | 8.0 mM (144 mg/dL) | [34,35] | |
| Australasian Diabetes In Pregnancy Society (ADIPS) | 5.1–6.9 mM (92–125 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5–11.0 mM (153–199 mg/dL) | Suggested an early OGTT (or HbA1c) with first antenatal blood or at the first antenatal visit (in the first trimester), register of glucose levels (National diabetes services scheme) | |
| Canadian Diabetes Association (CDA) | 5.3 mM (95 mg/dL) | 50 g | 10.6 mM (190 mg/dL) | 9.0 mM (162 mg/dL) | If abnormal, 75 g OGTT | [36] |
| 5.1 mM (92 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5 mM (153 mg/dL) | |||
| World Health Organization (WHO) | 5.1–6.9 mM (92–125 mg/dL) | 75 g | 10.0 mM (180 mg/dL) | 8.5–11.0 mM (153–199 mg/dL) | No established criteria for the diagnosis of diabetes based on the 1 h post-load value | [37] |
4. Extracellular Vesicles: New Players in Cell-to-Cell Communication
| Vesicle | Size | Biogenesis | Main Markers | Density | Content | Ref. |
|---|---|---|---|---|---|---|
| Small Extracellular Vesicles (sEVs)—previously identified as exosomes | 30–100 nm | Budding of the cellular plasma membrane and later inward invaginations of this endosomal membrane | ESCRT machinery (TSG101, Alix, HRS), Tetraspanins (e.g., CD9, CD63), RABs | 1.08–1.19 g/mL | Proteins, mRNA, miRNA, lipids | [43,44,45] |
| Ectosomes (microvesicles or microparticles *) | >100–1000 nm | Direct plasma membrane fission | Tubulin, CD40, Integrins, selectins | ~1.15 g/mL | Proteins, mRNA, miRNA, lipids | [43,46,47,48,49,50] |
| Apoptotic bodies | 500–5000 nm | Programmed cell death process | Annexin V, phosphatidylserine | 1.16–1.28 g/mL | Organelles, proteins, DNA, different RNA species, lipids | [51,52,53,54] |
5. Extracellular Vesicle-Associated Changes in the Pathophysiology of GDM
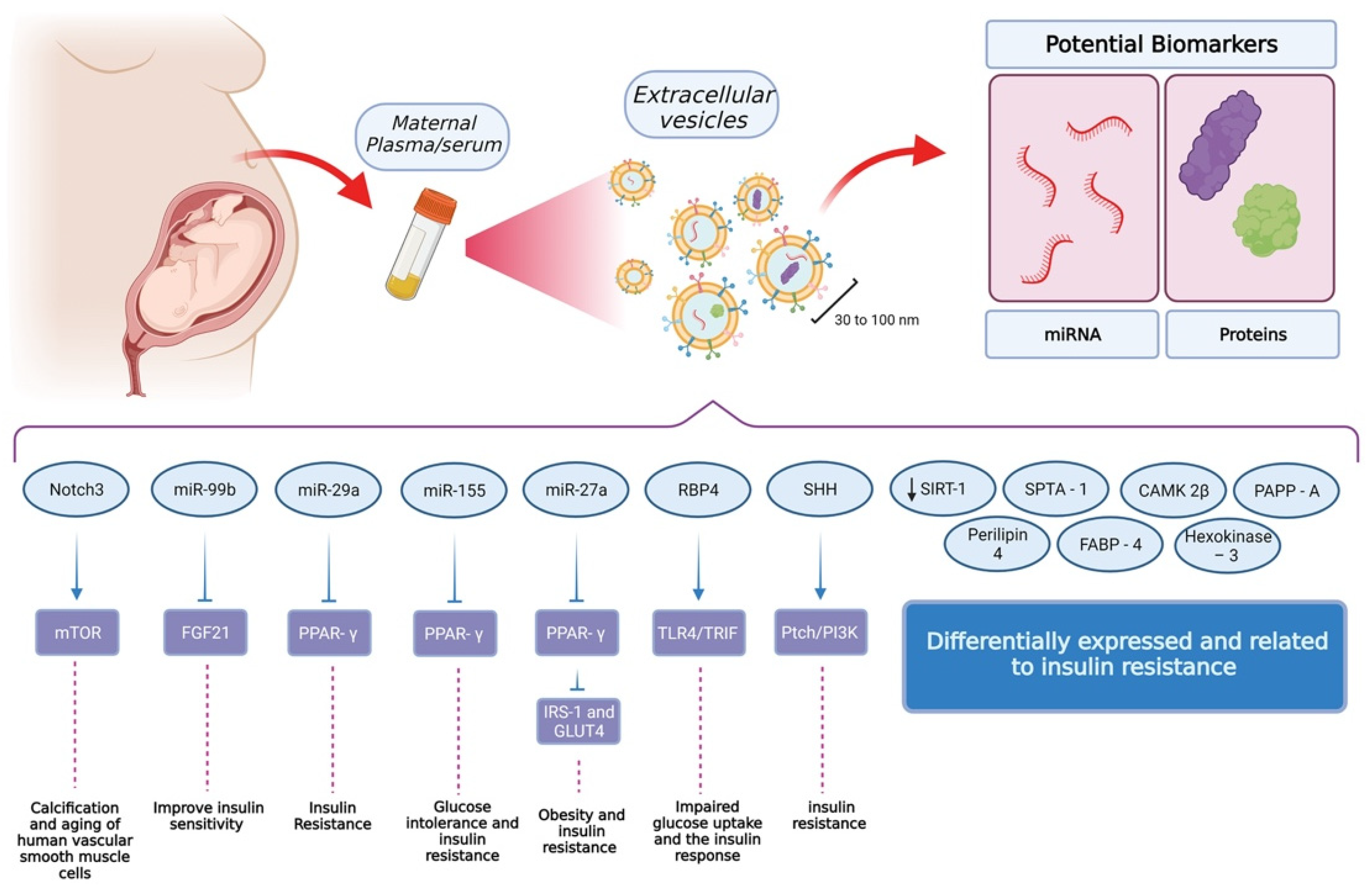
6. Extracellular Vesicle-Associated Signaling in GDM
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.M.; Dhatt, G.S.; Othman, Y. Gestational diabetes: Differences between the current international diagnostic criteria and implications of switching to IADPSG. J. Diabetes Its Complicat. 2015, 29, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; De Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S251–S260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomón, C.; Westermeier, F.; Puebla, C.; Arroyo, P.; Guzmán-Gutiérrez, E.; Pardo, F.; Leiva, A.; Casanello, P.; Sobrevia, L. Gestational Diabetes Reduces Adenosine Transport in Human Placental Microvascular Endothelium, an Effect Reversed by Insulin. PLoS ONE 2012, 7, e40578. [Google Scholar] [CrossRef]
- Cosson, E.; Carbillon, L.; Valensi, P. High Fasting Plasma Glucose during Early Pregnancy: A Review about Early Gestational Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 8921712. [Google Scholar] [CrossRef]
- Usami, T.; Yokoyama, M.; Ueno, M.; Iwama, N.; Sagawa, N.; Kawano, R.; Waguri, M.; Sameshima, H.; Hiramatsu, Y.; Sugiyama, T.; et al. Comparison of pregnancy outcomes between women with early-onset and late-onset gestational diabetes in a retrospective multi-institutional study in Japan. J. Diabetes Investig. 2019, 11, 216–222. [Google Scholar] [CrossRef]
- Utz, B.; Assarag, B.; Essolbi, A.; Barkat, A.; El Ansari, N.; Fakhir, B.; Delamou, A.; De Brouwere, V. Improving detection and initial management of gestational diabetes through the primary level of care in Morocco: Protocol for a cluster randomized controlled trial. Reprod. Health 2017, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [CrossRef]
- Karttunen, J.; Heiskanen, M.; Lipponen, A.; Poulsen, D.; Pitkänen, A. Extracellular Vesicles as Diagnostics and Therapeutics for Structural Epilepsies. Int. J. Mol. Sci. 2019, 20, 1259. [Google Scholar] [CrossRef] [Green Version]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfeky, O.; Longo, S.; Lai, A.; Rice, G.E.; Salomon, C. Influence of maternal BMI on the exosomal profile during gestation and their role on maternal systemic inflammation. Placenta 2017, 50, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.; Rodrigues, S.; Gomes, C.; Duarte, F.; Romao, M.; Leal, E.; Freire, P.; Neves, R.; Simões-Correia, J. Development of an optimized and scalable method for isolation of umbilical cord blood-derived small extracellular vesicles for future clinical use. Stem Cells Transl. Med. 2021, 10, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Palma, C.; Jellins, J.; Lai, A.; Salas, A.; Campos, A.; Sharma, S.; Duncombe, G.; Hyett, J.; Salomon, C. Extracellular Vesicles and Preeclampsia: Current Knowledge and Future Research Directions; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 455–482. [Google Scholar]
- James Allan, L.B.; Rosario, F.J.; Barner, K.; Lai, A.; Guanzon, D.; McIntyre, H.D.; Lappas, M.; Powell, T.L.; Salomon, C.; Jansson, T. Regulation of glucose homeostasis by small extracellular vesicles in normal pregnancy and in gestational diabetes. FASEB J. 2020, 34, 5724–5739. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, N.; Lai, A.; Nair, S.; Guanzon, D.; Scholz-Romero, K.; Palma, C.; McIntyre, H.D.; Lappas, M.; Salomon, C. Quantitative Proteomics by SWATH-MS Suggest an Association Between Circulating Exosomes and Maternal Metabolic Changes in Gestational Diabetes Mellitus. Proteomics 2018, 19, 1800164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, S.; Guanzon, D.; Jayabalan, N.; Lai, A.; Scholz-Romero, K.; Kalita De Croft, P.; Ormazabal, V.; Palma, C.; Diaz, E.; McCarthy, E.A.; et al. Extracellular vesicle-associated miRNAs are an adaptive response to gestational diabetes mellitus. J. Transl. Med. 2021, 19, 360. [Google Scholar] [CrossRef]
- Nair, S.; Jayabalan, N.; Guanzon, D.; Palma, C.; Scholz-Romero, K.; Elfeky, O.; Zuñiga, F.; Ormazabal, V.; Diaz, E.; Rice, G.; et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin. Sci. 2018, 132, 2451–2467. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadden, D.R. Prediabetes and the big baby. Diabet. Med. 2008, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bryson, C.L. Association between Gestational Diabetes and Pregnancy-induced Hypertension. Am. J. Epidemiol. 2003, 158, 1148–1153. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Gardea, M.O.; Gonzales-Pacheco, D.M.; Reader, D.M.; Thomas, A.M.; Wang, S.R.; Gregory, R.P.; Piemonte, T.A.; Thompson, K.L.; Moloney, L. Academy of Nutrition and Dietetics Gestational Diabetes Evidence-Based Nutrition Practice Guideline. J. Acad. Nutr. Diet. 2018, 118, 1719–1742. [Google Scholar] [CrossRef]
- Sweeting, A.N.; Ross, G.P.; Hyett, J.; Molyneaux, L.; Constantino, M.; Harding, A.J.; Wong, J. Gestational Diabetes Mellitus in Early Pregnancy: Evidence for Poor Pregnancy Outcomes Despite Treatment. Diabetes Care 2016, 39, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.C.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowan, J.A.; Budden, A.; Ivanova, V.; Hughes, R.C.; Sadler, L.C. Women with an HbA1c of 41–49 mmol/mol (5.9–6.6%): A higher risk subgroup that may benefit from early pregnancy intervention. Diabet. Med. 2016, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Nankervis, A.; Price, S.; Conn, J. Gestational diabetes mellitus: A pragmatic approach to diagnosis and management. Aust. J. Gen. Pract. 2018, 47, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; De Leiva, A.; Hod, M.; et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynecol. Obstet. 2015, 131, S173–S211. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [Green Version]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Summary of Revisions: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S4–S6. [CrossRef] [PubMed] [Green Version]
- RANZCOG. Diagnosis of Gestational Diabetes Mellitus (GDM); Royal Australian and New Zealand College of Obstetricians and Gynaecologists: Melbourne, Australia, 2017. [Google Scholar]
- Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline; Ministry of Health: Wellington, New Zealand, 2014.
- RACGP. Management of Type 2 Diabetes: A Handbook for General Practice; The Royal Australian College of General Practitioners Ltd.: East Melbourne, Australia, 2020. [Google Scholar]
- Nankervis, A.M.H.; Moses, R.; Ross, G.P.; Callaway, L.; Porter, C.; Jeffries, W.; Boorman, C.; De Vries, B.; McElduff, A. ADIPS Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia; Australasian Diabetes in Pregnancy Society: Sydney, Australia, 2014. [Google Scholar]
- Feig, D.S.; Berger, H.; Donovan, L.; Godbout, A.; Kader, T.; Keely, E.; Sanghera, R. Diabetes and Pregnancy. Can. J. Diabetes 2018, 42, S255–S282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.; Ehrlich, S.; Sridhar, S.; Darbinian, J.; Moore, S.; Ferrara, A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012, 35, 1492–1498. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.D. NICE guidance on diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet. Med. 2008, 25, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Iljas, J.D.; Guanzon, D.; Elfeky, O.; Rice, G.E.; Salomon, C. Review: Bio-compartmentalization of microRNAs in exosomes during gestational diabetes mellitus. Placenta 2017, 54, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poutsiaka, D.D.; Schroder, E.W.; Taylor, D.D.; Levy, E.M.; Black, P.H. Membrane vesicles shed by murine melanoma cells selectively inhibit the expression of Ia antigen by macrophages. J. Immunol. 1985, 134, 138–144. [Google Scholar]
- Jayachandran, M.; Miller, V.M.; Heit, J.A.; Owen, W.G. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J. Immunol. Methods 2012, 375, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, S.J.; Schaffer, J.E. Transport and Secretion Exosomes and Microvesicles. In Encyclopedia of Biological Chemistry III, 3rd ed.; Jez, J., Ed.; Elsevier: Oxford, UK, 2021; pp. 455–458. [Google Scholar] [CrossRef]
- Schwartzman, R.A.; Cidlowski, J.A. Apoptosis: The biochemistry and molecular biology of programmed cell death. Endocr. Rev. 1993, 14, 133–151. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-M.; Tseng, C.-H.; Chen, Y.-C.; Yu, W.-Y.; Ho, M.-Y.; Ho, C.-Y.; Lai, M.M.C.; Su, W.-C. Exosome-delivered and Y RNA-derived small RNA suppresses influenza virus replication. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Xue, F.T.; Li, Y.Y.; Liu, W.; Zhang, S. Exosomal piRNA sequencing reveals differences between heart failure and healthy patients. Eur Rev. Med. Pharmacol. Sci. 2018, 22, 7952–7961. [Google Scholar] [CrossRef]
- Onozato, M.; Tanaka, Y.; Arita, M.; Sakamoto, T.; Ichiba, H.; Sadamoto, K.; Kondo, M.; Fukushima, T. Amino acid analyses of the exosome-eluted fractions from human serum by HPLC with fluorescence detection. Pract. Lab. Med. 2018, 12, e00099. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, L.; Cui, Y.; Zhou, Y.; Yin, X.; Guo, J.; Zhang, G.; Wang, T.; He, Q.-Y. Cytoskeleton-centric protein transportation by exosomes transforms tumor-favorable macrophages. Oncotarget 2016, 7, 67387–67402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.; Samuel, M.; Kumar, S.; Mathivanan, S. Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2019, 1867, 140203. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Noerholm, M.; Balaj, L.; Limperg, T.; Salehi, A.; Zhu, L.D.; Hochberg, F.H.; Breakefield, X.O.; Carter, B.S.; Skog, J. RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, A.; Farber, E.L.; Rapoport, A.L.; Tejada, D.; Deniskin, R.; Akhmedov, N.B.; Farber, D.B. Transfer of MicroRNAs by Embryonic Stem Cell Microvesicles. PLoS ONE 2009, 4, e4722. [Google Scholar] [CrossRef] [Green Version]
- Menck, K.; Bleckmann, A.; Wachter, A.; Hennies, B.; Ries, L.; Schulz, M.; Balkenhol, M.; Pukrop, T.; Schatlo, B.; Rost, U.; et al. Characterisation of tumour-derived microvesicles in cancer patients’ blood and correlation with clinical outcome. J. Extracell Vesicles 2017, 6, 1340745. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Salomon, C.; Tapia, J.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J. Transl. Med. 2014, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Salomon, C.; Kobayashi, M.; Ashman, K.; Sobrevia, L.; Mitchell, M.D.; Rice, G.E. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS ONE 2013, 8, e79636. [Google Scholar] [CrossRef] [Green Version]
- Salomon, C.; Ryan, J.; Sobrevia, L.; Kobayashi, M.; Ashman, K.; Mitchell, M.; Rice, G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS ONE 2013, 8, e68451. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.D.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; Yee, S.; Scholz-Romero, K.; Kobayashi, M.; Vaswani, K.; Kvaskoff, D.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front. Pharmacol. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Catalano, P.M.; Huston, L.; Amini, S.B.; Kalhan, S.C. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am. J. Obstet. Gynecol. 1999, 180, 903–916. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Metzger, B.E.; Freinkel, N.; Bergman, R.N. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am. J. Obstet. Gynecol. 1990, 162, 1008–1014. [Google Scholar] [CrossRef]
- Sivan, E.; Chen, X.; Homko, C.J.; Reece, E.A.; Boden, G. Longitudinal study of carbohydrate metabolism in healthy obese pregnant women. Diabetes Care 1997, 20, 1470–1475. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.-C.; Cheng, P.; Shao, H.-G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Li, J.; Song, L.; Zhou, L.; Wu, J.; Sheng, C.; Chen, H.; Liu, Y.; Gao, S.; Huang, W. A MicroRNA Signature in Gestational Diabetes Mellitus Associated with Risk of Macrosomia. Cell. Physiol. Biochem. 2015, 37, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Du, X.; Xu, J.; Zhang, Y.; Tian, Y.; Liu, G.; Wang, X.; Ma, M.; Du, W.; Liu, Y.; et al. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020, 18, e3000603. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Diabetes Mellitus and Cardiovascular Risk Assessment in Mothers with a History of Gestational Diabetes Mellitus Based on Postpartal Expression Profile of MicroRNAs Associated with Diabetes Mellitus and Cardiovascular and Cerebrovascular Diseases. Int. J. Mol. Sci. 2020, 21, 2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose Tissue Exosome-Like Vesicles Mediate Activation of Macrophage-Induced Insulin Resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, C.; Kong, F. A Prospective Study of Maternal Plasma Concentrations of Retinol-Binding Protein 4 and Risk of Gestational Diabetes Mellitus. Ann. Nutr. Metab. 2019, 74, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Liu, J.; Han, N.; Zhao, Z.; Luo, S.; Wang, H. Exploring the mediating role of serum retinol-binding protein 4 in the relationship between sleep quality and insulin resistance in pregnant women. Diabetes Res. Clin. Pract. 2021, 176, 108866. [Google Scholar] [CrossRef]
- Song, M.; Han, L.; Chen, F.-F.; Wang, D.; Wang, F.; Zhang, L.; Wang, Z.-H.; Zhong, M.; Tang, M.-X.; Zhang, W. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell. Physiol. Biochem. 2018, 48, 1416–1432. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Meng, T.; Zhao, G.; Si, S. The effect of gestational diabetes on identification of key genes and pathways in human umbilical vein endothelial cell by integrated bioinformatics analysis. J. Obstet. Gynaecol. 2020, 41, 881–887. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Jin, X.; Zhang, Y.; Kang, X.; Zhang, Z.; Xu, M.; Qian, Z.; Ma, Z.; Gao, X.; et al. Adipose-specific knockdown of Sirt1 results in obesity and insulin resistance by promoting exosomes release. Cell Cycle 2019, 18, 2067–2082. [Google Scholar] [CrossRef]
- Beneventi, F.; Simonetta, M.; Lovati, E.; Albonico, G.; Tinelli, C.; Locatelli, E.; Spinillo, A. First trimester pregnancy-associated plasma protein-A in pregnancies complicated by subsequent gestational diabetes. Prenat. Diagn. 2011, 31, 523–528. [Google Scholar] [CrossRef]
- Gabreanu, G.R.; Angelescu, S. Erythrocyte membrane in type 2 diabetes mellitus. Discoveries 2016, 4, e60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, R.; Okura, T.; Fujioka, Y.; Sumi, K.; Matsuzawa, K.; Izawa, S.; Ueta, E.; Kato, M.; Taniguchi, S.-I.; Yamamoto, K. Serum fatty acid-binding protein 4 (FABP4) concentration is associated with insulin resistance in peripheral tissues, A clinical study. PLoS ONE 2017, 12, e0179737. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; de Vos, P.; Kuipers, J.; Sobrevia, L.; Faas, M.M. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta 2018, 66, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, S.; Wang, Y.J.; Wang, Y.; Zhong, J.Y.; He, J.Y.; Cui, X.J.; Zhan, J.K.; Liu, Y.S. Exosomal Notch3 from high glucose-stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life Sci. 2019, 232, 116582. [Google Scholar] [CrossRef]
- Kandzija, N.; Zhang, W.; Motta-Mejia, C.; Mhlomi, V.; McGowan-Downey, J.; James, T.; Cerdeira, A.S.; Tannetta, D.; Sargent, I.; Redman, C.W.; et al. Placental extracellular vesicles express active dipeptidyl peptidase IV; levels are increased in gestational diabetes mellitus. J. Extracell. Vesicles 2019, 8, 1617000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, L.J.; Varas-Godoy, M.; Monckeberg, M.; Realini, O.; Hernández, M.; Rice, G.; Romero, R.; Saavedra, J.F.; Illanes, S.E.; Chaparro, A. Oral extracellular vesicles in early pregnancy can identify patients at risk of developing gestational diabetes mellitus. PLoS ONE 2019, 14, e0218616. [Google Scholar] [CrossRef]
- Franzago, M.; Lanuti, P.; Fraticelli, F.; Marchioni, M.; Buca, D.; Di Nicola, M.; Liberati, M.; Miscia, S.; Stuppia, L.; Vitacolonna, E. Biological insight into the extracellular vesicles in women with and without gestational diabetes. J. Endocrinol. Investig. 2021, 44, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martínez, C.; Ricart, W.; Rieusset, J. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 2014, 37, 1375–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bork-Jensen, J.; Scheele, C.; Christophersen, D.V.; Nilsson, E.; Friedrichsen, M.; Fernandez-Twinn, D.S.; Grunnet, L.G.; Litman, T.; Holmstrom, K.; Vind, B.; et al. Glucose tolerance is associated with differential expression of microRNAs in skeletal muscle: Results from studies of twins with and without type 2 diabetes. Diabetologia 2015, 58, 363–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, C.; McIntyre, H.D.; Salomon, C. Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus. Biomedicines 2022, 10, 462. https://doi.org/10.3390/biomedicines10020462
Palma C, McIntyre HD, Salomon C. Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus. Biomedicines. 2022; 10(2):462. https://doi.org/10.3390/biomedicines10020462
Chicago/Turabian StylePalma, Carlos, H. David McIntyre, and Carlos Salomon. 2022. "Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus" Biomedicines 10, no. 2: 462. https://doi.org/10.3390/biomedicines10020462
APA StylePalma, C., McIntyre, H. D., & Salomon, C. (2022). Extracellular Vesicles—New Players in Cell-to-Cell Communication in Gestational Diabetes Mellitus. Biomedicines, 10(2), 462. https://doi.org/10.3390/biomedicines10020462







