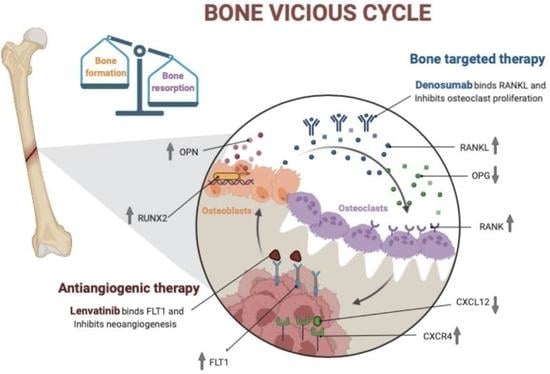
A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement and Case Series
2.2. Histological Analyses
2.3. Tissues and RNA Extraction
2.4. Reverse Transcription and Real-Time Quantitative PCR (RT-qPCR)
2.5. RT-qPCR Data Analysis
2.6. Isolation of Patient-Derived GCTB and DF Primary Cells
2.7. Establishment of GCTB and DF Patient-Derived Primary Cultures
2.8. Building of Collagen-Based Scaffold 3D Culture Model
2.9. Chemobiogram Analysis
2.10. Wound Healing Assay
2.11. Zebrafish Xenotransplant Model
2.12. Statistical Analysis
3. Results
3.1. Patient Clinical History
3.2. Diagnosis of GCTB and DF Case Series
3.3. Establishment of GCTB and DF Patient-Derived Primary Culture
3.4. Gene Expression Profiling in GCTB and DF Cases
3.5. Bone-Targeted Therapy and Tyrosine Kinase Inhibitor Assessment in monolayer and Tridimensional GCTB and DF Primary Culture Model Case Series
3.6. The Impact of Bone-Targeted Therapy and Tyrosine Kinase Inhibitor Combination on GCTB and DF Primary Cell Culture Migration
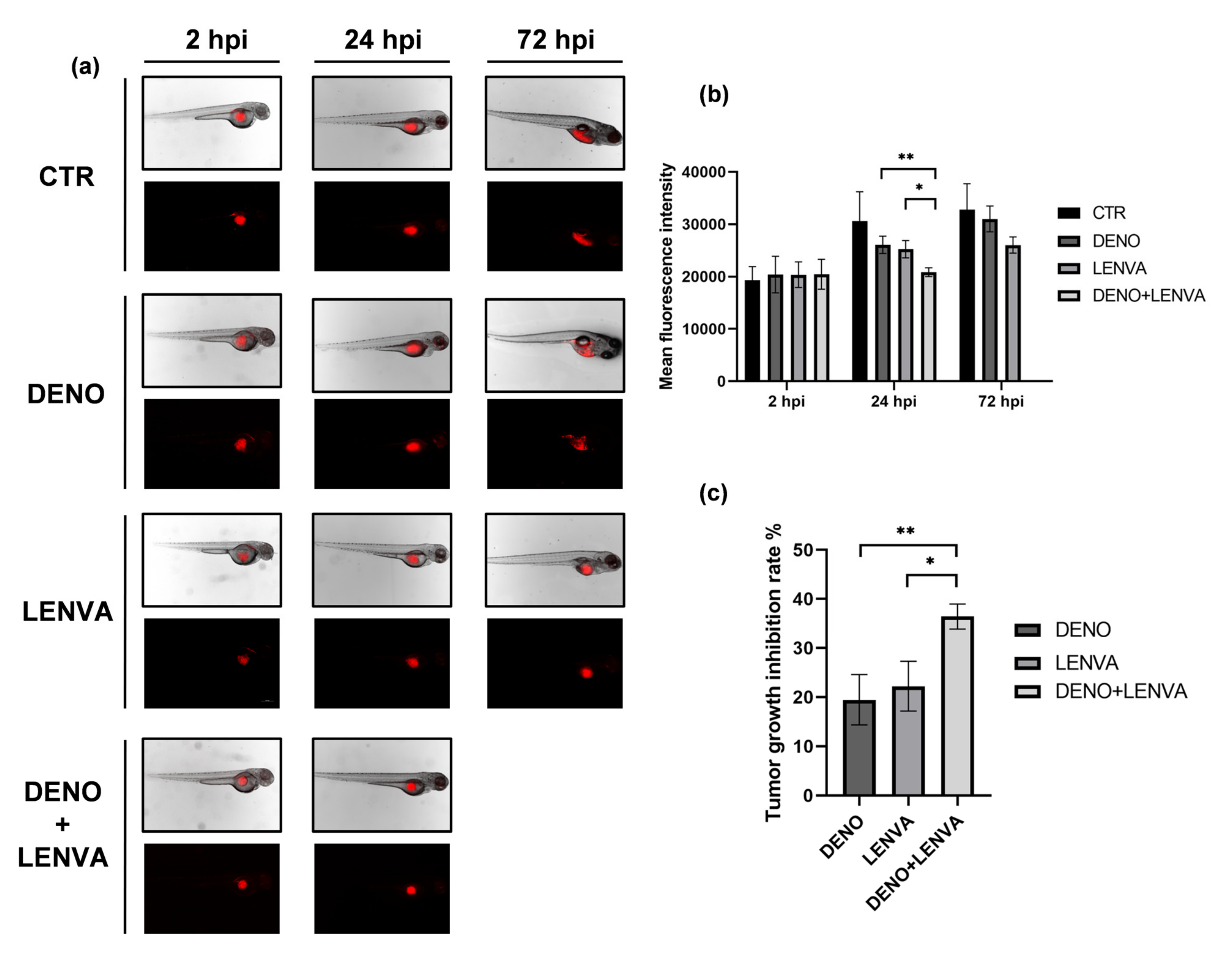
3.7. The Role of Bone-Targeted Therapy and Tyrosine Kinase Inhibitor in DF Primary Culture Xenotransplanted Zebrafish Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaffe, H.L. Giant-cell tumour (osteoclastoma) of bone: Its pathologic delimitation and the inherent clinical implications. Ann. R. Coll. Surg. Engl. 1953, 13, 343–355. [Google Scholar] [PubMed]
- WHO Classification of Tumours. Soft Tissue and Bone Tumours; IARC Press: Lyon, France, 2020; 368p. [Google Scholar]
- He, Y.; Cheng, D.; Lian, C.; Liu, Y.; Luo, W.; Wang, Y.; Ma, C.; Wu, Q.; Tian, P.; He, D.; et al. Serglycin induces osteoclastogenesis and promotes tumor growth in giant cell tumor of bone. Cell Death Dis. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Noguchi, R.; Tsuchiya, R.; Ono, T.; Sin, Y.; Akane, S.; Sugaya, J.; Mori, T.; Fukushima, S.; Yoshida, A.; et al. Establishment and characterization of novel patient-derived cell lines from giant cell tumor of bone. Hum. Cell 2021, 34, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-González, M.A.; Olivella, G.; Ramírez, N.; Soler-Salas, A.; Astacio, E.; Bibiloni, J.; Foy-Parilla, C. Giant cell tumor of bone at the proximal epiphysis of humerus in a skeletally immature patient: A case report. Int. J. Surg. Case Rep. 2020, 77, 560–564. [Google Scholar] [CrossRef]
- Puri, A.; Agarwal, M.G.; Shah, M.; Jambhekar, N.A.; Anchan, C.; Behle, S. Giant Cell Tumor of Bone in Children and Adolescents. J. Pediatr. Orthop. 2007, 27, 635–639. [Google Scholar] [CrossRef]
- Agrawal, A.C.; Verma, S.; Kar, B.; Sakale, H.; Choudhary, R. Mammoth Giant Cell Tumor of the First Metacarpal: A Case Report and Management Trends. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Lucasti, C.; Patel, D.; Hawayek, B.; Maraschiello, M.; Kowalski, J. Giant cell tumor of the thoracic spine causing acute paraplegia—a case report. J. Spine Surg. 2021, 7, 208–213. [Google Scholar] [CrossRef]
- Errani, C.; Ruggieri, P.; Asenzio, M.A.N.; Toscano, A.; Colangeli, S.; Rimondi, E.; Rossi, G.; Longhi, A.; Mercuri, M. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat. Rev. 2010, 36, 1–7. [Google Scholar] [CrossRef]
- Raskin, K.A.; Schwab, J.H.; Mankin, H.J.; Springfield, D.S.; Hornicek, F.J. Giant cell tumor of bone. J. Am. Acad. Orthop. Surg. 2013, 21, 118–126. [Google Scholar] [CrossRef]
- Enneking, W.F. A System of Staging Musculoskeletal Neoplasms. Clin. Orthop. Relat. Res. 1986, 204, 9–24. [Google Scholar] [CrossRef]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-cell tumor of bone. J. Bone Joint. Surg. Am. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Lucas, D.R. Giant Cell Tumor of Bone. Surg. Pathol. Clin. 2012, 5, 183–200. [Google Scholar] [CrossRef]
- Lipplaa, A.; Dijkstra, S.; Gelderblom, H. Challenges of denosumab in giant cell tumor of bone, and other giant cell-rich tumors of bone. Curr. Opin. Oncol. 2019, 31, 329–335. [Google Scholar] [CrossRef]
- Bridge, J.A.; Neff, J.R.; Bhatia, P.S.; Sanger, W.G.; Murphey, M.D. Cytogenetic findings and biologic behavior of giant cell tumors of bone. Cancer 1990, 65, 2697–2703. [Google Scholar] [CrossRef]
- Gamberi, G.; Benassi, M.S.; Böhling, T.; Ragazzini, P.; Molendini, L.; Sollazo, M.R.; Merli, M.; Ferrari, C.; Magagnoli, G.; Bertoni, F.; et al. Prognostic relevance of C-myc gene expression in giant-cell tumor of bone. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1998, 16, 1–7. [Google Scholar] [CrossRef]
- Balke, M.; Schremper, L.; Gebert, C.; Ahrens, H.; Streitbuerger, A.; Koehler, G.; Hardes, J.; Gosheger, G. Giant cell tumor of bone: Treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008, 134, 969–978. [Google Scholar] [CrossRef]
- Van der Heijden, L.; Dijkstra, P.D.; van de Sande, M.A.; Kroep, J.R.; Nout, R.A.; van Rijswijk, C.S.; Bovée, J.V.; Hogendoorn, P.C.; Gelderblom, H. The clinical approach toward giant cell tumor of bone. Oncol. 2014, 19, 550–561. [Google Scholar] [CrossRef] [Green Version]
- Kostenuik, P.J.; Nguyen, H.Q.; McCabe, J.; Warmington, K.S.; Kurahara, C.; Sun, N.; Chen, C.; Li, L.; Cattley, R.C.; Van, G.; et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 2009, 24, 182–195. [Google Scholar] [CrossRef]
- Chawla, S.; Henshaw, R.; Seeger, L.; Choy, E.; Blay, J.; Ferrari, S.; Kroep, J.; Grimer, R.; Reichardt, P.; Rutkowski, P.; et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013, 14, 901–908. [Google Scholar] [CrossRef]
- Thomas, D.; Henshaw, R.; Skubitz, K.; Chawla, S.; Staddon, A.; Blay, J.-Y.; Roudier, M.; Smith, J.; Ye, Z.; Sohn, W.; et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010, 11, 275–280. [Google Scholar] [CrossRef]
- Jaffe, H.L. Tumors and tumorous conditions of the bones and joints. Acad. Med. 1959, 34, 72. [Google Scholar] [CrossRef]
- Böhm, P.; Kröber, S.; Greschniok, A.; Laniado, M.; Kaiserling, E. Desmoplastic fibroma of the bone: A report of two patients, review of the literature, and therapeutic implications. Cancer 1996, 78, 1011–1023. [Google Scholar] [CrossRef]
- Ishizaka, T.; Susa, M.; Sato, C.; Horiuchi, K.; Koga, A.; Kumazawa, F.; Shimazaki, H.; Chiba, K. Desmoplastic fibroma of bone arising in the cortex of the proximal femur. J. Orthop. Sci. 2018, 26, 306–310. [Google Scholar] [CrossRef]
- Nedopil, A.; Raab, P.; Rudert, M. Desmoplastic Fibroma: A Case Report with Three Years of Clinical and Radiographic Observation and Review of the Literature. Open Orthop. J. 2013, 8, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Woods, T.R.; Cohen, D.M.; Islam, M.N.; Rawal, Y.; Bhattacharyya, I. Desmoplastic Fibroma of the Mandible: A Series of Three Cases and Review of Literature. Head Neck Pathol. 2014, 9, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Nag, H.L.; Kumar, R.; Bhan, S.; Awasthy, B.S.; Julka, P.K.; Ray, R.R. Radiotherapy for Desmoplastic Fibroma of Bone: A Case Report. J. Orthop. Surg. 2003, 11, 90–93. [Google Scholar] [CrossRef]
- Sanfilippo, N.J.; Wang, G.J.; Larner, J.M. Desmoplastic fibroma: A role for radiotherapy? S. Med. J. 1995, 88, 1267–1269. [Google Scholar] [CrossRef]
- Miserocchi, E.A.G.; Cocchi, C.; De Vita, A.; Liverani, C.; Spadazzi, C.; Calpona, S.; Di Menna, G.; Bassi, M.; Meccariello, G.; De Luca, G.; et al. Three-dimensional collagen-based scaffold model to study the microenvironment and drug-resistance mechanisms of oropharyngeal squamous cell carcinomas. Cancer Biol. Med. 2021, 18, 502–516. [Google Scholar] [CrossRef]
- De Vita, A.; Vanni, S.; Fausti, V.; Cocchi, C.; Recine, F.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Bassi, M.; Gessaroli, M.; et al. Deciphering the Genomic Landscape and Pharmacological Profile of Uncommon Entities of Adult Rhabdomyosarcomas. Int. J. Mol. Sci. 2021, 22, 11564. [Google Scholar] [CrossRef]
- Mercatali, L.; La Manna, F.; Miserocchi, G.; Liverani, C.; De Vita, A.; Spadazzi, C.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ghetti, M.; et al. Tumor-Stroma Crosstalk in Bone Tissue: The Osteoclastogenic Potential of a Breast Cancer Cell Line in a Co-Culture System and the Role of EGFR Inhibition. Int. J. Mol. Sci. 2017, 18, 1655. [Google Scholar] [CrossRef] [Green Version]
- Liverani, C.; Mercatali, L.; Spadazzi, C.; La Manna, F.; De Vita, A.; Riva, N.; Calpona, S.; Ricci, M.; Bongiovanni, A.; Gunelli, E.; et al. CSF-1 blockade impairs breast cancer osteoclastogenic potential in co-culture systems. Bone 2014, 66, 214–222. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Recine, F.; Miserocchi, G.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Vanni, S.; Liverani, C.; Farnedi, A.; Fabbri, F.; et al. The potential role of the extracellular matrix in the activity of trabectedin in UPS and L-sarcoma: Evidences from a patient-derived primary culture case series in tridimensional and zebrafish models. J. Exp. Clin. Cancer Res. 2021, 40, 165. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Mercatali, L.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Recine, F.; Bongiovanni, A.; Pieri, F.; Cavaliere, D.; Fausti, V.; et al. Establishment of a Primary Culture of Patient-derived Soft Tissue Sarcoma. J. Vis. Exp. JoVE 2018, 134, 56767. [Google Scholar] [CrossRef] [PubMed]
- Boss, D.S.; Glen, H.; Beijnen, J.H.; Keesen, M.; Morrison, R.; Tait, B.; Copalu, W.; Mazur, A.; Wanders, J.; O’Brien, J.P.; et al. A phase I study of E7080, a multitargeted tyrosine kinase inhibitor, in patients with advanced solid tumours. Br. J. Cancer 2012, 106, 1598–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xgeva INN-Denosumab—European Medicines Agency. Assesment Report for XGEVA International Non-Proprietary Name: Denosumab Procedure No. EMEA/H/C/002173; EMA: London, UK, 2011. [Google Scholar]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Q.; Alam, A.; Cui, J.; Suen, K.C.; Soo, A.P.; Eguchi, S.; Gu, J.; Ma, D. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Raikwar, N.S.; Liu, K.Z.; Thomas, C.P. Protein kinase C regulates FLT1 abundance and stimulates its cleavage in vascular endothelial cells with the release of a soluble PlGF/VEGF antagonist. Exp. Cell Res. 2013, 319, 2578–2587. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.A.; Puri, A. The current standing on the use of denosumab in giant cell tumour of the bone. J. Orthop. Surg. 2020, 28. [Google Scholar] [CrossRef]
- Nakano, K.; Takahashi, S. Current Molecular Targeted Therapies for Bone and Soft Tissue Sarcomas. Int. J. Mol. Sci. 2018, 19, 739. [Google Scholar] [CrossRef] [Green Version]
- Roitman, P.D.; Jauk, F.; Farfalli, G.L.; Albergo, J.I.; Aponte-Tinao, L.A. Denosumab-treated giant cell tumor of bone. Its histologic spectrum and potential diagnostic pitfalls. Hum. Pathol. 2017, 63, 89–97. [Google Scholar] [CrossRef]
- Rutkowski, P.; Gaston, L.; Borkowska, A.; Stacchiotti, S.; Gelderblom, H.; Baldi, G.G.; Palmerini, E.; Casali, P.; Gronchi, A.; Parry, M.; et al. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone—Multicenter analysis outside clinical trial. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1384–1390. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Wang, P. Lenvatinib in Management of Solid Tumors. Oncologist 2020, 25, e30. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.M.; Kashima, T.G.; Knowles, H.J.; Athanasou, N.A. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: Implications for giant cell tumour pathobiology. Lab. Investig. 2012, 92, 1398–1406. [Google Scholar] [CrossRef]
- Kumta, S.; Huang, L.; Cheng, Y.; Chow, L.; Lee, K.; Zheng, M. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003, 73, 1427–1436. [Google Scholar] [CrossRef]
- Gaspar, N.; Venkatramani, R.; Hecker-Nolting, S.; Melcon, S.G.; Locatelli, F.; Bautista, F.; Longhi, A.; Lervat, C.; Entz-Werle, N.; Casanova, M.; et al. Lenvatinib with etoposide plus ifosfamide in patients with refractory or relapsed osteosarcoma (ITCC-050): A multicentre, open-label, multicohort, phase 1/2 study. Lancet Oncol. 2021, 22, 1312–1321. [Google Scholar] [CrossRef]
- Evans, S.; Ramasamy, A.; Jeys, L.; Grimer, R. Desmoplastic fibroma of bone: A rare bone tumour. J. Bone Oncol. 2014, 3, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Traub, F.; Singh, J.; Dickson, B.; Leung, S.; Mohankumar, R.; Blackstein, M.E.; Razak, A.R.; Griffin, A.; Ferguson, P.C.; Wunder, J.S. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur. J. Cancer 2016, 59, 1–12. [Google Scholar] [CrossRef]
- Yayan, J. Denosumab for Effective Tumor Size Reduction in Patients with Giant Cell Tumors of the Bone: A Systematic Review and Meta-Analysis. Cancer Control 2020, 27. [Google Scholar] [CrossRef]
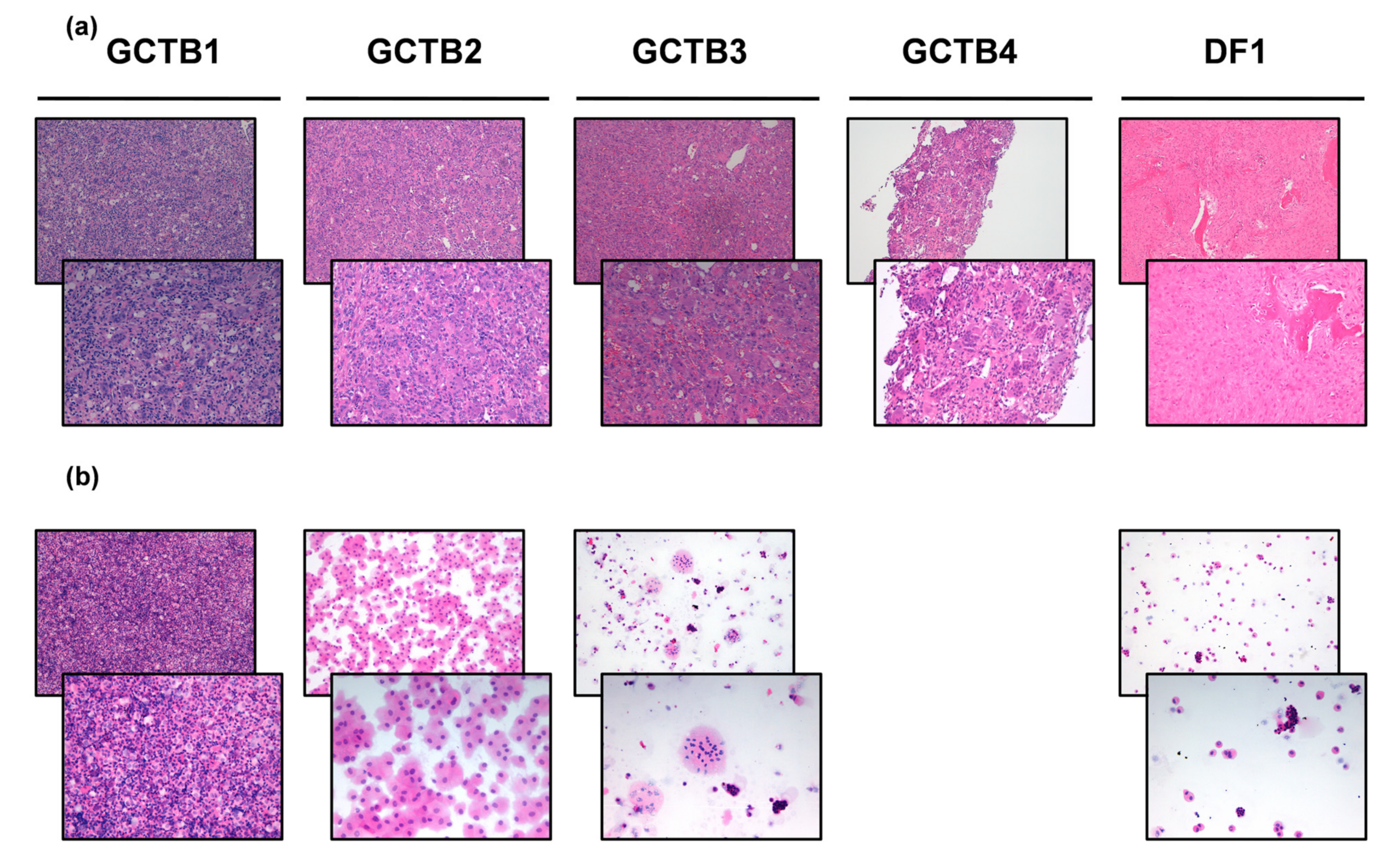



| Patient | Gender | Age at Surgery | Site | Size (cm) | Histological Subtype | IHC Analysis | Surgical Margins | Radiotherapy Postsurgery | Chemotherapy Pre/Post Surgery | Follow-Up Months |
|---|---|---|---|---|---|---|---|---|---|---|
| GCTB1 | F | 45 | right proximal tibia | Abundant fragments of giant cell tumor, areas of necrosis and fragments of newly formed bone tissue | na | R1 | na | Neoadjuvant Denosumab 120 mg with PR and right inferior mandibular ONJ | 42 | |
| GCTB2 | F | 39 | right distal tibia | 9 × 8 × 2 | Fragments consisting of mononuclear cells with rare mitosis and numerous giant cells. Necrosis and remodeled marginal bone trabeculae were observed | na | R0 | na | na | 19 |
| GCTB3 | M | 43 | right distal ulna | 5 × 3 × 1 | Fragments of giant cell tumor | na | R0 | na | na | 10 |
| GCTB4 | M | 47 | left sciatic bone and posterior pillar of the acetabulum | Compact proliferation sections of osteoclastic giant cells mixed with mononuclear elements with similar nuclear characteristics. Cell proliferation was covered by a fine capillary network. There were no atypical elements in interstitial proliferation nor aspects of fibrosis or granulating tissue | CD163 +S100 - | na | na | Adjuvant denosumab 120 mg with CR | 90 | |
| DF1 | M | 24 | left femoral head and neck | 4.5 × 2 × 1 | fragments of desmoplastic fibroma | na | R0 | na | na | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, A.; Vanni, S.; Miserocchi, G.; Fausti, V.; Pieri, F.; Spadazzi, C.; Cocchi, C.; Liverani, C.; Calabrese, C.; Casadei, R.; et al. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines 2022, 10, 372. https://doi.org/10.3390/biomedicines10020372
De Vita A, Vanni S, Miserocchi G, Fausti V, Pieri F, Spadazzi C, Cocchi C, Liverani C, Calabrese C, Casadei R, et al. A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines. 2022; 10(2):372. https://doi.org/10.3390/biomedicines10020372
Chicago/Turabian StyleDe Vita, Alessandro, Silvia Vanni, Giacomo Miserocchi, Valentina Fausti, Federica Pieri, Chiara Spadazzi, Claudia Cocchi, Chiara Liverani, Chiara Calabrese, Roberto Casadei, and et al. 2022. "A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences" Biomedicines 10, no. 2: 372. https://doi.org/10.3390/biomedicines10020372
APA StyleDe Vita, A., Vanni, S., Miserocchi, G., Fausti, V., Pieri, F., Spadazzi, C., Cocchi, C., Liverani, C., Calabrese, C., Casadei, R., Recine, F., Gurrieri, L., Bongiovanni, A., Ibrahim, T., & Mercatali, L. (2022). A Rationale for the Activity of Bone Target Therapy and Tyrosine Kinase Inhibitor Combination in Giant Cell Tumor of Bone and Desmoplastic Fibroma: Translational Evidences. Biomedicines, 10(2), 372. https://doi.org/10.3390/biomedicines10020372