Environmental Nanoparticles Reach Human Fetal Brains
Abstract
:1. Introduction
2. Materials and Methods
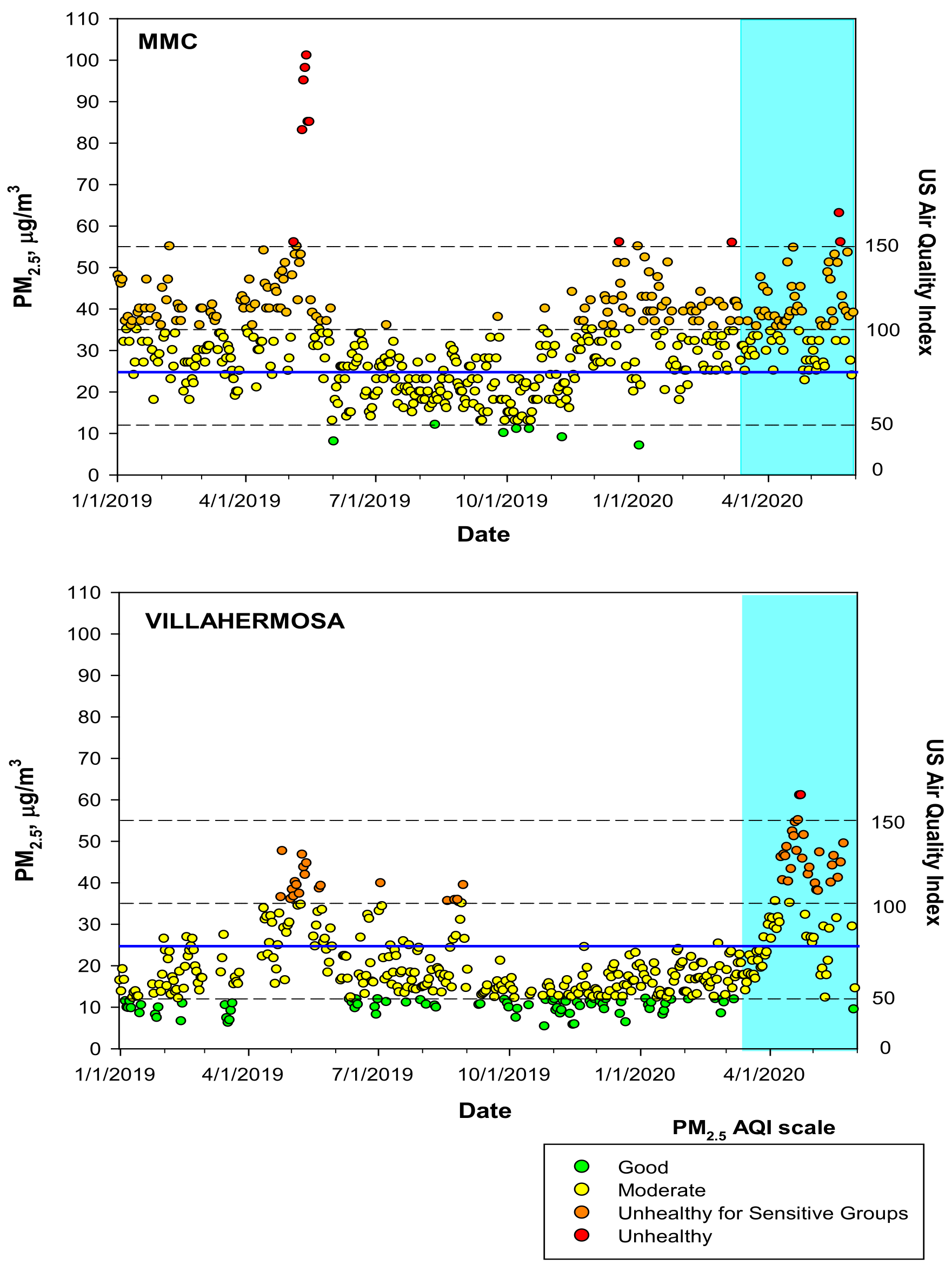
2.1. Study Cities and Air Quality
2.2. Preeclamptic, Normal Term, and Postconceptional Weeks (PCW) 12–15 Placentas and Products
3. Results
3.1. Air Pollution
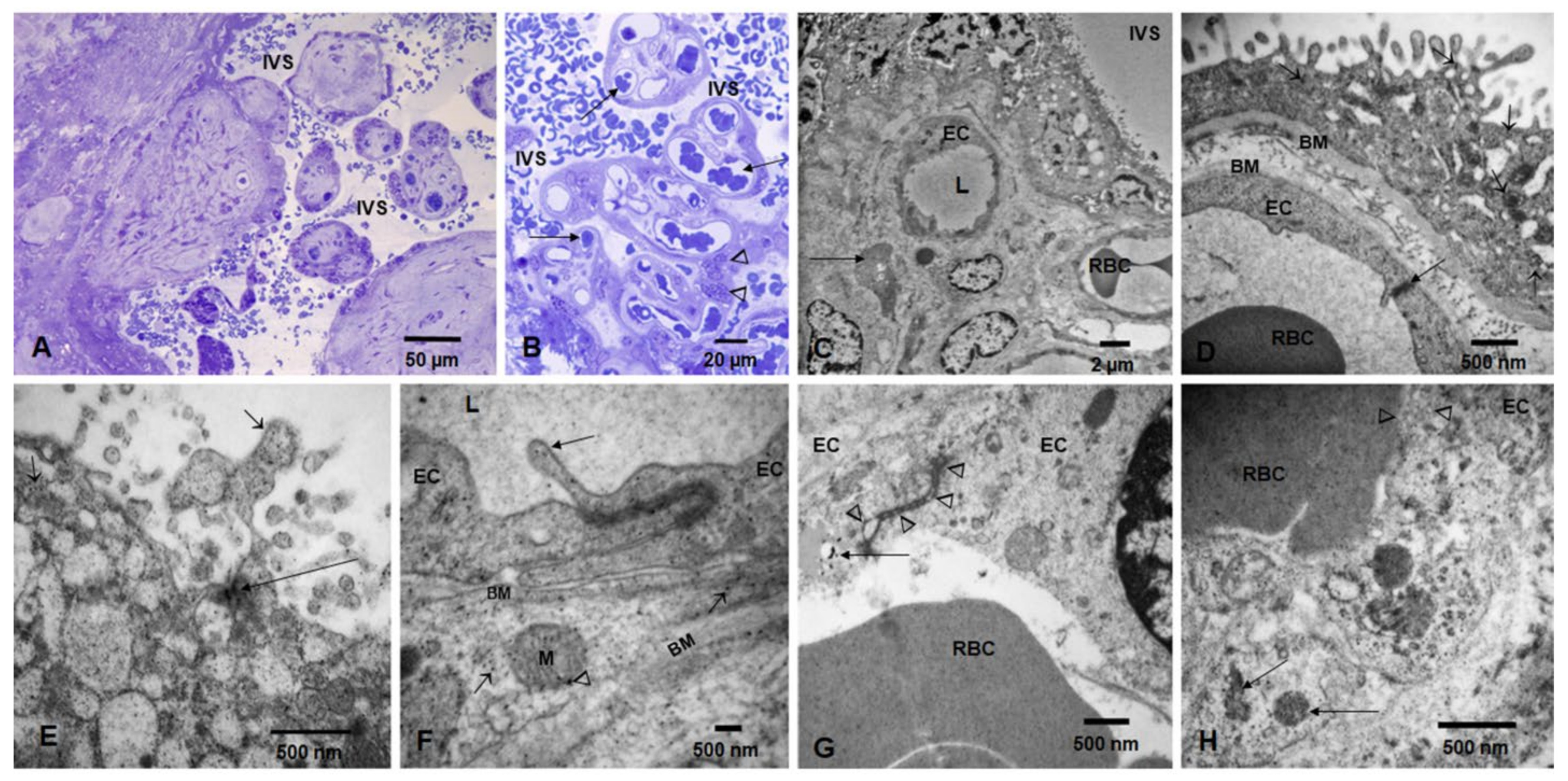
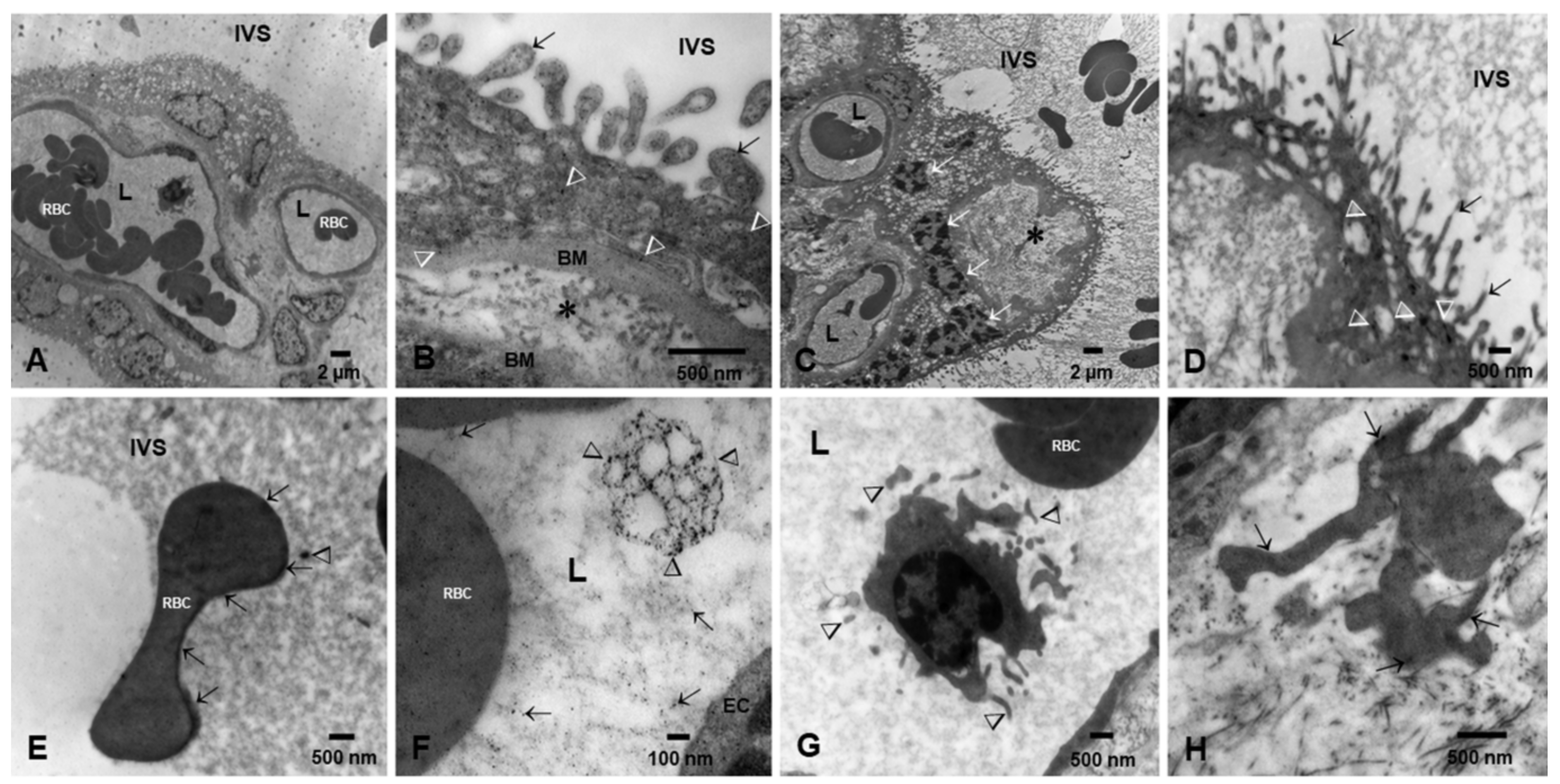
3.2. Placental Light Microscopy and Transmission Electron Microscopy (TEM)
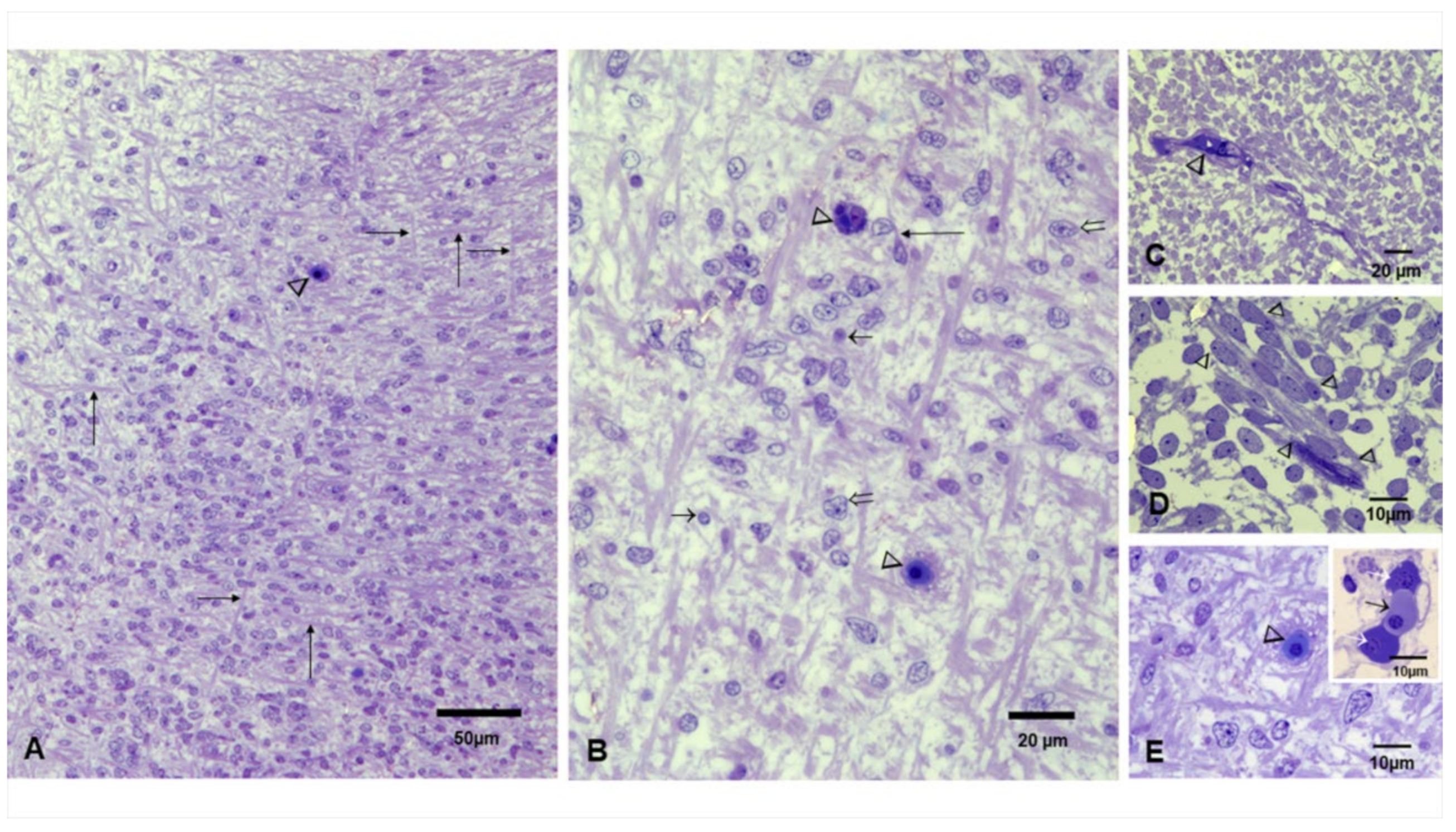
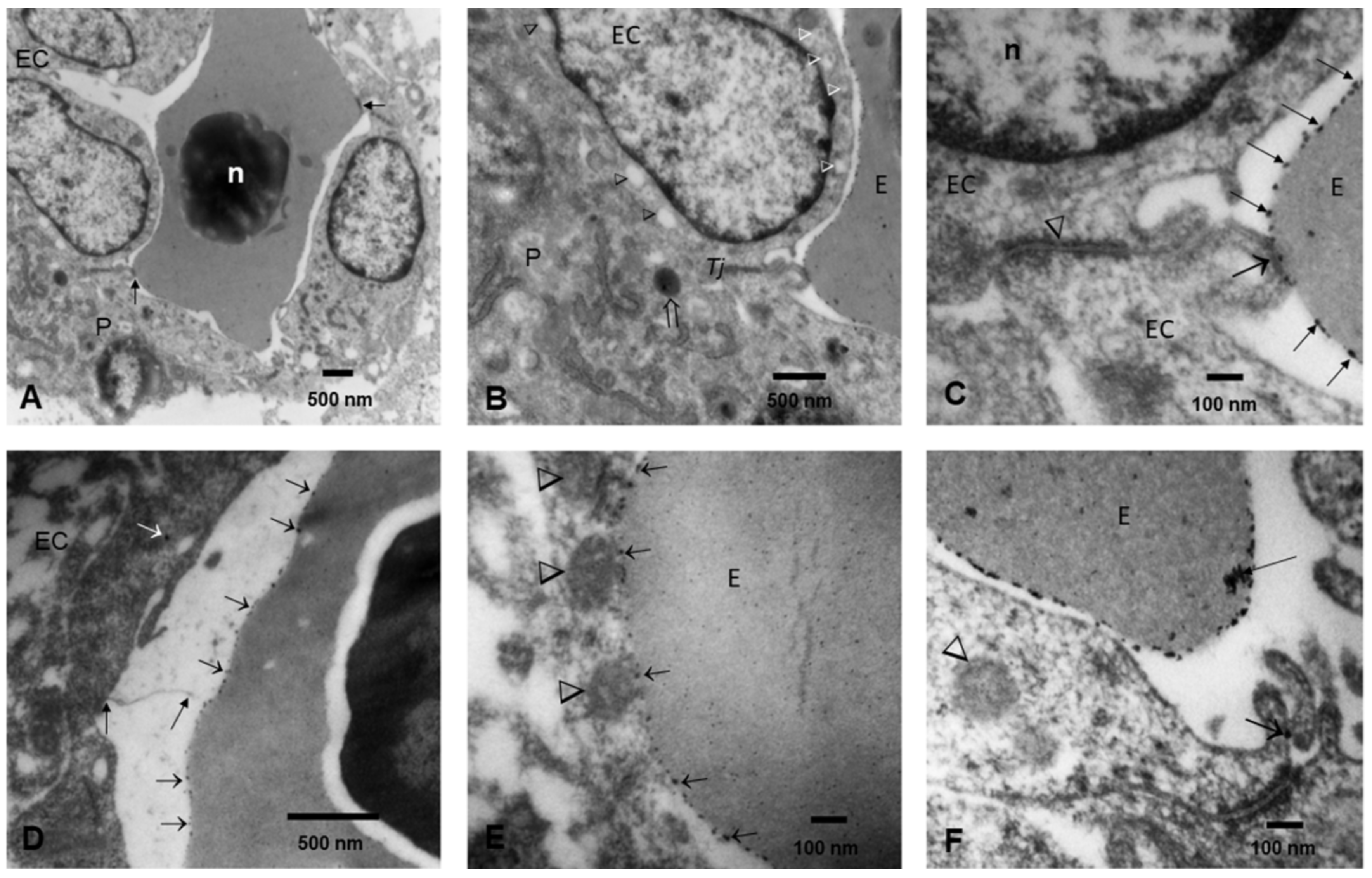
3.3. Fetal Brains Light Microscopy and TEM
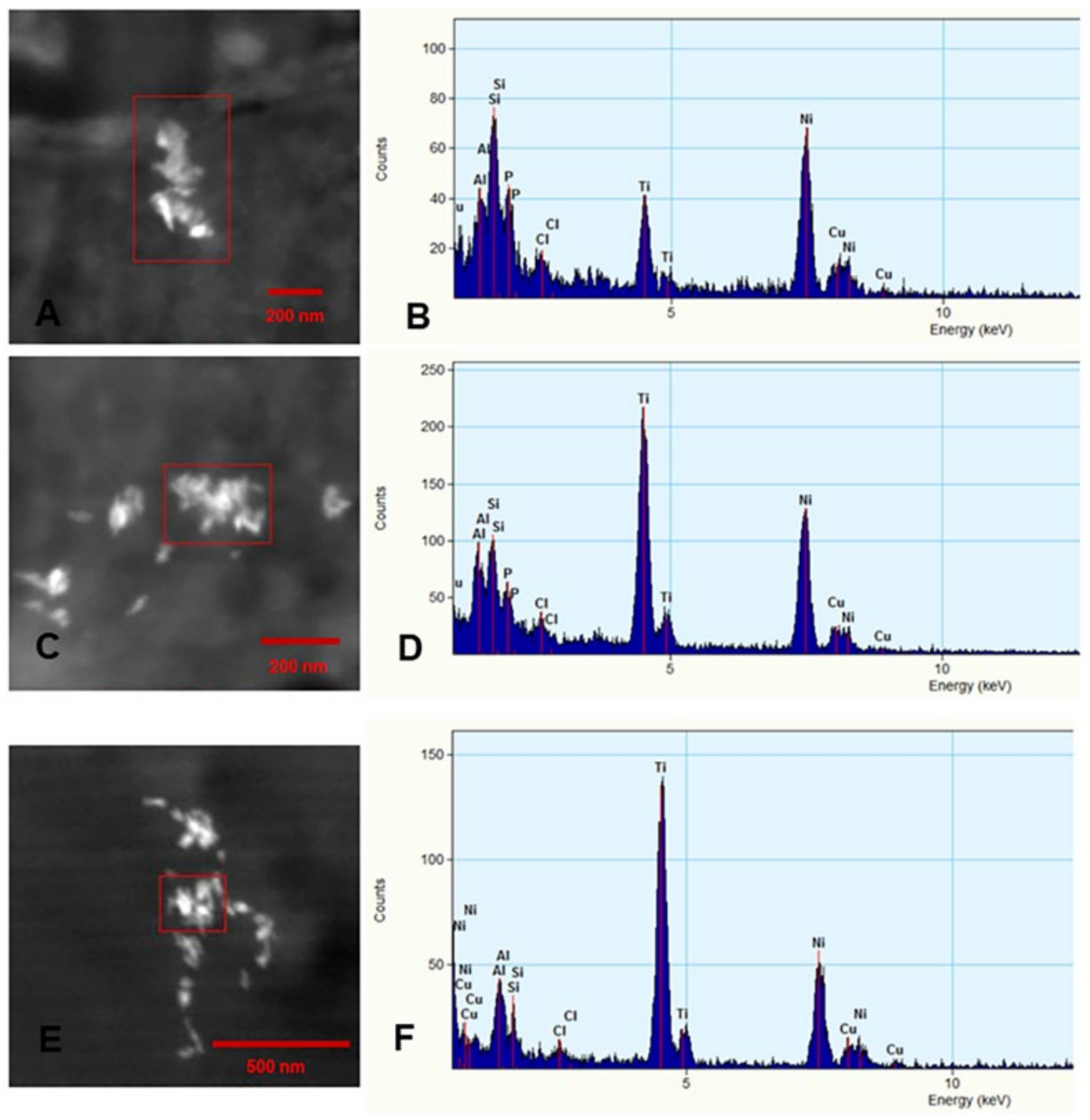
3.4. Energy Dispersive X-ray Spectrometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, J.D.; Bhatt, D.L.; Rajagopalan, S.; Balmes, J.R.; Brauer, M.; Breysse, P.N.; Brown, A.G.; Carnethon, M.R.; Cascio, W.E.; Collman, G.W.; et al. Cardiopulmonary Impact of Particulate Air Pollution in High-Risk Populations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2878–2894. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Mudway, I.; Booth, A.; Peters, J.; Anstey, K.J. Putting Fine Particulate Matter and Dementia in the Wider Context of Noncommunicable Disease: Where are We Now and What Should We Do Next: A Systematic Review. Neuroepidemiology 2021, 55, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Reynoso-Robles, R.; González-Maciel, A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: The culprit of Alzheimer and Parkinson’s diseases. Environ. Res. 2019, 176, 108574. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Stommel, E.W.; Rajkumar, R.P.; Mukherjee, P.S.; Ayala, A. Particulate Air Pollution and Risk of Neuropsychiatric Outcomes. What We Breathe, Swallow, and Put on Our Skin Matters. Int. J. Environ. Res. Public Health 2021, 18, 11568. [Google Scholar] [CrossRef] [PubMed]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [Green Version]
- Cleland, S.E.; West, J.J.; Jia, Y.; Reid, S.; Raffuse, S.; O’Neill, S.; Serre, M.L. Estimating Wildfire Smoke Concentrations during the October 2017 California Fires through BME Space/Time Data Fusion of Observed, Modeled, and Satellite-Derived PM2.5. Environ. Sci. Technol. 2020, 54, 13439–13447. [Google Scholar] [CrossRef]
- Yu, C.; Pasternak, D.; Lee, J.; Yang, M.; Bell, T.; Bower, K.; Wu, H.; Liu, D.; Reed, C.; Bauguitte, S.; et al. Characterizing the Particle Composition and Cloud Condensation Nuclei from Shipping Emission in Western Europe. Environ. Sci. Technol. 2020, 54, 15604–15612. [Google Scholar] [CrossRef]
- Saikawa, E.; Wu, Q.; Zhong, M.; Avramov, A.; Ram, K.; Stone, E.A.; Stockwell, C.E.; Jayarathne, T.; Panday, A.K.; Yokelson, R.J. Garbage Burning in South Asia: How Important Is It to Regional Air Quality? Environ. Sci. Technol. 2020, 54, 9928–9938. [Google Scholar] [CrossRef]
- Bongaerts, E.; Nawrot, T.S.; Van Pee, T.; Ameloot, M.; Bové, H. Translocation of (ultra)fine particles and nanoparticles across the placenta; a systematic review on the evidence of in vitro, ex vivo, and in vivo studies. Part. Fibre Toxicol. 2020, 17, 1–26. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Huang, F.; Liu, X.; Wang, Z.; Yan, B. Breakthrough of ZrO2 nanoparticles into fetal brains depends on developmental stage of maternal placental barrier and fetal blood-brain-barrier. J. Hazard. Mater. 2020, 402, 123563. [Google Scholar] [CrossRef]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of metallic nanoparticles. WIREs Nanomed. Nanobiotechnol. 2014, 7, 189–217. [Google Scholar] [CrossRef]
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the field forward! Nanomedicine 2016, 11, 941–957. [Google Scholar] [CrossRef] [PubMed]
- Bové, H.; Bongaerts, E.; Slenders, E.; Bijnens, E.M.; Saenen, N.D.; Gyselaers, W.; Van Eyken, P.; Plusquin, M.; Roeffaers, M.B.J.; Ameloot, M.; et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 2019, 10, 3866. [Google Scholar] [CrossRef] [PubMed]
- Aengenheister, L.; Dugershaw, B.B.; Manser, P.; Wichser, A.; Schoenenberger, R.; Wick, P.; Hesler, M.; Kohl, Y.; Straskraba, S.; Suter, M.J.-F.; et al. Investigating the accumulation and translocation of titanium dioxide nanoparticles with different surface modifications in static and dynamic human placental transfer models. Eur. J. Pharm. Biopharm. 2019, 142, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Jia, J.; Wang, Z.; Sharma, V.K.; Yan, B. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol. Environ. Saf. 2019, 182, 109439. [Google Scholar] [CrossRef]
- Guillard, A.; Gaultier, E.; Cartier, C.; Devoille, L.; Noireaux, J.; Chevalier, L.; Morin, M.; Grandin, F.; Lacroix, M.Z.; Coméra, C.; et al. Basal Ti level in the human placenta and meconium and evidence of a materno-foetal transfer of food-grade TiO2 nanoparticles in an ex vivo placental perfusion model. Part. Fibre Toxicol. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Liu, N.M.; Miyashita, L.; Maher, B.A.; McPhail, G.; Jones, C.J.; Barratt, B.; Thangaratinam, S.; Karloukovski, V.; Ahmed, I.A.; Aslam, Z.; et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. 2020, 751, 142235. [Google Scholar] [CrossRef] [PubMed]
- Dugershaw, B.B.; Aengenheister, L.; Hansen, S.S.K.; Hougaard, K.S.; Buerki-Thurnherr, T. Recent insights on indirect mechanisms in developmental toxicity of nanomaterials. Part. Fibre Toxicol. 2020, 17, 31. [Google Scholar] [CrossRef]
- Barker, D.J.P. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. [Google Scholar] [CrossRef]
- Fanni, D.; Gerosa, C.; Rais, M.; Ravarino, A.; Van Eyken PFanos, V.; Faa, G. The role of neuropathological markers in the interpretation of neuropsychiatric disorders: Focus on fetal and perinatal programming. Neurosci. Lett. 2018, 669, 75–82. [Google Scholar] [CrossRef]
- Lamballais, S.; Adank, M.C.; Hussainali, R.F.; Schalekamp-Timmermans, S.; Vernooij, M.W.; Luik, A.I.; Steegers, E.A.P.; Ikram, M.A. Design and overview of the Origins of Alzheimer’s Disease Across the Life course (ORACLE) study. Eur. J. Epidemiol. 2020, 36, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Leon, R.; Chalak, L.F. Placental origins of neonatal diseases: Toward a precision medicine approach. Pediatr. Res. 2020, 89, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Arogbokun, O.; Rosen, E.; Keil, A.P.; Milne, G.L.; Barrett, E.; Nguyen, R.; Bush, N.R.; Swan, S.H.; Sathyanarayana, S.; Ferguson, K.K. Maternal Oxidative Stress Biomarkers in Pregnancy and Child Growth from Birth to Age 6. J. Clin. Endocrinol. Metab. 2021, 106, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Katsuragi, S. Relationship between Birth Weight and Future Cardiovascular Risks in Japanese in View of the Developmental Origins of Health and Disease (DOHad) Theory. J. Atheroscler. Thromb. 2022, 29, 146–147. [Google Scholar] [CrossRef]
- Amgalan, A.; Andescavage, N.; Limperopoulos, C. Prenatal origins of neuropsychiatric diseases. Acta Paediatr. 2021, 110, 1741–1749. [Google Scholar] [CrossRef]
- Chu, A.H.Y.; Godfrey, K.M. Gestational Diabetes Mellitus and Developmental Programming. Ann. Nutr. Metab. 2021, 76 (Suppl. 3), 4–15. [Google Scholar] [CrossRef]
- Ursini, G.; Punzi, G.; Langworthy, B.W.; Chen, Q.; Xia, K.; Cornea, E.A.; Goldman, B.D.; Styner, M.A.; Knickmeyer, R.C.; Gilmore, J.H.; et al. Placental genomic risk scores and early neurodevelopmental outcomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2019789118. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P. Biofabricated zinc oxide nanoparticles impair cognitive function via modulating oxidative stress and acetylcholinesterase level in mice. Environ. Toxicol. 2020, 36, 572–585. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Sałek, M.; Gewartowska, M.; Sidoryk-Węgrzynowicz, M.; Strużyńska, L. Early Postnatal Exposure to a Low Dose of Nanoparticulate Silver Induces Alterations in Glutamate Transporters in Brain of Immature Rats. Int. J. Mol. Sci. 2020, 21, 8977. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814. [Google Scholar] [CrossRef]
- Virgintino, D.; Maiorano, E.; Errede, M.; Vimercati, A.; Greco, P.; Selvaggi, L.; Roncali, L.; Bertossi, M. Astroglia-microvessel relationship in the developing human telencephalon. Int. J. Dev. Biol. 1998, 42, 1165–1168. [Google Scholar]
- Virgintino, D.; Girolamo, F.; Errede, M.; Capobianco, C.; Robertson, D.; Stallcup, W.B.; Perris, R.; Roncali, L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 2007, 10, 35–45. [Google Scholar] [CrossRef]
- Bertossi, M.; Virgintino, D.; Errede, M.; Roncali, L. Immunohistochemical and Ultrastructural Characterization of Cortical Plate Microvasculature in the Human Fetus Telencephalon. Microvasc. Res. 1999, 58, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Girolamo, F.; Virgintino, D. High-Resolution Confocal Imaging of Pericytes in Human Fetal Brain Microvessels. Methods Mol. Biol. 2020, 2206, 143–150. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidoryk-Wegrzynowicz, M.; Strużyńska, L. Dysfunctional glia: Contributors to neurodegenerative disorders. Neural Regen. Res. 2012, 16, 218–222. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef]
- Torres-Jardón, R. Politicas públicas y su efecto en la calidad del aire de la Zona metropolitana de la Ciudad de Mexico. In Transversalidad de la Politica del Aire en Mexico.G.S.; Nunez, S., Ed.; Instituto de Investigaciones Dr. Jose Maria Luis Mora: Ciudad de México, Mexico, 2018; pp. 43–74. [Google Scholar]
- Caudillo, L.; Salcedo, D.; Peralta, O.; Castro, T.; Alvarez-Ospina, H. Nanoparticle size distributions in Mexico City. Atmos. Pollut. Res. 2019, 11, 78–84. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metalrich magnetic nanoparticles (and engineered Tirich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Hernández-Luna, J.; Ávila-Cervantes, R.; Macías-Escobedo, E.; González-González, O.; González-Maciel, A.; García-Hernández, K.; et al. Mild Cognitive Impairment and Dementia Involving Multiple Cognitive Domains in Mexican Urbanites. J. Alzheimers Dis. 2019, 68, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- SERNAMAP. Programa de Gestión para Mejorar la Calidad del Aire del Estado de Tabasco 2018–2027. Secretaría de Energía, Recursos Naturales y Protección Ambiental de Tabasco. 2018. Available online: https://tabasco.gob.mx/proaire-tabasco-2018-2027 (accessed on 29 December 2021).
- Gatti, A.M.; Montanari, S.; Ferrero, S.; Lavezzi, A.M. Silver nanoparticles in the fetal brain: New perspectives in understanding the pathogenesis of unexplained stillbirths. Nanomedicine 2021, 16, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Onoda, A.; Kawasaki, T.; Tsukiyama, K.; Takeda, K.; Umezawa, M. Carbon nanoparticles induce endoplasmic reticulum stress around blood vessels with accumulation of misfolded proteins in the developing brain of offspring. Sci. Rep. 2020, 10, 10028. [Google Scholar] [CrossRef]
- Coccini, T.; Pignatti, P.; Spinillo, A.; De Simone, U. Developmental Neurotoxicity Screening for Nanoparticles Using Neuron-Like Cells of Human Umbilical Cord Mesenchymal Stem Cells: Example with Magnetite Nanoparticles. Nanomaterials 2020, 10, 1607. [Google Scholar] [CrossRef]
- Mallard, C.; Ek, C.J.; Vexler, Z.S. The myth of the immature barrier systems in the developing brain: Role in perinatal brain injury. J. Physiol. 2018, 596, 5655–5664. [Google Scholar] [CrossRef]
- Volkamer, R.; Sheehy, P.; Molina, L.T.; Molina, M.J. Oxidative capacity of the Mexico City atmosphere—Part 1: A radical source perspective. Atmos. Chem. Phys. 2010, 10, 6969–6991. [Google Scholar] [CrossRef] [Green Version]
- SEDEMA. Secretaría del Medio Ambiente de la Ciudad de México. Inventario de Emisiones de la Ciudad de México 2016. Ciudad de México. Septiembre. 2018. Available online: http://www.aire.cdmx.gob.mx/descargas/publicaciones/flippingbook/inventario-emisiones-2016/mobile/#p=1 (accessed on 29 December 2021).
- Molina, L.T.; Velasco, E.; Retama, A.; Zavala, M. Experience from Integrated Air Quality Management in the Mexico City Metropolitan Area and Singapore. Atmosphere 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Velasco, E.; Retama, A.; Segovia, E.; Ramos, R. Particle exposure and inhaled dose while commuting by public transport in Mexico City. Atmos. Environ. 2019, 219, 117044. [Google Scholar] [CrossRef]
- Mugica-Alvarez, V.; Figueroa-Lara, J.D.J.; Romo, M.A.R.; Sepúlveda-Sánchez, J.; López-Moreno, T. Concentrations and properties of airborne particles in the Mexico City subway system. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
- McDonald-Buller, E.; McGaughey, G.; Grant, J.; Shah, T.; Kimura, Y.; Yarwood, G. Emissions and Air Quality Implications of Up-stream and Midstream Oil and Gas Operations in Mexico. Atmosphere 2021, 12, 1696. [Google Scholar] [CrossRef]
- INEC. Determinación de Factores de Emisión para Emisiones Fugitivas de la Industria Petrolera en México Instituto Nacional de Ecología y Cambio Climático. México. DF. Enero. 2013. Available online: https://www.gob.mx/inecc/documentos/determinacion-de-factores-de-emision-para-emisiones-fugitivas-de-la-industria-petrolera-en-mexico (accessed on 29 December 2021).
- Villasenor, R.; Magdaleno, M.; Quintanar, A.; Gallardo, J.; López, M.; Jurado, R.; Miranda, A.; Aguilar, M.; Melgarejo, L.; Palmerín, E.; et al. An air quality emission inventory of offshore operations for the exploration and production of petroleum by the Mexican oil industry. Atmos. Environ. 2003, 37, 3713–3729. [Google Scholar] [CrossRef]
- Pinkus-Rendón, M.J.; Contreras-Sánchez, A. Impacto socioambiental de la industria petrolera en Tabasco: El caso de la Chontalpa. LiminaR 2012, 10, 122–144. [Google Scholar] [CrossRef]
- Del Angel, E.; Vera, R.; Corvo, F. Atmospheric corrosion of galvanized steel in different environments in Chile and Mexico. Int. J. Electrochem. Sci. 2015, 10, 7985–8004. [Google Scholar]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostović, I.; Sedmak, G.; Judaš, M. Neural histology and neurogenesis of the human fetal and infant brain. NeuroImage 2018, 188, 743–773. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Morgan, T.K.; Bednarek, P.; Morita, M.; Burton, G.J.; Lo, J.; Frias, A.E. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: New insights from contrast-enhanced ultrasound and tissue histopathology. Hum. Reprod. 2017, 32, 2382–2393. [Google Scholar] [CrossRef]
- Baron, M.H.; Isern, J.; Fraser, S.T. The embryonic origins of erythropoiesis in mammals. Blood 2012, 119, 4828–4837. [Google Scholar] [CrossRef] [Green Version]
- Dalal, C.; Saha, A.; Jana, N.R. Nanoparticle Multivalency Directed Shifting of Cellular Uptake Mechanism. J. Phys. Chem. C 2016, 120, 6778–6786. [Google Scholar] [CrossRef] [Green Version]
- Panja, P.; Jana, N.R. Arginine-Terminated Nanoparticles of <10 nm Size for Direct Membrane Penetration and Protein Delivery for Straight Access to Cytosol and Nucleus. J. Phys. Chem. Lett. 2020, 11, 2363–2368. [Google Scholar] [CrossRef]
- Debnath, K.; Pal, S.; Jana, N.R. Chemically Designed Nanoscale Materials for Controlling Cellular Processes. Acc. Chem. Res. 2021, 54, 2916–2927. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goasdoue, K.; Miller, S.M.; Coldtz, P.B.; Björkmam, S.T. Review: The blood-brain barrier; protecting the developing fetal brain. Placenta 2017, 54, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Edreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.; Habgood, M.D.; Møllgård, K.; Ebauer, H.-C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Tang, M. The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system. Nanomedicine 2018, 13, 233–249. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nikkhah, M. TiO2 Nanoparticles as Potential Promoting Agents of Fibrillation of α-Synuclein, a Parkinson’s Disease-Related Protein. Iran. J. Biotechnol. 2017, 15, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Semerád, J.; Moeder, M.; Filip, J.; Pivokonský, M.; Filipová, A.; Cajthaml, T. Oxidative stress in microbes after exposure to iron nanoparticles: Analysis of aldehydes as oxidative damage products of lipids and proteins. Environ. Sci. Pollut. Res. Int. 2019, 26, 33670–33682. [Google Scholar] [CrossRef]
- Vereda, F.; De Vicente, J.; Hidalgo-Álvarez, R. Physical Properties of Elongated Magnetic Particles: Magnetization and Friction Coefficient Anisotropies. ChemPhysChem 2009, 10, 1165–1179. [Google Scholar] [CrossRef]
- Abu-Bakr, A.F.; Zubarev, A.Y. On the theory of magnetic hyperthermia: Clusterization of nanoparticles. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2020, 378, 20190251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; Del Bianco, L. Mixing iron oxide nanoparticles with different shape and size for tunable magneto-heating performance. Nanoscale 2021, 13, 5714–5729. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Jabalera, Y.; Chico-Lozano, M.A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Reactive oxygen species (ROS) production in HepG2 cancer cell line through the application of localized alternating magnetic field. J. Mater. Chem. B 2020, 8, 7667–7676. [Google Scholar] [CrossRef] [PubMed]
- Pacakova, B.; Kubickova, S.; Salas, G.; Mantlikova, A.R.; Marciello, M.; Morales, M.P.; Niznansky, D.; Vejpravova, J. The internal structure of magnetic nanoparticles determines the magnetic response. Nanoscale 2017, 9, 5129–5140. [Google Scholar] [CrossRef]
- Pall, M.L. Wi-Fi is an important threat to human health. Environ. Res. 2018, 164, 405–416. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.; Yin, H.; Peng, Y.; Liu, S.; Yao, S.; Zhang, L. Chemical conjugation of FITC to track silica nanoparticles in vivo and in vitro: An emerging method to assess the reproductive toxicity of industrial nanomaterials. Environ. Int. 2021, 152, 106497. [Google Scholar] [CrossRef]
- Li, L.; Deng, Y.; Meng, X.; Chang, H.; Ling, C.; Li, D.; Wang, Q.; Lu, T.; Yang, Y.; Song, G.; et al. Genotoxicity evaluation of silica nanoparticles in murine: A systematic review and meta-analysis. Toxicol. Mech. Methods 2021, 32, 1–17. [Google Scholar] [CrossRef]
- Sungur, S.; Kaya, P.; Koroglu, M. Determination of titanium dioxide nanoparticles used in various foods. Food Addit. Contam. 2020, 13, 260–267. [Google Scholar] [CrossRef]
- Tiede, K.; Boxall, A.B.; Tear, S.P.; Lewis, J.; David, H.; Hassellöv, M. Detection and characterization of engineered nanoparticles in food and the environment. Food Addit. Contam. Part A 2008, 25, 795–821. [Google Scholar] [CrossRef]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.R.; Sangani, N.B.; Fernandes, T.G.; Diogo, M.M.; Curfs, L.M.G.; Reutelingsperger, C.P. Extracellular Vesicles in CNS Developmental Disorders. Int. J. Mol. Sci. 2020, 21, 9428. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Radnaa, E.; Tantengco, O.A.G.; Kechichian, T.; Kammala, A.K.; Sheller-Miller, S.; Taylor, B.D.; Menon, R. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cells. Biol. Reprod. 2021, 105, 464–480. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, G.; Zeng, M.; Feng, W.; Liu, J. Overview of extracellular vesicles in the pathogenesis of preeclampsia. Biol. Reprod. 2021, 105, 32–39. [Google Scholar] [CrossRef]
- Condrat, C.; Varlas, V.; Duică, F.; Antoniadis, P.; Danila, C.; Cretoiu, D.; Suciu, N.; Crețoiu, S.; Voinea, S. Pregnancy-Related Extracellular Vesicles Revisited. Int. J. Mol. Sci. 2021, 22, 3904. [Google Scholar] [CrossRef]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef]
- Daniel, S.; Kloog, I.; Factor-Litvak, P.; Levy, A.; Lunenfeld, E.; Kioumourtzoglou, M.-A. Risk for preeclampsia following exposure to PM2.5 during pregnancy. Environ. Int. 2021, 156, 106636. [Google Scholar] [CrossRef]
- Bearblock, E.; Aiken, C.E.; Burton, G.J. Air pollution and pre-eclampsia; Associations and potential mechanisms. Placenta 2020, 104, 188–194. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsubara, Y.; Uchikura, Y.; Sugiyama, T. Pathophysiology of Preeclampsia: The Role of Exosomes. Int. J. Mol. Sci. 2021, 22, 2572. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A. White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathol. 2017, 134, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Chedotal, A.; Richards, L.J. Wiring the Brain: The Biology of Neuronal Guidance. Cold Spring Harb. Perspect. Biol. 2010, 2, a001917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravishankar, S.; Redline, R.W. The placenta. Handb. Clin. Neurol. 2019, 162, 57–66. [Google Scholar]
- Barron, A.; McCarthy, C.M.; O’Keeffe, G.W. Preeclampsia and Neurodevelopmental Outcomes: Potential Pathogenic Roles for Inflammation and Oxidative Stress? Mol. Neurobiol. 2021, 58, 2734–2756. [Google Scholar] [CrossRef]
- Morton-Bermea, O.; Garza-Galindo, R.; Hernández-Álvarez, E.; Ordoñez-Godínez, S.L.; Amador-Muñoz, O.; Beramendi-Orosco, L. Atmospheric PM 2.5 Mercury in the Metropolitan Area of Mexico City. Bull. Environ. Contam. Toxicol. 2018, 100, 588–592. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Pamphlett, R. The prevalence of inorganic mercury in human cells increases during aging but decreases in the very old. Sci. Rep. 2021, 11, 16714. [Google Scholar] [CrossRef]
- Cernichiari, E.; Brewer, R.; Myers, G.J.; Marsh, D.O.; Lapham, L.W.; Cox, C.; Shamlaye, C.F.; Berlin, M.; Davidson, P.W.; Clarkson, T.W. Monitoring methylmercury during pregnancy: Maternal hair predicts fetal brain exposure. Neuro Toxicol. 1995, 16, 705–710. [Google Scholar]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Bowman, K.; Branfireun, B.; Chetalat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S.; et al. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, V.; Sprovieri, F. Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Tissues | Residency | Women Age | Pregnancy Weeks |
|---|---|---|---|
| Term Normal Placentas n = 7 | MMC | 27.7 ± 7.29 y | 39.12 ± 2.44 |
| Preeclampsia placentas n = 7 | MMC | 25.42 ± 6.5 y | 37.02 ± 2.47 |
| Term Normal Placentas n = 12 | Villahermosa | 26.91 ± 4.1 y | 38.25 ± 1.48 |
| Preeclampsia placentas n = 13 | Villahermosa | 22.07 ± 6.96 y | 36.32 ± 3.14 |
| Early placentas n = 19 * | Villahermosa | 27.21 ± 6.50 y | 12–15 weeks |
| 12–15 week products n = 55 | Villahermosa | 26.01 ± 6.27 y | 12–15 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; Pérez-Calatayud, Á.A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.d.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. https://doi.org/10.3390/biomedicines10020410
Calderón-Garcidueñas L, Pérez-Calatayud ÁA, González-Maciel A, Reynoso-Robles R, Silva-Pereyra HG, Ramos-Morales A, Torres-Jardón R, Soberanes-Cerino CdJ, Carrillo-Esper R, Briones-Garduño JC, et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines. 2022; 10(2):410. https://doi.org/10.3390/biomedicines10020410
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Ángel Augusto Pérez-Calatayud, Angélica González-Maciel, Rafael Reynoso-Robles, Héctor G. Silva-Pereyra, Andrea Ramos-Morales, Ricardo Torres-Jardón, Candelario de Jesús Soberanes-Cerino, Raúl Carrillo-Esper, Jesús Carlos Briones-Garduño, and et al. 2022. "Environmental Nanoparticles Reach Human Fetal Brains" Biomedicines 10, no. 2: 410. https://doi.org/10.3390/biomedicines10020410
APA StyleCalderón-Garcidueñas, L., Pérez-Calatayud, Á. A., González-Maciel, A., Reynoso-Robles, R., Silva-Pereyra, H. G., Ramos-Morales, A., Torres-Jardón, R., Soberanes-Cerino, C. d. J., Carrillo-Esper, R., Briones-Garduño, J. C., & Conde-Gutiérrez, Y. d. S. (2022). Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines, 10(2), 410. https://doi.org/10.3390/biomedicines10020410







