Tempol Preserves Endothelial Progenitor Cells in Male Mice with Ambient Fine Particulate Matter Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. PM Exposure and Animal Model
2.2. Flow Cytometry Analysis for EPCs, Intracellular ROS Level and Cell Apoptosis
2.3. Measurement of Inflammatory Cytokines
2.4. Western Blot
2.5. Statistical Analysis
3. Results
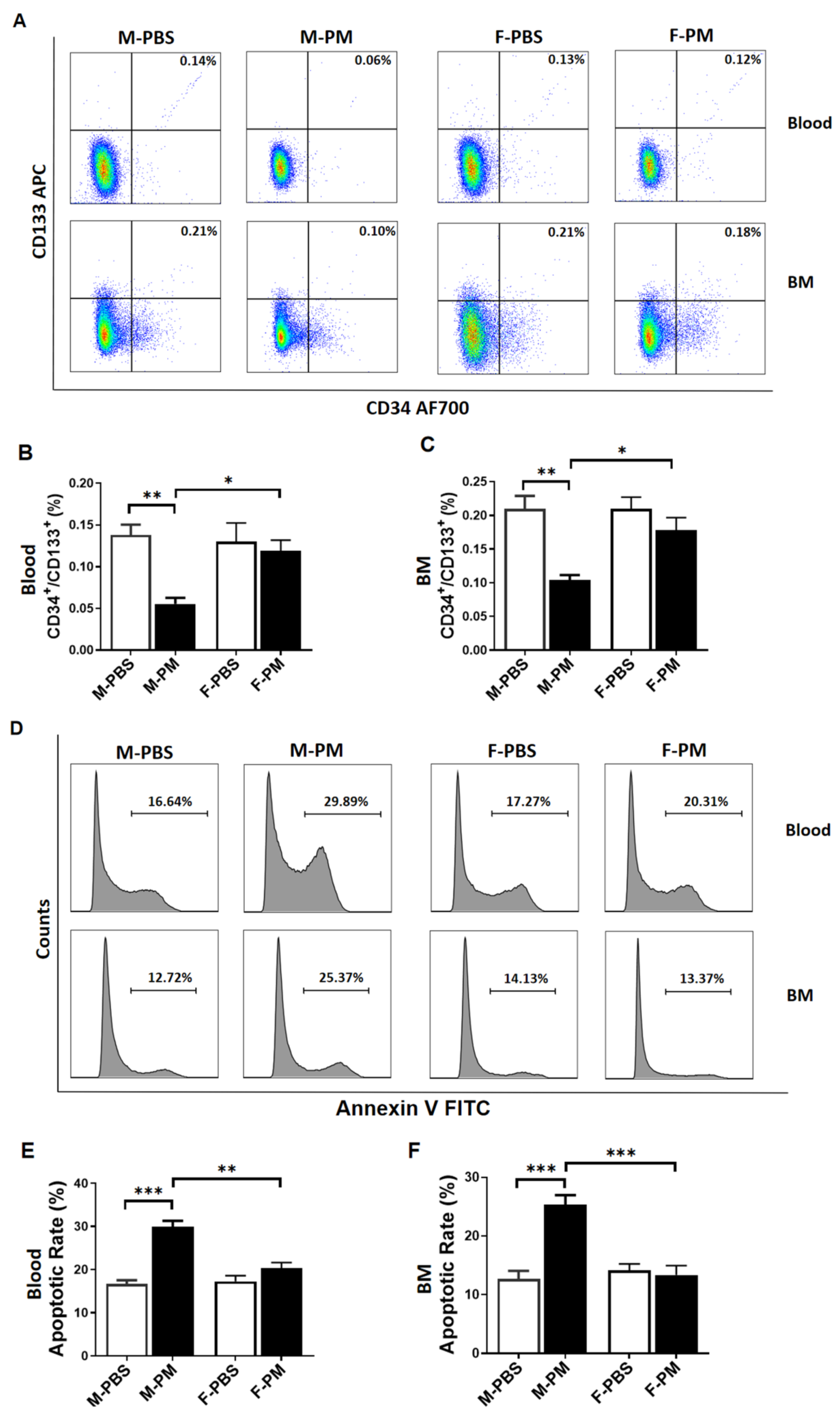
3.1. Levels of Circulating and Bone Marrow EPCs Were Decreased with Increased Apoptosis in Male Mice with PM Exposure
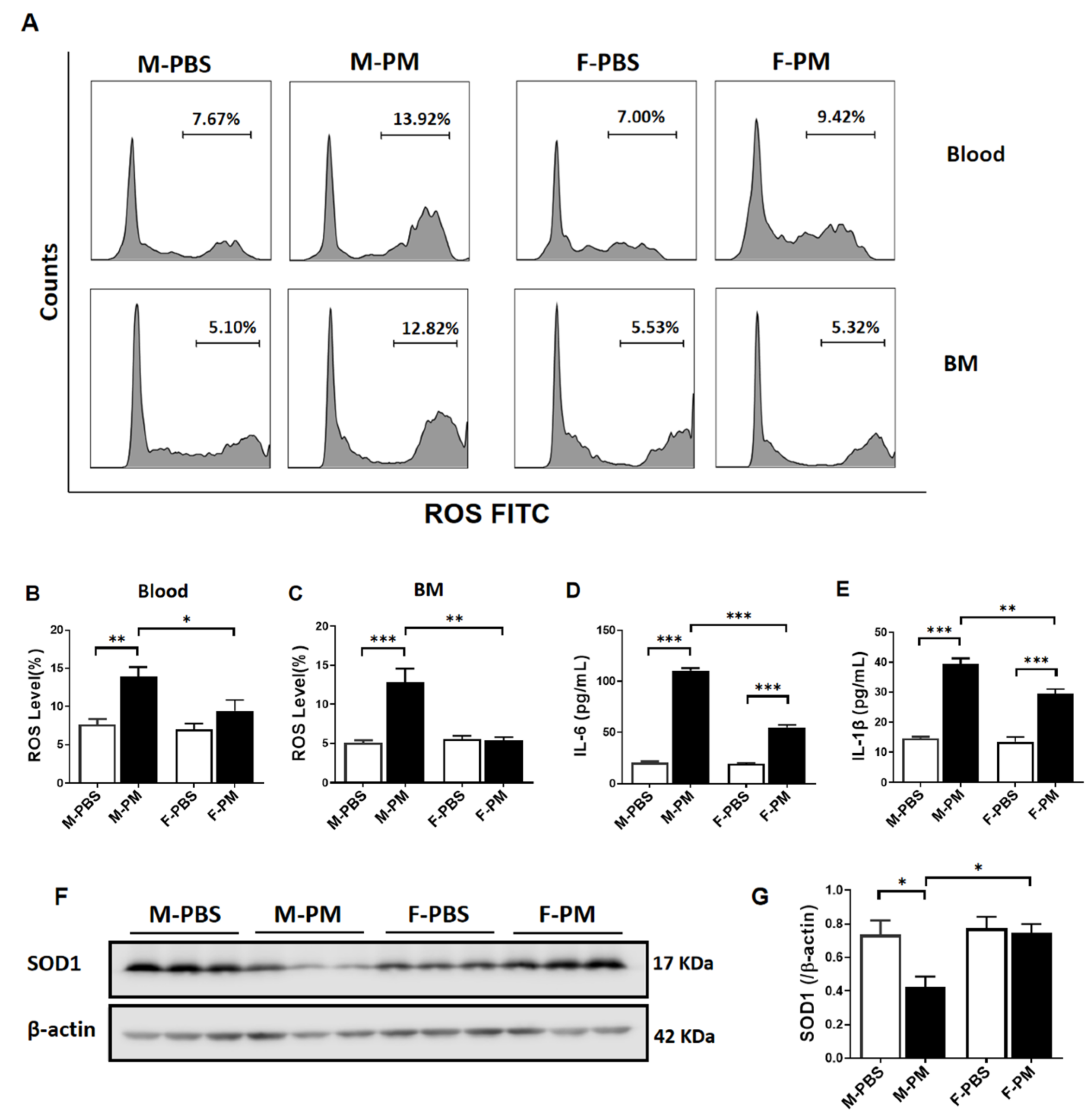
3.2. Cytokines and ROS Levels Were Significantly Increased in Male Mice with PM Exposure with Decreased Pulmonary SOD1 Expression
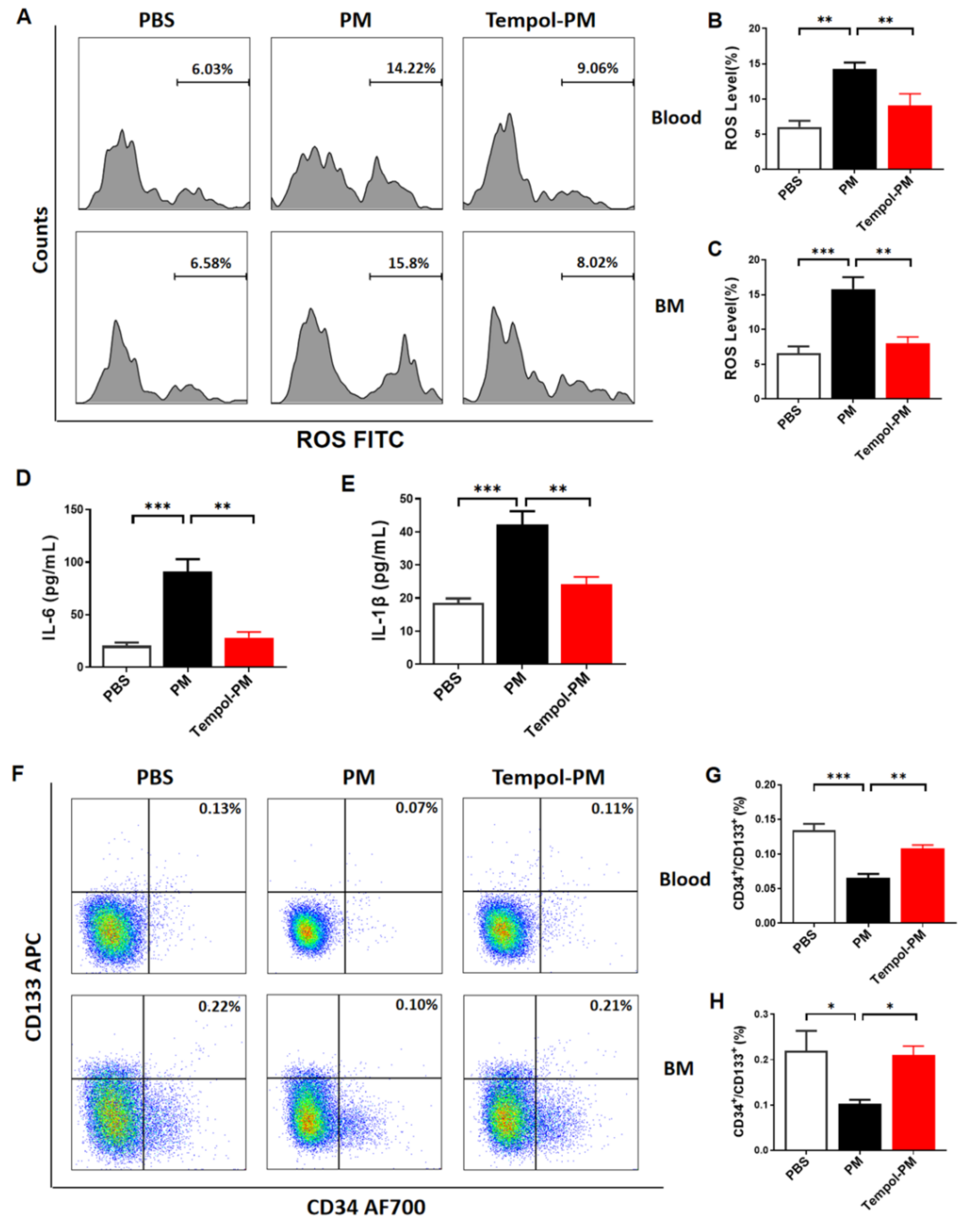
3.3. Treatment with SOD Mimic Tempol Prevented PM Exposure-Induced Production of Cytokines and ROS and Reduction of EPCs in Male Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, M.R.; Newby, D.E. Air pollution and cardiovascular disease: Car sick. Cardiovasc. Res. 2020, 116, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meo, S.A.; Suraya, F. Effect of environmental air pollution on cardiovascular diseases. Eur. Rev. Med. Pharm. Sci. 2015, 19, 4890–4897. [Google Scholar]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Gimbrone, M.J.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; O’Toole, T.E.; Conklin, D.J.; Hill, B.G.; Haberzettl, P. Endothelial progenitor cells as critical mediators of environmental air pollution-induced cardiovascular toxicity. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1440–H1455. [Google Scholar] [CrossRef]
- Altabas, V.; Altabas, K.; Kirigin, L. Endothelial progenitor cells (EPCs) in ageing and age-related diseases: How currently available treatment modalities affect EPC biology, atherosclerosis, and cardiovascular outcomes. Mech. Ageing. Dev. 2016, 159, 49–62. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef]
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef]
- O’Toole, T.E.; Hellmann, J.; Wheat, L.; Haberzettl, P.; Lee, J.; Conklin, D.J.; Bhatnagar, A.; Pope, C.R. Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ. Res. 2010, 107, 200–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberzettl, P.; Conklin, D.J.; Abplanalp, W.T.; Bhatnagar, A.; O’Toole, T.E. Inhalation of Fine Particulate Matter Impairs Endothelial Progenitor Cell Function Via Pulmonary Oxidative Stress. Arter. Thromb. Vasc. Biol. 2018, 38, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox. Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Dabla, P.K. Oxidative stress and antioxidants in hypertension-a current review. Curr. Hypertens. Rev. 2015, 11, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Y.; Zhu, Q.; Cui, Y.; Hao, H.; Wang, M.; Cowan, P.J.; Korthuis, R.J.; Li, G.; Sun, Q.; et al. Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. Int. J. Mol. Sci. 2021, 22, 7200. [Google Scholar] [CrossRef]
- Jantzen, K.; Jensen, A.; Kermanizadeh, A.; Elholm, G.; Sigsgaard, T.; Moller, P.; Roursgaard, M.; Loft, S. Inhalation of House Dust and Ozone Alters Systemic Levels of Endothelial Progenitor Cells, Oxidative Stress, and Inflammation in Elderly Subjects. Toxicol. Sci. 2018, 163, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Soberanes, S.; Urich, D.; Baker, C.M.; Burgess, Z.; Chiarella, S.E.; Bell, E.L.; Ghio, A.J.; De Vizcaya-Ruiz, A.; Liu, J.; Ridge, K.M.; et al. Mitochondrial complex III-generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J. Biol. Chem. 2009, 284, 2176–2186. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.; Lakey, P.; Weber, R.J.; Shiraiwa, M. Oxidative Potential of Particulate Matter and Generation of Reactive Oxygen Species in Epithelial Lining Fluid. Environ. Sci. Technol. 2019, 53, 12784–12792. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Phungphong, S.; Kijtawornrat, A.; Wattanapermpool, J.; Bupha-Intr, T. Improvement in cardiac function of ovariectomized rats by antioxidant tempol. Free Radic. Biol. Med. 2020, 160, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, U.; Christensen, F.H.; Buus, N.H. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol. Ther. 2009, 122, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.A.; Marwaha, A.; Tallam, L.S.; Lokhandwala, M.F. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 2005, 54, 2219–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, E.H.; Fukuda, N.; Matsumoto, T.; Kobayashi, N.; Katakawa, M.; Yamamoto, C.; Tsunemi, A.; Suzuki, R.; Ueno, T.; Matsumoto, K. Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect. Hypertens. Res. 2007, 30, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.; Correia, T.; Pereira, R.; Da, S.R.; Augusto, O.; Queiroz, R.F. Tempol reduces inflammation and oxidative damage in cigarette smoke-exposed mice by decreasing neutrophil infiltration and activating the Nrf2 pathway. Chem. Biol. Interact. 2020, 329, 109210. [Google Scholar] [CrossRef] [PubMed]
- Nocun, M.S.; Schantz, M.M. Determination of selected oxygenated polycyclic aromatic hydrocarbons (oxy-PAHs) in diesel and air particulate matter standard reference materials (SRMs). Anal. Bioanal. Chem. 2013, 405, 5583–5593. [Google Scholar] [CrossRef]
- Hoffmann, D.S.; Weydert, C.J.; Lazartigues, E.; Kutschke, W.J.; Kienzle, M.F.; Leach, J.E.; Sharma, J.A.; Sharma, R.V.; Davisson, R.L. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 2008, 51, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Fleenor, B.S.; Seals, D.R.; Zigler, M.L.; Sindler, A.L. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 2012, 11, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Martinez, G.L.; Ortiz, M.C.; Galindo, M.; Sanchez, J.M.; Sancho-Rodriguez, N.; Albaladejo, O.M.; Rodriguez, M.M.; Rodriguez, F. Role of heme oxygenase in the regulation of the renal hemodynamics in a model of sex dependent programmed hypertension by maternal diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022. [Google Scholar] [CrossRef]
- Liberda, E.N.; Cuevas, A.K.; Qu, Q.; Chen, L.C. The acute exposure effects of inhaled nickel nanoparticles on murine endothelial progenitor cells. Inhal. Toxicol. 2014, 26, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic. Biol. Med. 2018, 128, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Haberzettl, P.; Lee, J.; Duggineni, D.; McCracken, J.; Bolanowski, D.; O’Toole, T.E.; Bhatnagar, A.; Conklin, D.J. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ. Health Perspect. 2012, 120, 848–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianconi, V.; Sahebkar, A.; Kovanen, P.; Bagaglia, F.; Ricciuti, B.; Calabro, P.; Patti, G.; Pirro, M. Endothelial and cardiac progenitor cells for cardiovascular repair: A controversial paradigm in cell therapy. Pharmacol. Ther. 2018, 181, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Rossig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kamper, U.; Dimmeler, S.; Zeiher, A.M. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.P.; Lin, F.Y.; Huang, P.H.; Chen, Y.L.; Chen, W.C.; Chen, H.Y.; Huang, Y.C.; Liao, W.L.; Huang, H.C.; Liu, P.L.; et al. Endothelial progenitor cell dysfunction in cardiovascular diseases: Role of reactive oxygen species and inflammation. Biomed. Res. Int. 2013, 2013, 845037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Liberda, E.N.; Qu, S.; Guo, X.; Li, X.; Zhang, J.; Meng, J.; Yan, B.; Li, N.; Zhong, M.; et al. The role of metal components in the cardiovascular effects of PM2.5. PLoS ONE 2013, 8, e83782. [Google Scholar] [CrossRef]
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox. Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Guellich, A.; Damy, T.; Conti, M.; Claes, V.; Samuel, J.L.; Pineau, T.; Lecarpentier, Y.; Coirault, C. Tempol prevents cardiac oxidative damage and left ventricular dysfunction in the PPAR-alpha KO mouse. Am. J. Physiol. Heart. Circ. Physiol. 2013, 304, H1505–H1512. [Google Scholar] [CrossRef] [Green Version]
- Metz, J.M.; Smith, D.; Mick, R.; Lustig, R.; Mitchell, J.; Cherakuri, M.; Glatstein, E.; Hahn, S.M. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin. Cancer Res. 2004, 10, 6411–6417. [Google Scholar] [CrossRef] [Green Version]
- Ramick, M.G.; Brian, M.S.; Matthews, E.L.; Patik, J.C.; Seals, D.R.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Apocynin and Tempol ameliorate dietary sodium-induced declines in cutaneous microvascular function in salt-resistant humans. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H97–H103. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Cassis, L.A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Gao, X.M.; Moore, X.L.; Kiriazis, H.; Su, Y.; Ming, Z.; Lim, Y.L.; Dart, A.M.; Du, X.J. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J. Mol. Cell Cardiol. 2007, 43, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, A.M.; Kadokami, T.; Lemster, B.; Frye, C.S.; McTiernan, C.F.; Feldman, A.M. Morphological and functional changes in cardiac myocytes isolated from mice overexpressing TNF-alpha. Am. J. Physiol. Heart. Circ. Physiol. 2003, 284, H960–H969. [Google Scholar] [CrossRef] [PubMed]
- Zemskova, M.; Kurdyukov, S.; James, J.; McClain, N.; Rafikov, R.; Rafikova, O. Sex-specific stress response and HMGB1 release in pulmonary endothelial cells. PLoS ONE 2020, 15, e0231267. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Itoh, N.; Askarinam, S.; Hill, H.; Arnold, A.P.; Voskuhl, R.R. XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2014, 111, 2806–2811. [Google Scholar] [CrossRef] [Green Version]
- Tamosiuniene, R.; Manouvakhova, O.; Mesange, P.; Saito, T.; Qian, J.; Sanyal, M.; Lin, Y.C.; Nguyen, L.P.; Luria, A.; Tu, A.B.; et al. Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ. Res. 2018, 122, 1689–1702. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell. Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Barp, J.; Araujo, A.S.; Fernandes, T.R.; Rigatto, K.V.; Llesuy, S.; Bello-Klein, A.; Singal, P. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater oxidative stress in healthy young men compared with premenopausal women. Arter. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Ji, L.L.; Liu, T.Y.; Wang, Z.T. Evaluation of gender-related differences in various oxidative stress enzymes in mice. Chin. J. Physiol. 2011, 54, 385–390. [Google Scholar] [PubMed]
- Cacabelos, D.; Ramirez-Nunez, O.; Granado-Serrano, A.B.; Torres, P.; Ayala, V.; Moiseeva, V.; Povedano, M.; Ferrer, I.; Pamplona, R.; Portero-Otin, M.; et al. Early and gender-specific differences in spinal cord mitochondrial function and oxidative stress markers in a mouse model of ALS. Acta Neuropathol. Commun. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumenko, N.; Pollari, E.; Kurronen, A.; Giniatullina, R.; Shakirzyanova, A.; Magga, J.; Koistinaho, J.; Giniatullin, R. Gender-Specific Mechanism of Synaptic Impairment and Its Prevention by GCSF in a Mouse Model of ALS. Front. Cell. Neurosci. 2011, 5, 26. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, A.; Chen, Z.; Cui, Y.; Hao, H.; Domeier, T.L.; Sun, Q.; Liu, Z. Tempol Preserves Endothelial Progenitor Cells in Male Mice with Ambient Fine Particulate Matter Exposure. Biomedicines 2022, 10, 327. https://doi.org/10.3390/biomedicines10020327
Liu X, Wang A, Chen Z, Cui Y, Hao H, Domeier TL, Sun Q, Liu Z. Tempol Preserves Endothelial Progenitor Cells in Male Mice with Ambient Fine Particulate Matter Exposure. Biomedicines. 2022; 10(2):327. https://doi.org/10.3390/biomedicines10020327
Chicago/Turabian StyleLiu, Xuanyou, Aimin Wang, Zhiheng Chen, Yuqi Cui, Hong Hao, Timothy L. Domeier, Qinghua Sun, and Zhenguo Liu. 2022. "Tempol Preserves Endothelial Progenitor Cells in Male Mice with Ambient Fine Particulate Matter Exposure" Biomedicines 10, no. 2: 327. https://doi.org/10.3390/biomedicines10020327
APA StyleLiu, X., Wang, A., Chen, Z., Cui, Y., Hao, H., Domeier, T. L., Sun, Q., & Liu, Z. (2022). Tempol Preserves Endothelial Progenitor Cells in Male Mice with Ambient Fine Particulate Matter Exposure. Biomedicines, 10(2), 327. https://doi.org/10.3390/biomedicines10020327





