Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Isolation of Bone Marrow Neutrophils
2.2. Isolation of Gut Microbiota-Derived Small EVs
2.3. Neutrophil Stimulation Protocol
2.4. Characterization of Small EVs by Tunable Resistive Pulse Sensing (TRPS)
2.5. Characterization of Small EVs by Transmission Electron Microscopy (TEM)
2.6. Measurement of Endotoxin Levels and Lipoteichoic Acid (LTA) Concentration
2.7. Antibodies
2.8. SDS-PAGE Western Blotting
2.9. Assessment of the Total Protein Concentration
2.10. Cytokine and Chemokine Measurements
2.11. Measurement of Reactive Oxygen Species (ROS)
2.12. In Vitro Transmigration Assay
2.13. In Vitro Phagocytosis Assay
2.14. Analysis of Cell Viability-MTT Assay
2.15. RNA Isolation and Real-Time qPCR
2.16. Statistical Analysis
3. Results
3.1. Murine Microbiota-Derived Small EV Purification and Characterization
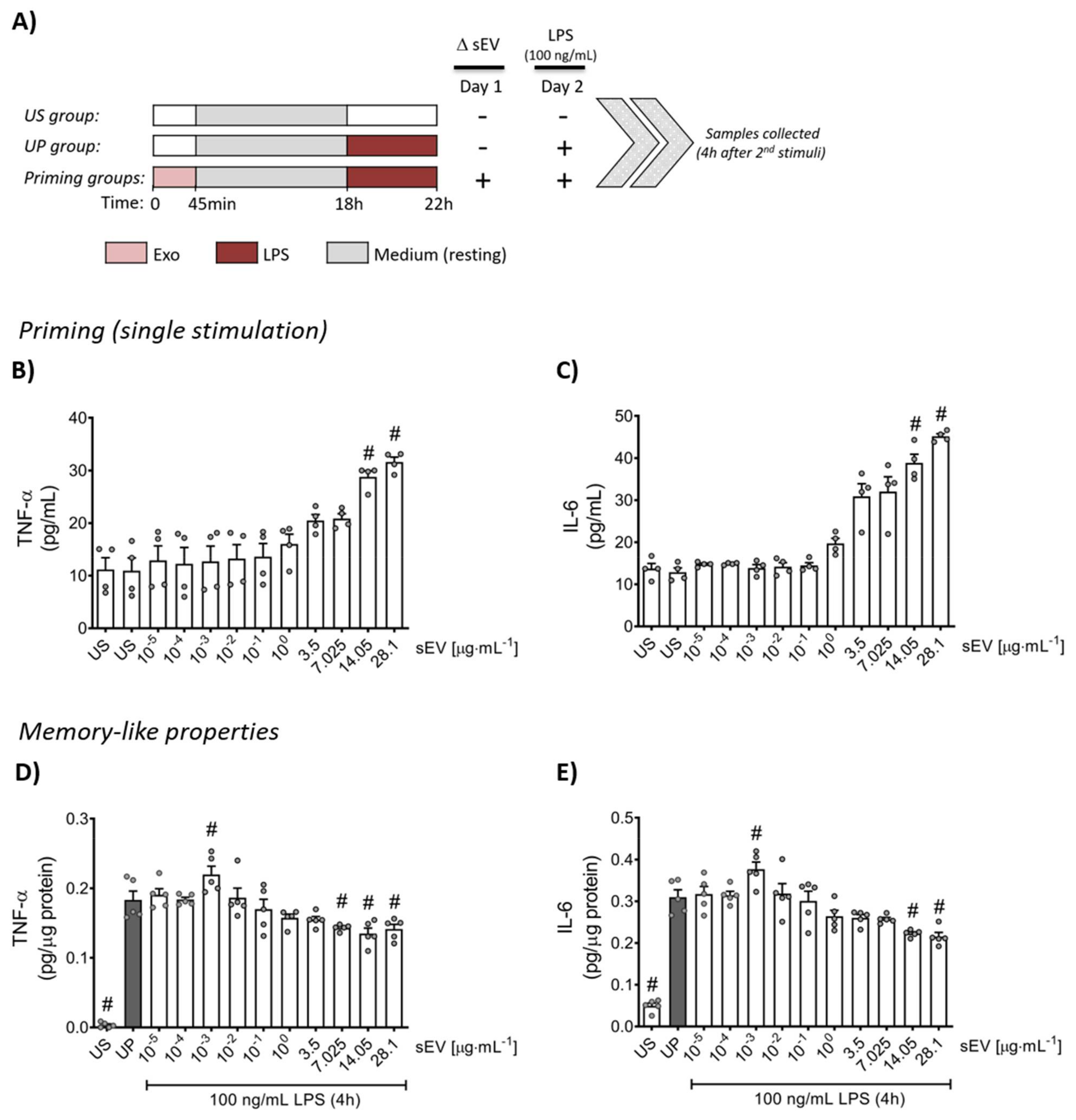
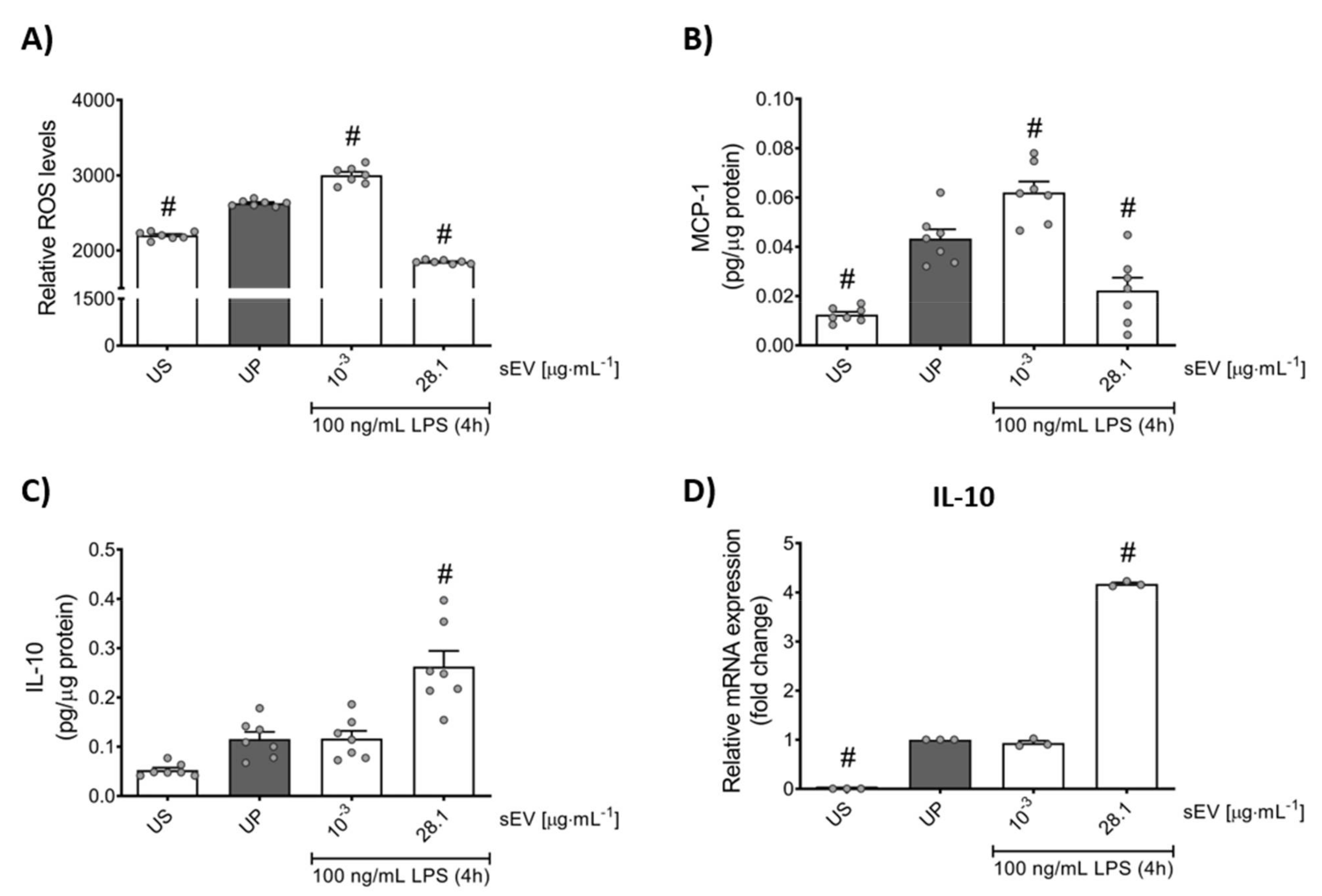
3.2. Microbiota-Derived Small EVs Promote Dose-Dependent Memory-like Inflammatory Features in Murine Bone Marrow Neutrophils
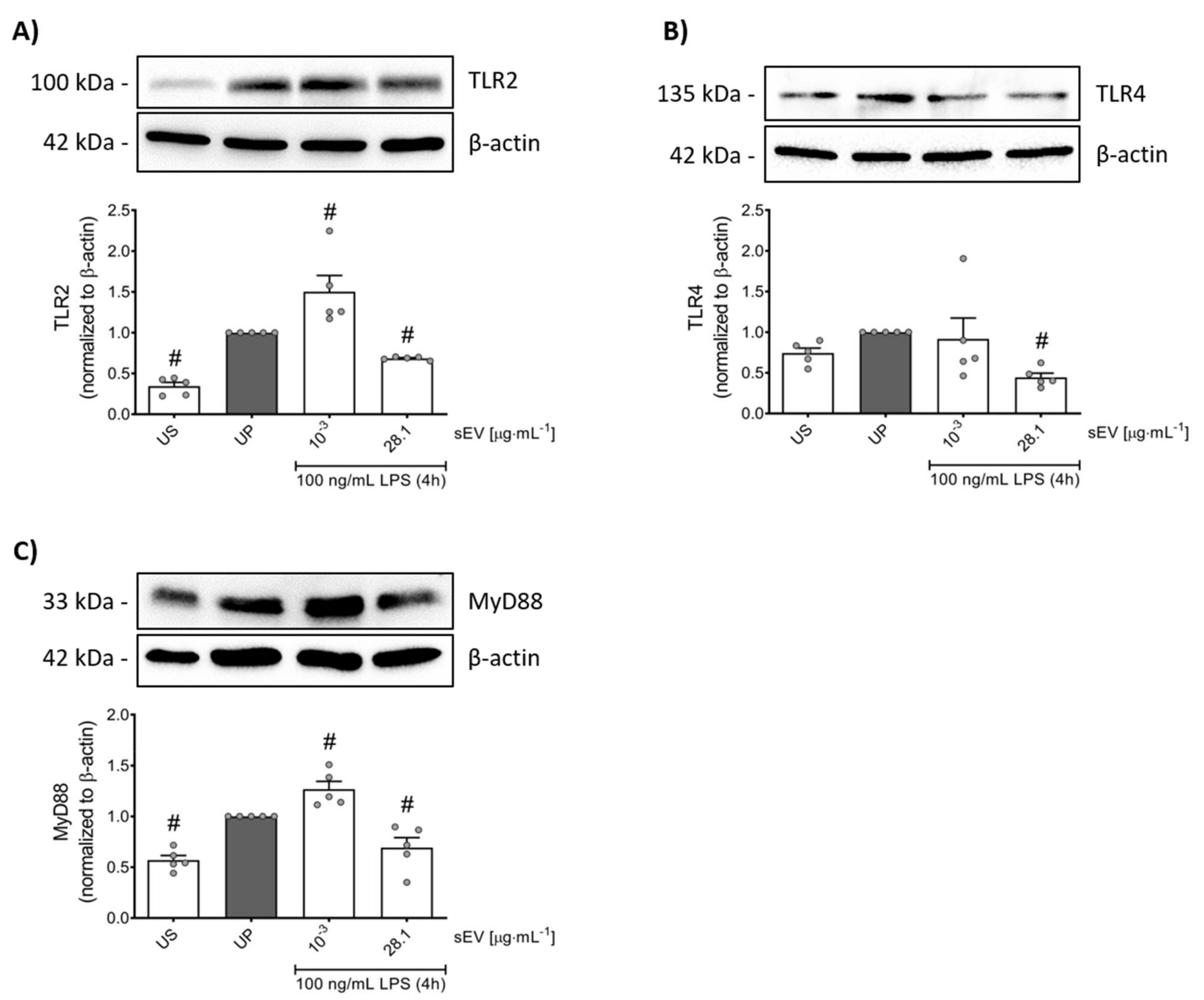
3.3. Small EV-Priming Is Promoted by TLR2/MyD88 Activation
3.4. Small EV-Priming Alters Migratory Activities and Phagocytic Capacity of Murine Bone Marrow Neutrophils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as Emerging Therapeutic Targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil Recruitment and Function in Health and Inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Neutrophil in Vascular Inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the Site of Inflammation: The Leukocyte Adhesion Cascade Updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils Cascading Their Way to Inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Mócsai, A. Diverse Novel Functions of Neutrophils in Immunity, Infammation, and Beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.J. Innate Immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An Introduction to Immunology and Immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A.; Medzhitov, R. Control of Adaptive Immunity by the Innate Immune System. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained Immunity, Tolerance, Priming and Differentiation: Distinct Immunological Processes. Nat. Immunol. 2021, 22, 928. [Google Scholar] [CrossRef]
- Netea, M.G.; van der Meer, J.W.M. Trained Immunity: An Ancient Way of Remembering. Cell Host Microbe 2017, 21, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Quintin, J.; Cheng, S.; van der Meer, J.W.; Netea, M.G. Innate Immune Memory: Towards a Better Understanding of Host Defense Mechanisms. Curr. Opin. Immunol. 2014, 29C, 1–7. [Google Scholar] [CrossRef]
- West, M.A.; Heagy, W. Endotoxin Tolerance: A Review. Crit. Care Med. 2002, 30, S64–S73. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Fan, H.K.; Cook, J.A. Molecular Mechanisms of Endotoxin Tolerance. J. Endotoxin Res. 2004, 10, 71–84. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida Albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin Induces NOD2-Dependent Nonspecific Protection from Reinfection via Epigenetic Reprogramming of Monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Quintin, J.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Lajqi, T.; Lang, G.-P.; Haas, F.; Williams, D.L.; Hudalla, H.; Bauer, M.; Groth, M.; Wetzker, R.; Bauer, R. Memory-Like Inflammatory Responses of Microglia to Rising Doses of LPS: Key Role of PI3Kγ. Front. Immunol. 2019, 10, 2492. [Google Scholar] [CrossRef] [Green Version]
- Lajqi, T.; Pöschl, J.; Frommhold, D.; Hudalla, H. The Role of Microbiota in Neutrophil Regulation and Adaptation in Newborns. Front. Immunol. 2020, 11, 568685. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, X.; Feng, Y.; Yu, J. Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis. Front. Immunol. 2020, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Lajqi, T.; Stojiljkovic, M.; Williams, D.L.; Hudalla, H.; Bauer, M.; Witte, O.W.; Wetzker, R.; Bauer, R.; Schmeer, C. Memory-Like Responses of Brain Microglia Are Controlled by Developmental State and Pathogen Dose. Front. Immunol. 2020, 11, 546415. [Google Scholar] [CrossRef]
- Yuan, R.; Geng, S.; Li, L. Molecular Mechanisms That Underlie the Dynamic Adaptation of Innate Monocyte Memory to Varying Stimulant Strength of TLR Ligands. Front. Immunol. 2016, 7, 497. [Google Scholar] [CrossRef] [Green Version]
- Maitra, U.; Gan, L.; Chang, S.; Li, L. Low-Dose Endotoxin Induces Inflammation by Selectively Removing Nuclear Receptors and Activating CCAAT/Enhancer-Binding Protein Delta. J. Immunol. 2011, 186, 4467–4473. [Google Scholar] [CrossRef]
- Maitra, U.; Deng, H.; Glaros, T.; Baker, B.; Capelluto, D.G.S.; Li, Z.; Li, L. Molecular Mechanisms Responsible for the Selective and Low-Grade Induction of Proinflammatory Mediators in Murine Macrophages by Lipopolysaccharide. J. Immunol. 2012, 189, 1014–1023. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Maitra, U.; Morris, M.; Li, L. Molecular Mechanism Responsible for the Priming of Macrophage Activation. J. Biol. Chem. 2013, 288, 3897–3906. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.F.; et al. The Persistence of Low-Grade Inflammatory Monocytes Contributes to Aggravated Atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Stojiljkovic, M.; Wetzker, R. Toxin-Induced Hormesis May Restrain Aging. Biogerontology 2019, 20, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-Mediated Aerobic Glycolysis as Metabolic Basis for Trained Immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajqi, T.; Marx, C.; Hudalla, H.; Haas, F.; Große, S.; Wang, Z.Q.; Heller, R.; Bauer, M.; Wetzker, R.; Bauer, R. The Role of the Pathogen Dose and PI3Kγ in Immunometabolic Reprogramming of Microglia for Innate Immune Memory. Int. J. Mol. Sci. 2021, 22, 2578. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Andrés, J.; Novakovic, B.; Li, Y.; Scicluna, B.P.; Gresnigt, M.S.; Arts, R.J.W.; Oosting, M.; Moorlag, S.J.C.F.M.; Groh, L.A.; Zwaag, J.; et al. The Itaconate Pathway Is a Central Regulatory Node Linking Innate Immune Tolerance and Trained Immunity. Cell Metab. 2019, 29, 211–220.e5. [Google Scholar] [CrossRef] [Green Version]
- Jentho, E.; Lajqi, T.; Yang, K.; Winkler, R.; Stojiljkovic, M.; Wetzker, R.; Bauer, M. Pathogen-Induced Hormetic Responses. In The Science of Hormesis in Health and Longevity; Rattan, S.I.S., Kyriazis, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–170. [Google Scholar]
- Ferrante, A. Tumor Necrosis Factor Alpha Potentiates Neutrophil Antimicrobial Activity: Increased Fungicidal Activity against Torulopsis Glabrata and Candida Albicans and Associated Increases in Oxygen Radical Production and Lysosomal Enzyme Release. Infect. Immun. 1989, 57, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Yektaei-Karin, E.; Moshfegh, A.; Lundahl, J.; Berggren, V.; Hansson, L.-O.; Marchini, G. The Stress of Birth Enhances in Vitro Spontaneous and IL-8-Induced Neutrophil Chemotaxis in the Human Newborn. Pediatr. Allergy Immunol. 2007, 18, 643–651. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of Peptidoglycan from the Microbiota by Nod1 Enhances Systemic Innate Immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef] [Green Version]
- Sridharan, G.V.; Choi, K.; Klemashevich, C.; Wu, C.; Prabakaran, D.; Pan, L.B.; Steinmeyer, S.; Mueller, C.; Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and Quantification of Bioactive Microbiota Metabolites in the Mouse Gut. Nat. Commun. 2014, 5, 5492. [Google Scholar] [CrossRef] [Green Version]
- Lajqi, T.; Braun, M.; Kranig, S.A.; Frommhold, D.; Johannes, P.; Hudalla, H. LPS Induces Opposing Memory-like Inflammatory Responses in Mouse Bone Marrow Neutrophils. Int. J. Mol. Sci. 2021, 22, 9803. [Google Scholar] [CrossRef]
- Lajqi, T.; Frommhold, D.; Braun, M.; Kranig, S.A.; Pöschl, J.; Hudalla, H. Gram-Positive Staphylococcus Aureus LTA Promotes Distinct Memory-like Effects in Murine Bone Marrow Neutrophils. Preprints 2021, 2021120009. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-Schrepping, J.; Li, X.; Hatzioannou, A.; Grinenko, T.; Hagag, E.; Sinha, A.; Has, C.; Dietz, S.; et al. Innate Immune Training of Granulopoiesis Promotes Anti-Tumor Activity. Cell 2020, 183, 771–785. [Google Scholar] [CrossRef]
- Hergott, C.B.; Roche, A.M.; Tamashiro, E.; Clarke, T.B.; Bailey, A.G.; Laughlin, A.; Bushman, F.D.; Weiser, J.N. Peptidoglycan from the Gut Microbiota Governs the Lifespan of Circulating Phagocytes at Homeostasis. Blood 2016, 127, 2460–2471. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.-E.; Scheiermann, C.; et al. Neutrophil Ageing Is Regulated by the Microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef]
- Zhang, D.; Frenette, P.S. Cross Talk between Neutrophils and the Microbiota. Blood 2019, 133, 2168–2177. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The Microbiota Regulates Neutrophil Homeostasis and Host Resistance to Escherichia Coli K1 Sepsis in Neonatal Mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Dietz, S.; Schwarz, J.; Rühle, J.; Schaller, M.; Fehrenbacher, B.; Marmé, A.; Schmid, E.; Peter, A.; Poets, C.F.; Gille, C.; et al. Extracellular Vesicles Released by Myeloid-Derived Suppressor Cells from Pregnant Women Modulate Adaptive Immune Responses. Cell. Immunol. 2021, 361, 104276. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Frommhold, D.; Kamphues, A.; Hepper, I.; Pruenster, M.; Lukić, I.K.; Socher, I.; Zablotskaya, V.; Buschmann, K.; Lange-Sperandio, B.; Schymeinsky, J.; et al. RAGE and ICAM-1 Cooperate in Mediating Leukocyte Recruitment during Acute Inflammation in Vivo. Blood 2010, 116, 841–849. [Google Scholar] [CrossRef]
- Schymeinsky, J.; Sindrilaru, A.; Frommhold, D.; Sperandio, M.; Gerstl, R.; Then, C.; Mócsai, A.; Scharffetter-Kochanek, K.; Walzog, B. The Vav Binding Site of the Non–Receptor Tyrosine Kinase Syk at Tyr 348 Is Critical for Β2 Integrin (CD11/CD18)–Mediated Neutrophil Migration. Blood 2006, 108, 3919–3927. [Google Scholar] [CrossRef]
- Blundell, E.L.C.J.; Vogel, R.; Platt, M. Particle-by-Particle Charge Analysis of DNA-Modified Nanoparticles Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 1082–1090. [Google Scholar] [CrossRef] [Green Version]
- Blundell, E.L.C.J.; Mayne, L.J.; Lickorish, M.; Christie, S.D.R.; Platt, M. Protein Detection Using Tunable Pores: Resistive Pulses and Current Rectification. Faraday Discuss. 2016, 193, 487–505. [Google Scholar] [CrossRef] [Green Version]
- Blundell, E.L.C.J.; Healey, M.J.; Holton, E.; Sivakumaran, M.; Manstana, S.; Platt, M. Characterisation of the Protein Corona Using Tunable Resistive Pulse Sensing: Determining the Change and Distribution of a Particle’s Surface Charge. Anal. Bioanal. Chem. 2016, 408, 5757–5768. [Google Scholar] [CrossRef] [Green Version]
- Balta, E.; Hardt, R.; Liang, J.; Kirchgessner, H.; Orlik, C.; Jahraus, B.; Hillmer, S.; Meuer, S.; Hübner, K.; Wabnitz, G.H.; et al. Spatial Oxidation of L-Plastin Downmodulates Actin-Based Functions of Tumor Cells. Nat. Commun. 2019, 10, 4073. [Google Scholar] [CrossRef] [Green Version]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harb. Protoc. 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.; Domiguéz-Andrés, J.; Netea, M.G. The Role of Cell Metabolism in Innate Immune Memory. J. Innate Immun. 2021, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Latz, E.; Mills, K.H.G.; O'Neill, L.A.J. Innate Immune Memory: A Paradigm Shift in Understanding Host Defense. Nat. Immunol. 2015, 16, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, P.; Majcherczyk, P.A. Proinflammatory Activity of Cell-Wall Constituents from Gram-Positive Bacteria. Scand. J. Infect. Dis. 2003, 35, 632–641. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, K.H.; Kim, Y.S.; Hong, B.S.; Kim, O.Y.; Kim, J.H.; Yoon, C.M.; Koh, G.Y.; Kim, Y.K.; Gho, Y.S. Outer Membrane Vesicles Derived from Escherichia Coli Induce Systemic Inflammatory Response Syndrome. PLoS ONE 2010, 5, e11334. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Sullivan, C.J.; Lonergan, N.E.; Stanley, S.; Soult, M.C.; Britt, L.D. Circulating Bacterial Membrane Vesicles Cause Sepsis in Rats. Shock 2012, 37, 621–628. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, J.; Lee, C.; Park, H.T.; Kim, J.W.; Kim, O.Y.; Kim, S.R.; Rådinger, M.; Jung, H.Y.; Park, J.; et al. Sepsis-like Systemic Inflammation Induced by Nano-Sized Extracellular Vesicles from Feces. Front. Microbiol. 2018, 9, 1735. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, M.J.; Kesty, N.C. Bacterial Outer Membrane Vesicles and the Host-Pathogen Interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef] [Green Version]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Tulkens, J.; De Wever, O.; Hendrix, A. Analyzing Bacterial Extracellular Vesicles in Human Body Fluids by Orthogonal Biophysical Separation and Biochemical Characterization. Nat. Protoc. 2020, 15, 40–67. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Lathwal, S.; Yerneni, S.S.; Boye, S.; Muza, U.L.; Takahashi, S.; Sugimoto, N.; Lederer, A.; Das, S.R.; Campbell, P.G.; Matyjaszewski, K. Engineering Exosome Polymer Hybrids by Atom Transfer Radical Polymerization. Proc. Natl. Acad. Sci. USA 2021, 118, e2020241118. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Sekhar Nanda, H.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Pharmacokinetics of Exosomes—An Important Factor for Elucidating the Biological Roles of Exosomes and for the Development of Exosome-Based Therapeutics. J. Pharm. Sci. 2017, 106, 2265–2269. [Google Scholar] [CrossRef] [Green Version]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Artis, D. Epithelial-Cell Recognition of Commensal Bacteria and Maintenance of Immune Homeostasis in the Gut. Nat. Rev. Immunol. 2008, 8, 411–420. [Google Scholar] [CrossRef]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate Immune Memory in the Brain Shapes Neurological Disease Hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef]
- Kalafati, L.; Mitroulis, I.; Verginis, P.; Chavakis, T.; Kourtzelis, I. Neutrophils as Orchestrators in Tumor Development and Metastasis Formation. Front. Oncol. 2020, 10, 581457. [Google Scholar] [CrossRef]
- van der Valk, F.M.; Bekkering, S.; Kroon, J.; Yeang, C.; Van den Bossche, J.; van Buul, J.D.; Ravandi, A.; Nederveen, A.J.; Verberne, H.J.; Scipione, C.; et al. Oxidized Phospholipids on Lipoprotein(a) Elicit Arterial Wall Inflammation and an Inflammatory Monocyte Response in Humans. Circulation 2016, 134, 611–624. [Google Scholar] [CrossRef]
- Théroude, C.; Reverte, M.; Heinonen, T.; Ciarlo, E.; Schrijver, I.T.; Antonakos, N.; Maillard, N.; Pralong, F.; Le Roy, D.; Roger, T. Trained Immunity Confers Prolonged Protection From Listeriosis. Front. Immunol. 2021, 12, 723393. [Google Scholar] [CrossRef]
- Schaar, V.; De Vries, S.P.W.; Perez Vidakovics, M.L.A.; Bootsma, H.J.; Larsson, L.; Hermans, P.W.M.; Bjartell, A.; Mörgelin, M.; Riesbeck, K. Multicomponent Moraxella Catarrhalis Outer Membrane Vesicles Induce an Inflammatory Response and Are Internalized by Human Epithelial Cells. Cell. Microbiol. 2011, 13, 432–449. [Google Scholar] [CrossRef]
- Van Bergenhenegouwen, J.; Kraneveld, A.D.; Rutten, L.; Kettelarij, N.; Garssen, J.; Vos, A.P. Extracellular Vesicles Modulate Host-Microbe Responses by Altering TLR2 Activity and Phagocytosis. PLoS ONE 2014, 9, e89121. [Google Scholar] [CrossRef]
- Fábrega, M.J.; Aguilera, L.; Giménez, R.; Varela, E.; Cañas, M.A.; Antolín, M.; Badía, J.; Baldomà, L. Activation of Immune and Defense Responses in the Intestinal Mucosa by Outer Membrane Vesicles of Commensal and Probiotic Escherichia Coli Strains. Front. Microbiol. 2016, 7, 705. [Google Scholar] [CrossRef] [Green Version]
- Molina-Tijeras, J.A.; Gálvez, J.; Rodríguez-Cabezas, M.E. The Immunomodulatory Properties of Extracellular Vesicles Derived from Probiotics: A Novel Approach for the Management of Gastrointestinal Diseases. Nutrients 2019, 11, 1038. [Google Scholar] [CrossRef] [Green Version]
- Useckaite, Z.; Ward, M.P.; Trappe, A.; Reilly, R.; Lennon, J.; Davage, H.; Matallanas, D.; Cassidy, H.; Dillon, E.T.; Brennan, K.; et al. Increased Extracellular Vesicles Mediate Inflammatory Signalling in Cystic Fibrosis. Thorax 2020, 75, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Vince, R.V.; Chrismas, B.; Midgley, A.W.; McNaughton, L.R.; Madden, L.A. Hypoxia Mediated Release of Endothelial Microparticles and Increased Association of S100A12 with Circulating Neutrophils. Oxid. Med. Cell. Longev. 2009, 2, 2–6. [Google Scholar] [CrossRef]
- Coffey, M.J.; Nielsen, S.; Wemheuer, B.; Kaakoush, N.O.; Garg, M.; Needham, B.; Pickford, R.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Gut Microbiota in Children With Cystic Fibrosis: A Taxonomic and Functional Dysbiosis. Sci. Rep. 2019, 9, 18593. [Google Scholar] [CrossRef]
- Dayama, G.; Priya, S.; Niccum, D.E.; Khoruts, A.; Blekhman, R. Interactions between the Gut Microbiome and Host Gene Regulation in Cystic Fibrosis. Genome Med. 2020, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulos, A.; Kalluri, R. Emerging Role of Bacterial Extracellular Vesicles in Cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabroe, I.; Dower, S.K.; Whyte, M.K.B. The Role of Toll-like Receptors in the Regulation of Neutrophil Migration, Activation, and Apoptosis. Clin. Infect. Dis. 2005, 41 (Suppl. S7), S421–S426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castoldi, A.; Braga, T.T.; Correa-Costa, M.; Aguiar, C.F.; Bassi, Ê.J.; Correa-Silva, R.; Elias, R.M.; Salvador, F.; Moraes-Vieira, P.M.; Cenedeze, M.A.; et al. TLR2, TLR4 and the Myd88 Signaling Pathway Are Crucial for Neutrophil Migration in Acute Kidney Injury Induced by Sepsis. PLoS ONE 2012, 7, e37584. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Freitas, A.; Souto, F.O.; Spiller, F.; Paula-Neto, H.; Silva, J.S.; Gazzinelli, R.T.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Regulation of Chemokine Receptor by Toll-like Receptor 2 Is Critical to Neutrophil Migration and Resistance to Polymicrobial Sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4018–4023. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, F.; Means, T.K.; Luster, A.D. Toll-like Receptors Stimulate Human Neutrophil Function. Blood 2003, 102, 2660–2669. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Athman, J.J.; Wang, Y.; McDonald, D.J.; Boom, W.H.; Harding, C.V.; Wearsch, P.A. Bacterial Membrane Vesicles Mediate the Release of Mycobacterium Tuberculosis Lipoglycans and Lipoproteins from Infected Macrophages. J. Immunol. 2015, 195, 1044–1053. [Google Scholar] [CrossRef] [Green Version]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’Cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial Membrane Vesicles Transport Their DNA Cargo into Host Cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef]
- Li, S.P.; Lin, Z.X.; Jiang, X.Y.; Yu, X.Y. Exosomal Cargo-Loading and Synthetic Exosome-Mimics as Potential Therapeutic Tools. Acta Pharmacol. Sin. 2018, 39, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Hagai, T.; Chen, X.; Miragaia, R.J.; Rostom, R.; Gomes, T.; Kunowska, N.; Henriksson, J.; Park, J.E.; Proserpio, V.; Donati, G.; et al. Gene Expression Variability across Cells and Species Shapes Innate Immunity. Nature 2018, 563, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in Innate Immune Response between Man and Mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef] [Green Version]


| Gene Name | Primer Sequence (5′–3′) | |
|---|---|---|
| IL-10 (Interleukin 10) | Forward: Reverse: | ACCAGCTGGACAACATACTGC TCACTCTTCACCTGCTCCACT |
| CD11a (Integrin α-L) | Forward: Reverse: | AGATCGAGTCCGGACCCACAG GGCAGTGATAGAGGCCTCCCG |
| CD32 (Cluster of differentiation 32) | Forward: Reverse: | AATCCTGCCGTTCCTACTGATC GTGTCACCGTGTCTTCCTTGAG |
| GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) | Forward: Reverse: | CATGGCCTTCCGTGTTTCCTA CCTGCTTCACCACCTTCTTGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajqi, T.; Köstlin-Gille, N.; Hillmer, S.; Braun, M.; Kranig, S.A.; Dietz, S.; Krause, C.; Rühle, J.; Frommhold, D.; Pöschl, J.; et al. Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils. Biomedicines 2022, 10, 442. https://doi.org/10.3390/biomedicines10020442
Lajqi T, Köstlin-Gille N, Hillmer S, Braun M, Kranig SA, Dietz S, Krause C, Rühle J, Frommhold D, Pöschl J, et al. Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils. Biomedicines. 2022; 10(2):442. https://doi.org/10.3390/biomedicines10020442
Chicago/Turabian StyleLajqi, Trim, Natascha Köstlin-Gille, Stefan Hillmer, Maylis Braun, Simon A. Kranig, Stefanie Dietz, Christian Krause, Jessica Rühle, David Frommhold, Johannes Pöschl, and et al. 2022. "Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils" Biomedicines 10, no. 2: 442. https://doi.org/10.3390/biomedicines10020442
APA StyleLajqi, T., Köstlin-Gille, N., Hillmer, S., Braun, M., Kranig, S. A., Dietz, S., Krause, C., Rühle, J., Frommhold, D., Pöschl, J., Gille, C., & Hudalla, H. (2022). Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils. Biomedicines, 10(2), 442. https://doi.org/10.3390/biomedicines10020442







