Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient
2.2. HBV RT Sequence Analysis
2.3. Construction of HBV Reverse Transcriptase (RT) Mutant Replicons
2.4. Cell Culture, Transfection, and Drug Treatment
2.5. Southern Blot Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Quantitative Real-Time PCR (RT-qPCR)
2.8. Statistical Analysis
3. Results
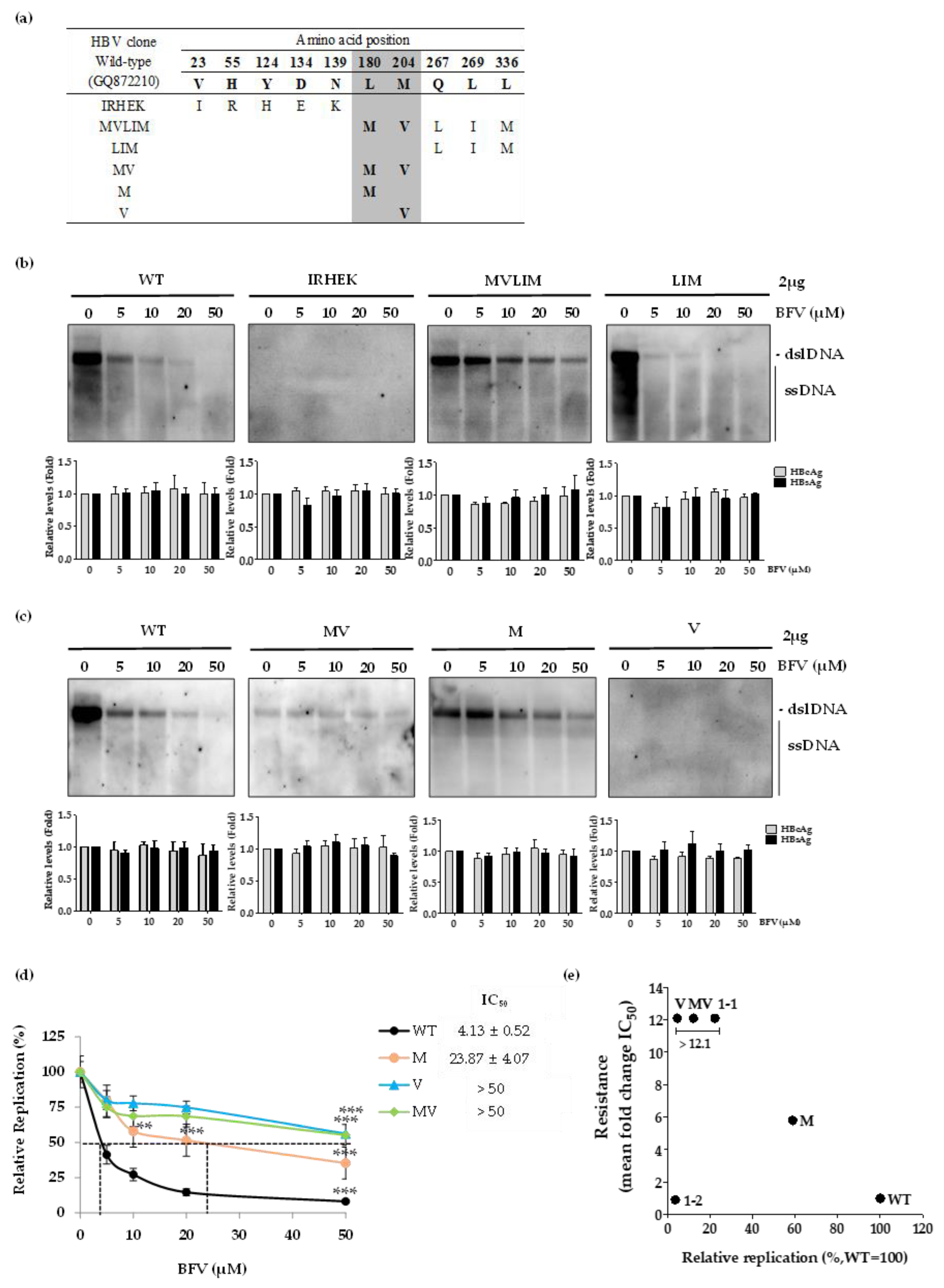
3.1. Mutation Profile of HBV RT Domain Isolated from a Patient Treated with BSV
3.2. Patient-Derived HBV RT Mutant Harboring IRHEKMVLIM Is Resistant to BFV Treatment
3.3. HBV RT rtL180M and rtM204V Mutations Are Associated with BFV Resistance
3.4. Susceptibility of Patient-Derived and BFV-Resistant Clones to Other Antiviral Agents
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Wang, S.; Yi, Y.; Zhang, J.; Duan, Z.; Yuan, K.; Liu, W.; Li, J.; Zhu, Y. The Hepatitis B Surface Antigen Binding Protein: An Immunoglobulin G Constant Region-Like Protein That Interacts With HBV Envelop Proteins and Mediates HBV Entry. Front. Cell. Infect. Microbiol. 2018, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Bartholomeusz, A.; Locarnini, S. HBV drug resistance: Mechanisms, detection and interpretation. J. Hepatol. 2006, 44, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.N.; Hu, J. Unveiling the roles of HBV polymerase for new antiviral strategies. Future Virol. 2015, 10, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Budzinska, M.A.; Vondran, F.W.R.; Shackel, N.A.; Urban, S. Hepatitis B Virus DNA Integration Occurs Early in the Viral Life Cycle in an In Vitro Infection Model via Sodium Taurocholate Cotransporting Polypeptide-Dependent Uptake of Enveloped Virus Particles. J. Virol. 2018, 92, e02007-17. [Google Scholar] [CrossRef]
- Ko, C.; Chakraborty, A.; Chou, W.M.; Hasreiter, J.; Wettengel, J.M.; Stadler, D.; Bester, R.; Asen, T.; Zhang, K.; Wisskirchen, K.; et al. Hepatitis B virus genome recycling and de novo secondary infection events maintain stable cccDNA levels. J. Hepatol. 2018, 69, 1231–1241. [Google Scholar] [CrossRef]
- Dusseaux, M.; Masse-Ranson, G.; Darche, S.; Ahodantin, J.; Li, Y.; Fiquet, O.; Beaumont, E.; Moreau, P.; Riviere, L.; Neuveut, C.; et al. Viral Load Affects the Immune Response to HBV in Mice With Humanized Immune System and Liver. Gastroenterology 2017, 153, 1647–1661.e9. [Google Scholar] [CrossRef]
- Konig, A.; Yang, J.; Jo, E.; Park, K.H.P.; Kim, H.; Than, T.T.; Song, X.; Qi, X.; Dai, X.; Park, S.; et al. Efficient long-term amplification of hepatitis B virus isolates after infection of slow proliferating HepG2-NTCP cells. J. Hepatol. 2019, 71, 289–300. [Google Scholar] [CrossRef]
- Yasutake, Y.; Hattori, S.I.; Tamura, N.; Matsuda, K.; Kohgo, S.; Maeda, K.; Mitsuya, H. Structural features in common of HBV and HIV-1 resistance against chirally-distinct nucleoside analogues entecavir and lamivudine. Sci. Rep. 2020, 10, 3021. [Google Scholar] [CrossRef]
- Seifer, M.; Patty, A.; Serra, I.; Li, B.; Standring, D.N. Telbivudine, a nucleoside analog inhibitor of HBV polymerase, has a different in vitro cross-resistance profile than the nucleotide analog inhibitors adefovir and tenofovir. Antivir. Res. 2009, 81, 147–155. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, N.D.; Seong, B.L. Discovery and development of anti-HBV agents and their resistance. Molecules 2010, 15, 5878–5908. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Park, Y.K.; Ahn, S.H.; Cho, E.S.; Choe, W.H.; Lee, C.H.; Kim, B.K.; Ko, S.Y.; Choi, H.S.; Park, E.S.; et al. Identification and characterization of clevudine-resistant mutants of hepatitis B virus isolated from chronic hepatitis B patients. J. Virol. 2010, 84, 4494–4503. [Google Scholar] [CrossRef] [PubMed]
- Korean Association for the Study of the Liver. KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Kim, W.; Jung, Y.K.; Yang, J.M.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Efficacy and Safety of Besifovir Dipivoxil Maleate Compared With Tenofovir Disoproxil Fumarate in Treatment of Chronic Hepatitis B Virus Infection. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 1850–1859.e4. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Ahn, S.H.; Lee, K.S.; Um, S.H.; Cho, M.; Yoon, S.K.; Lee, J.W.; Park, N.H.; Kweon, Y.O.; Sohn, J.H.; et al. Phase IIb multicentred randomised trial of besifovir (LB80380) versus entecavir in Asian patients with chronic hepatitis B. Gut 2014, 63, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.A.; Kim, S.R.; Kim, T.E.; Kim, J.R.; Lee, S.Y.; Huh, W.; Ko, J.W. Pharmacokinetic comparison of the maleate and free base formulations of LB80380, a novel nucleotide analog, in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 2012, 50, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Fung, J.; Lai, C.L.; Yuen, M.F. LB80380: A promising new drug for the treatment of chronic hepatitis B. Expert Opin. Investig. Drugs 2008, 17, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Ahn, S.H.; Lee, K.S.; Um, S.H.; Cho, M.; Yoon, S.K.; Lee, J.W.; Park, N.H.; Kweon, Y.O.; Sohn, J.H.; et al. Two-year treatment outcome of chronic hepatitis B infection treated with besifovir vs. entecavir: Results from a multicentre study. J. Hepatol. 2015, 62, 526–532. [Google Scholar] [CrossRef]
- Song, D.S.; Kim, W.; Ahn, S.H.; Yim, H.J.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Continuing besifovir dipivoxil maleate versus switching from tenofovir disoproxil fumarate for treatment of chronic hepatitis B: Results of 192-week phase 3 trial. Clin. Mol. Hepatol. 2021, 27, 346–359. [Google Scholar] [CrossRef]
- Lee, A.R.; Cho, J.Y.; Kim, J.C.; Dezhbord, M.; Choo, S.Y.; Ahn, C.H.; Kim, N.Y.; Shin, J.J.; Park, S.; Park, E.S.; et al. Distinctive HBV Replication Capacity and Susceptibility to Tenofovir Induced by a Polymerase Point Mutation in Hepatoma Cell Lines and Primary Human Hepatocytes. Int. J. Mol. Sci. 2021, 22, 1606. [Google Scholar] [CrossRef]
- Lucifora, J.; Salvetti, A.; Marniquet, X.; Mailly, L.; Testoni, B.; Fusil, F.; Inchauspe, A.; Michelet, M.; Michel, M.L.; Levrero, M.; et al. Detection of the hepatitis B virus (HBV) covalently-closed-circular DNA (cccDNA) in mice transduced with a recombinant AAV-HBV vector. Antivir. Res. 2017, 145, 14–19. [Google Scholar] [CrossRef]
- Park, E.S.; Lee, A.R.; Kim, D.H.; Lee, J.H.; Yoo, J.J.; Ahn, S.H.; Sim, H.; Park, S.; Kang, H.S.; Won, J.; et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J. Hepatol. 2019, 70, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, E.S.; Koo, J.E.; Park, Y.K.; Lee, A.R.; Dezhbord, M.; Cho, E.S.; Ahn, S.H.; Kim, D.H.; Lee, J.H.; et al. Entecavir-resistant hepatitis B virus decreases surface antigenicity: A full genome and functional characterization. Liver Int. 2020, 40, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Hou, W.; Wo, J.E.; Liu, K.Z. Character of HBV (hepatitis B virus) polymerase gene rtM204V/I and rtL180M mutation in patients with lamivudine resistance. J. Zhejiang Univ. Sci. B 2005, 6, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Watashi, K.; Kato, T.; Muramatsu, M.; Wakita, T.; Tamura, N.; Hattori, S.I.; Maeda, K.; Mitsuya, H.; Yasutake, Y.; et al. Biochemical and Structural Properties of Entecavir-Resistant Hepatitis B Virus Polymerase with L180M/M204V Mutations. J. Virol. 2021, 95, e0240120. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Sarkar, N.; Saha, D.; Guha, S.K.; Saha, B.; Chakrabarti, S.; Chakravarty, R. High incidence of lamivudine-resistance-associated vaccine-escape HBV mutants among HIV-coinfected patients on prolonged antiretroviral therapy. Antivir. Ther. 2015, 20, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Li, X.; Niu, M.; Chen, R.; Shao, J.; Si, L.; Luo, D.; Lin, Y.; Li, L.; et al. Hepatitis B virus mutation pattern rtL180M+A181C+M204V may contribute to entecavir resistance in clinical practice. Emerg. Microbes Infect. 2019, 8, 354–365. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Chen, Y.U.; Zheng, S.; Zhou, L.I.; Lu, F.; Duan, Z. Lamivudine-resistant rtL180M and rtM204I/V are persistently dominant during combination rescue therapy with entecavir and adefovir for hepatitis B. Exp. Ther. Med. 2016, 11, 2293–2299. [Google Scholar] [CrossRef][Green Version]
- Suzuki, F.; Sezaki, H.; Hosaka, T.; Suzuki, Y.; Fujiyama, S.; Kawamura, Y.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Arase, Y.; et al. Virologic analysis of tenofovir resistance in a patient with chronic hepatitis B experiencing viral breakthrough during combination treatment with tenofovir disoproxil fumarate and entecavir. Hepatol. Res. 2021, 51, 503–508. [Google Scholar] [CrossRef]
- Tenney, D.J.; Rose, R.E.; Baldick, C.J.; Pokornowski, K.A.; Eggers, B.J.; Fang, J.; Wichroski, M.J.; Xu, D.; Yang, J.; Wilber, R.B.; et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 2009, 49, 1503–1514. [Google Scholar] [CrossRef]
- Yuen, M.F.; Gane, E.J.; Kim, D.J.; Weilert, F.; Yuen Chan, H.L.; Lalezari, J.; Hwang, S.G.; Nguyen, T.; Flores, O.; Hartman, G.; et al. Antiviral Activity, Safety, and Pharmacokinetics of Capsid Assembly Modulator NVR 3-778 in Patients with Chronic HBV Infection. Gastroenterology 2019, 156, 1392–1403.e7. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, Z.; Cheng, J.; Wu, S.; Luo, Y.; Chang, J.; Hu, J.; Guo, J.T. Hepatitis B Virus Core Protein Dephosphorylation Occurs during Pregenomic RNA Encapsidation. J. Virol. 2018, 92, e02139-17. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.C. Another oral antiviral treatment, but still far away from hepatitis B virus cure. Clin. Mol. Hepatol. 2021, 27, 281–282. [Google Scholar] [CrossRef]
- Yuen, M.F.; Han, K.H.; Um, S.H.; Yoon, S.K.; Kim, H.R.; Kim, J.; Kim, C.R.; Lai, C.L. Antiviral activity and safety of LB80380 in hepatitis B e antigen-positive chronic hepatitis B patients with lamivudine-resistant disease. Hepatology 2010, 51, 767–776. [Google Scholar] [CrossRef] [PubMed]
- You, C.R.; Lee, S.W.; Jang, J.W.; Yoon, S.K. Update on hepatitis B virus infection. World J. Gastroenterol. 2014, 20, 13293–13305. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.M.; Espiritu, C.; Vogel, R.; Ren, S.; Lau, V.; Kelly, M.; Kuduk, S.D.; Hartman, G.D.; Flores, O.A.; Klumpp, K. Preclinical Characterization of NVR 3-778, a First-in-Class Capsid Assembly Modulator against Hepatitis B Virus. Antimicrob. Agents Chemother. 2019, 63, e01734-18. [Google Scholar] [CrossRef]
- Song, J.E.; Park, J.Y. Besifovir dipivoxil maleate: A novel antiviral agent with low toxicity and high genetic barriers for chronic hepatitis B. Expert Opin. Pharm. 2021, 21, 2427–2433. [Google Scholar] [CrossRef]
- Yim, H.J.; Kim, W.; Ahn, S.H.; Yang, J.M.; Jang, J.Y.; Kweon, Y.O.; Cho, Y.K.; Kim, Y.J.; Hong, G.Y.; Kim, D.J.; et al. Besifovir Dipivoxil Maleate 144-Week Treatment of Chronic Hepatitis B: An Open-Label Extensional Study of a Phase 3 Trial. Am. J. Gastroenterol 2020, 115, 1217–1225. [Google Scholar] [CrossRef]
- Yim, H.J.; Kim, W.; Ahn, S.H.; Jung, Y.K.; Um, S.H.; Sohn, J.H.; Jang, J.Y.; Kim, D.J.; Park, E.S.; Jin, S.Y.; et al. Besifovir Therapy Improves Hepatic Histology and Reduces Covalently Closed Circular DNA in Chronic Hepatitis B Patients. J. Gastroenterol. Hepatol. 2021. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jgh.15710 (accessed on 16 January 2022).



| Variables | Values | Standard Levels |
|---|---|---|
| HBeAg | Positive | - |
| HBeAg antibody | Negative | - |
| HBV DNA (IU/mL) | 6,954,754.3 | - |
| bilirubin (mg/dL) | 0.81 | 0.2~1.2 |
| AST (IU/L) | 66 | 5~40 |
| ALT (IU/L) | 101 | 0~40 |
| Prothrombin time (s) | 11.6 | 9.5~13.5 |
| White blood cell (103/μL) | 5.46 | 4~10 |
| Serum albumin (mg/dL) | 4.5 | 3.3~5.2 |
| Hemoglobin (g/dL) | 13.6 | 12~16 |
| Platelet (103/μL) | 157 | 150~450 |
| HBV Clone Wild-Type (GQ872210) | Amino Acid Position | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 | 38 | 54 | 55 | 68 | 110 | 123 | 124 | 134 | 139 | 180 | 191 | 204 | 207 | 226 | 244 | 266 | 267 | 269 | 285 | 303 | 317 | 329 | 333 | 336 | |
| V | T | T | H | S | R | N | Y | D | N | L | V | M | V | N | G | V | Q | L | K | C | S | A | K | L | |
| 1-1,14,19,21,22 | I | R | H | E | K | M | V | L | I | M | |||||||||||||||
| 1-2,16 | A | I | H | I | L | A | T | ||||||||||||||||||
| 1-15,24 | G | D | H | L | I | Q | |||||||||||||||||||
| 1-23,30 | |||||||||||||||||||||||||
| 1-3 | I | S | R | H | E | K | M | V | L | I | M | ||||||||||||||
| 1-4 | I | R | H | E | K | M | V | L | L | I | M | ||||||||||||||
| 1-17 | P | ||||||||||||||||||||||||
| 1-25 | S | ||||||||||||||||||||||||
| 1-31 | G | D | H | L | I | R | R | Q | |||||||||||||||||
| Clone | Region | Mutation in the Corresponding Gene | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | RT | V 23 | T 38 | T 42 | H 55 | L 102 | Y 124 | D 134 | N 139 | Y 141 | G 165 | L 180 | V 191 | M 204 | N 226 | V 266 | Q 267 | L 269 | S 317 | A 329 | L 336 |
| S | L 15 | Q 30 | S 34 | T 47 | L 94 | T 116 | I 126 | T 131 | M 133 | A 157 | W 172 | W 182 | I 195 | I 218 | - | - | - | - | - | - | |
| 1-1 | RT | I | R | H | E | K | M | V | L | I | M | ||||||||||
| S | L | A | S | N | T | M | |||||||||||||||
| 1-2 | RT | A | I | H | I | L | A | T | |||||||||||||
| S | S | T | T | D | * | * | |||||||||||||||
| Clone | Replication Ability (%) | IC50 (μM) | Fold Resistance (/WT IC50) |
|---|---|---|---|
| WT | 100 | 4.13 ± 0.52 | 1.00 |
| 1-1 | 22.36 ± 0.08 | >50 | >12.1 |
| 1-2 | 3.75 ± 1.59 | 3.83 ± 0.6 | 0.92 |
| M | 58.84 ± 0.82 | 23.87 ± 4.07 | 5.8 |
| V | 4.6 ± 0.39 | >50 | >12.1 |
| MV | 12.21 ± 1.02 | >50 | >12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.C.; Lee, H.Y.; Lee, A.R.; Dezhbord, M.; Lee, D.R.; Kim, S.H.; Won, J.; Park, S.; Kim, N.Y.; Shin, J.J.; et al. Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient. Biomedicines 2022, 10, 282. https://doi.org/10.3390/biomedicines10020282
Kim JC, Lee HY, Lee AR, Dezhbord M, Lee DR, Kim SH, Won J, Park S, Kim NY, Shin JJ, et al. Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient. Biomedicines. 2022; 10(2):282. https://doi.org/10.3390/biomedicines10020282
Chicago/Turabian StyleKim, Jong Chul, Hye Young Lee, Ah Ram Lee, Mehrangiz Dezhbord, Da Rae Lee, Seong Ho Kim, Juhee Won, Soree Park, Na Yeon Kim, Jae Jin Shin, and et al. 2022. "Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient" Biomedicines 10, no. 2: 282. https://doi.org/10.3390/biomedicines10020282
APA StyleKim, J. C., Lee, H. Y., Lee, A. R., Dezhbord, M., Lee, D. R., Kim, S. H., Won, J., Park, S., Kim, N. Y., Shin, J. J., Kim, S. G., Kim, Y. S., Yoo, J.-J., & Kim, K.-H. (2022). Identification and Characterization of Besifovir-Resistant Hepatitis B Virus Isolated from a Chronic Hepatitis B Patient. Biomedicines, 10(2), 282. https://doi.org/10.3390/biomedicines10020282






