Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Blood Sampling and Laboratory Analysis
2.3. Statistics
3. Results
3.1. CRP, PTX3 and Neprilysin in STEMI Patients
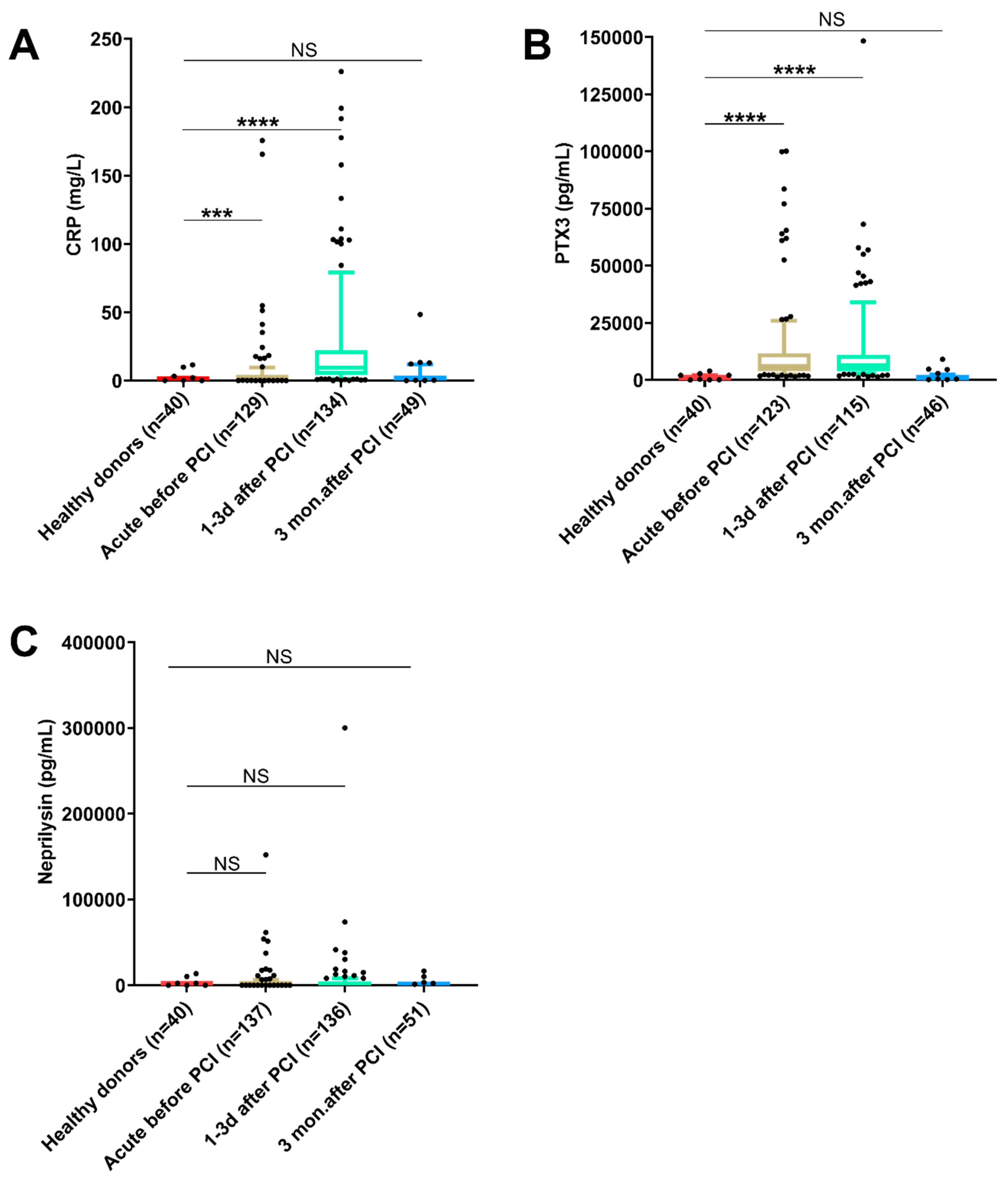
3.2. Differences in Biomarker Levels as Compared with Healthy Donors
3.3. Differences in Biomarker Levels between Patients with Patent and Occluded Culprit Vessels
3.4. Differences in Biomarker Levels between Patients with and without Thrombus Aspiration
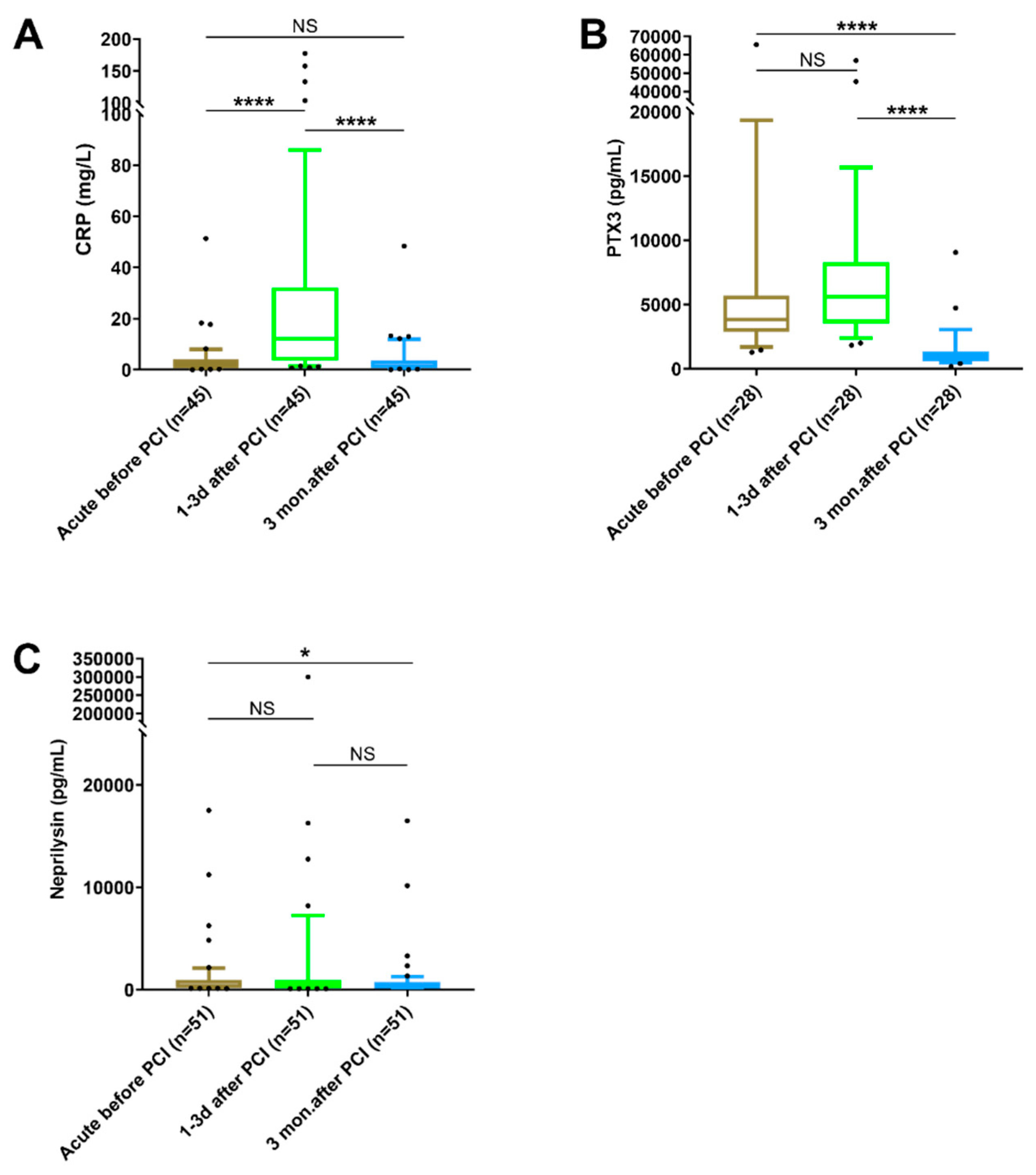
3.5. Changes in Biomarker Levels at Different Time Points
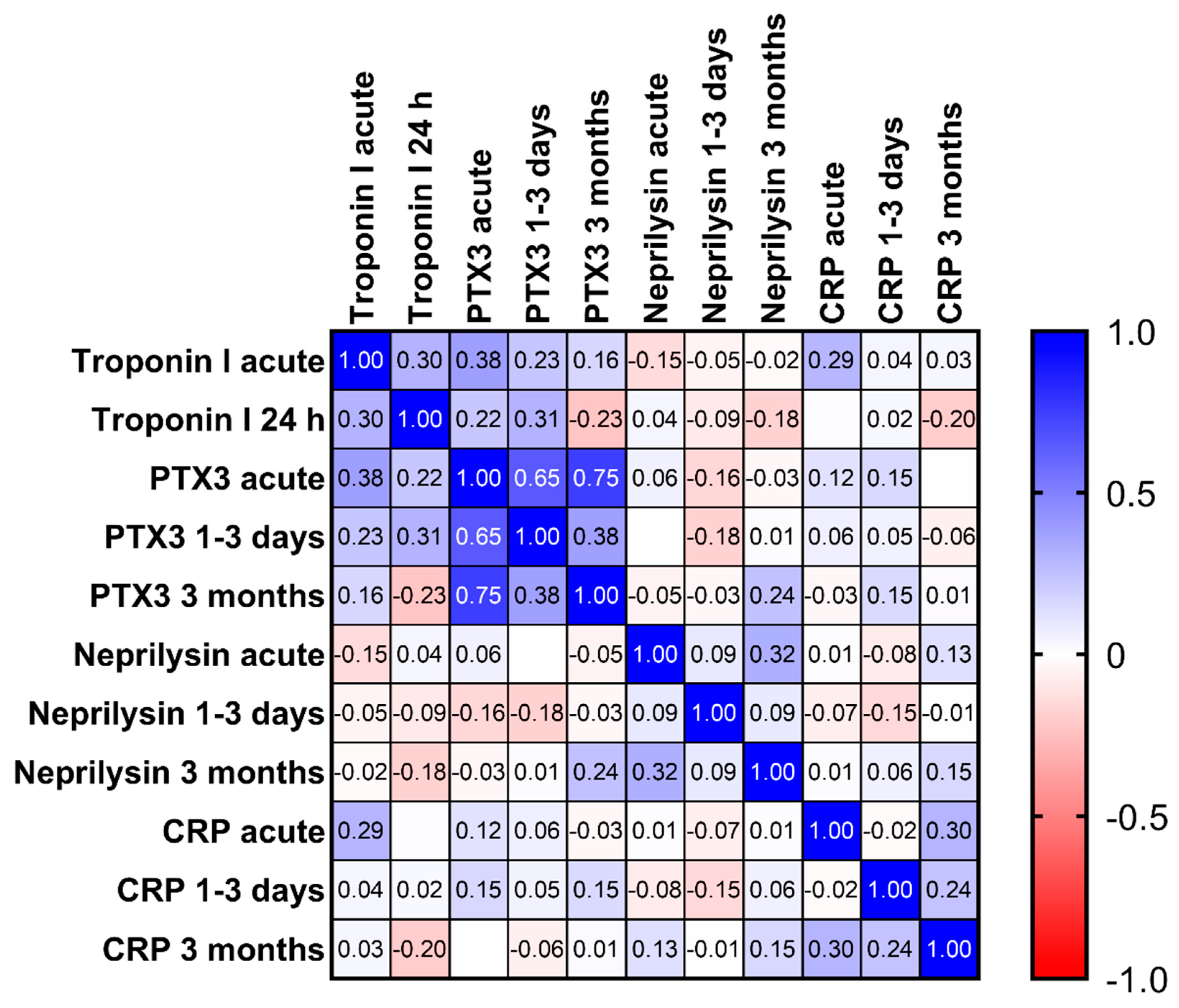
3.6. Correlation between Biomarkers
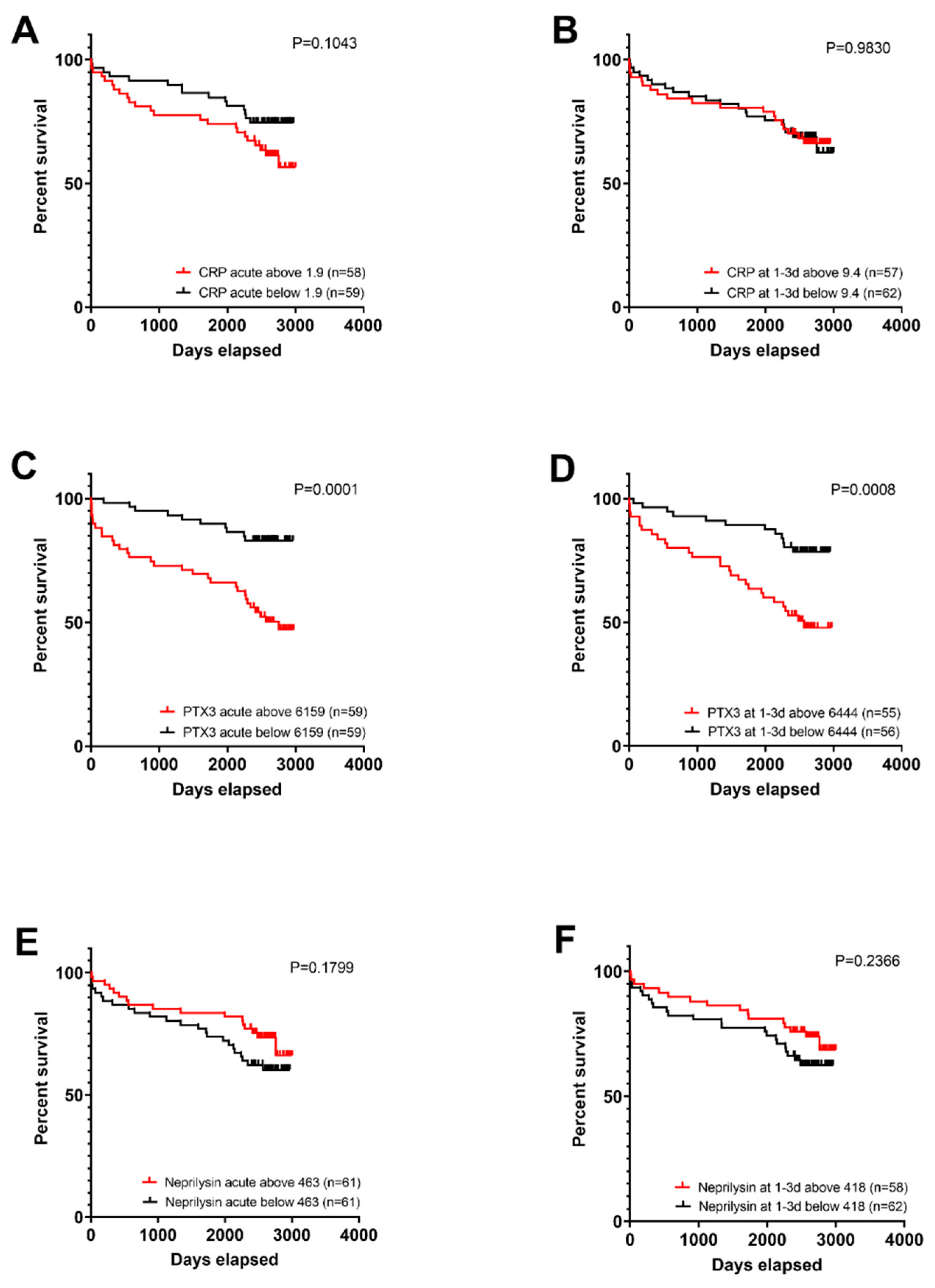
3.7. Survival Analysis of Patients with Elevated Levels of Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, J.D.; Kiefer, J.; Braig, D.; Loseff-Silver, J.; Potempa, L.A.; Eisenhardt, S.U.; Peter, K. Dissociation of C-Reactive Protein Localizes and Amplifies Inflammation: Evidence for a Direct Biological Role of C-Reactive Protein and Its Conformational Changes. Front. Immunol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.S.; Kurdi, M.I.; Al Aseri, Z.; Suriya, M.O. CRP levels are higher in patients with ST elevation than non-ST elevation acute coronary syndrome. Arq. Bras. Cardiol. 2011, 96, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Damen, S.A.J.; Cramer, G.E.; Dieker, H.J.; Gehlmann, H.; Aengevaeren, W.R.M.; Oude Ophuis, T.J.M.; Fokkert, M.J.; Dikkeschei, L.D.; Vroemen, W.H.; Verheugt, F.W.; et al. A multi-site coronary sampling study on CRP in non-STEMI: Novel insights into the inflammatory process in acute coronary syndromes. Atherosclerosis 2018, 278, 117–123. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Paffen, E.; DeMaat, M.P. C-reactive protein in atherosclerosis: A causal factor? Cardiovasc Res. 2006, 71, 30–39. [Google Scholar] [CrossRef]
- Kritchevsky, S.B.; Cesari, M.; Pahor, M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc. Res. 2005, 66, 265–275. [Google Scholar] [CrossRef]
- Helseth, R.; Solheim, S.; Opstad, T.; Hoffmann, P.; Arnesen, H.; Seljeflot, I. The time profile of Pentraxin 3 in patients with acute ST-elevation myocardial infarction and stable angina pectoris undergoing percutaneous coronary intervention. Mediat. Inflamm. 2014, 2014, 608414. [Google Scholar] [CrossRef]
- Staubli, S.M.; Schäfer, J.; Rosenthal, R.; Zeindler, J.; Oertli, D.; Nebiker, C.A. The role of CRP and Pentraxin 3 in the prediction of systemic inflammatory response syndrome and death in acute pancreatitis. Sci. Rep. 2019, 9, 18340. [Google Scholar] [CrossRef]
- Temelli, B.; Yetkin Ay, Z.; Savaş, H.B.; Aksoy, F.; Kumbul Doğuç, D.; Uskun, E.; Varol, E. Circulation levels of acute phase proteins pentraxin 3 and serum amyloid A in atherosclerosis have correlations with periodontal inflamed surface area. J. Appl. Oral. Sci. 2018, 26, e20170322. [Google Scholar] [CrossRef] [PubMed]
- Bottazzi, B.; Inforzato, A.; Messa, M.; Barbagallo, M.; Magrini, E.; Garlanda, C.; Mantovani, A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016, 64, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Imamura, T.; Kitamura, K.; Ogawa, T.; Fujimoto, S.; Kakitsubata, Y.; Ishikawa, T.; Asada, Y.; Kodama, T. Associations of Plasma Pentraxin 3 and Monocyte Chemoattractant Protein-1 Concentrations with Cardiovascular Disease in Patients with Chronic Kidney Disease. Ren. Fail. 2011, 33, 398–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of pentraxin 3 with cardiovascular disease and all-cause death: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Napoleone, E.; Di Santo, A.; Peri, G.; Mantovani, A.; De Gaetano, G.; Donati, M.B.; Lorenzet, R. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: Another link between inflammation and clotting activation. J. Leukoc. Biol. 2004, 76, 203–209. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Barallat, J.; Richards, A.M. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J. Am. Coll. Cardiol. 2016, 68, 639–653. [Google Scholar] [CrossRef]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 2013, 15, 1062–1073. [Google Scholar]
- D’Elia, E.; Iacovoni, A.; Vaduganathan, M.; Lorini, F.L.; Perlini, S.; Senni, M. Neprilysin inhibition in heart failure: Mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 2017, 19, 710–717. [Google Scholar] [CrossRef]
- Kaplinsky, E. Sacubitril/valsartan in heart failure: Latest evidence and place in therapy. Ther. Adv. Chronic Dis. 2016, 7, 278–290. [Google Scholar] [CrossRef]
- Scardovi, A.B.; Boccanelli, A. Valsartan/Sacubitril in heart failure and hypotension: How, when and why. Eur. Heart J. Suppl. 2019, 21 (Suppl. B), B88–B89. [Google Scholar] [CrossRef] [PubMed]
- Fröbert, O.; Lagerqvist, B.; Gudnason, T.; Thuesen, L.; Svensson, R.; Olivecrona, G.K.; James, S.K. Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am. Heart J. 2010, 160, 1042–1048. [Google Scholar] [PubMed]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Befekadu, R.; Christiansen, K.; Larsson, A.; Grenegard, M. Increased plasma cathepsin S and trombospondin-1 in patients with acute ST-segment elevation myocardial infarction. Cardiol. J. 2019, 26, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I.; Broder, M.L.; Karp, D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J. Clin. Investig. 1978, 61, 235–242. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Butt, N.; Bache-Mathiesen, L.K.; Ushakova, A.; Nordrehaug, J.E.; Jensen, S.E.; Munk, P.S.; Danchin, N.; Dubois-Rande, J.L.; Hansen, H.S.; Paganelli, F.; et al. Pentraxin 3 in primary percutaneous coronary intervention for ST elevation myocardial infarction is associated with early irreversible myocardial damage: Kinetic profile, relationship to interleukin 6 and infarct size. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 302–312. [Google Scholar] [CrossRef]
- Latini, R.; Maggioni, A.P.; Peri, G.; Gonzini, L.; Lucci, D.; Mocarelli, P.; Vago, L.; Pasqualini, F.; Signorini, S.; Soldateschi, D.; et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation 2004, 110, 2349–2354. [Google Scholar] [CrossRef]
- Jaillon, S.; Peri, G.; Delneste, Y.; Fremaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef]
- Ljuca, F.; Hadžiefendić, B.; Jahić, E.; Tihić, N.; Lukić, S. Pentraxin 3 might be better prognostic serum marker than IL-6, IL-10, and high-sensitivity C-reactive protein for major adverse cardiovascular events in patients with ST-elevation myocardial infarction after bare-metal stent implantation. Saudi Med. J. 2019, 40, 1202–1208. [Google Scholar] [CrossRef]
- Haibo, L.; Xiaofang, G.; Chunming, W.; Jie, Y.; Guozhong, C.; Limei, Z.; Yong, C.; Yu, F.; Yingchun, B.; Wangjun, Y.; et al. Prognostic value of plasma pentraxin-3 levels in patients with stable coronary artery disease after drug-eluting stent implantation. Mediat. Inflamm. 2014, 2014, 963096. [Google Scholar] [CrossRef] [PubMed]
- Bernelin, H.; Mewton, N.; Si-Mohamed, S.; Croisille, P.; Rioufol, G.; Bonnefoy-Cudraz, E.; Douek, P.; Dufay, N.; Amaz, C.; Jossan, C.; et al. Neprilysin levels at the acute phase of ST-elevation myocardial infarction. Clin. Cardiol. 2019, 42, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, Y.; Wen, J.; Li, W.; Xu, Y. Elevated plasma level of pentraxin-3 predicts in-hospital and 30-day clinical outcomes in patients with non-ST-segment elevation myocardial infarction who have undergone percutaneous coronary intervention. Cardiology 2014, 129, 178–188. [Google Scholar] [CrossRef] [PubMed]
| Age, Years (mean, range) | 69 (43–94) |
|---|---|
| Female/male | 46/104 |
| Prior myocardial infarction | 17% |
| Smoking, current | 34% |
| BMI (kg/m2).(mean, range) | 27.1 (16.3–39.1) |
| Hyperlipidemia (according to patient anamnesis) | 52% |
| Diabetes mellitus (any type) | 18% |
| (according to patient anamnesis) | 46% |
| Unstable angina | 10% |
| PPT (103/µL, mean ±SD) | 228 ± 62 |
| WBC (103/µL, mean ±SD) | 10.8 ± 4.1 |
| Lesion type | |
| Occluded | 70% |
| Patent (partially open) | 30% |
| Medication (at admission before PCI) | |
| ASA (only) | 22% |
| ASA+clopidogrel | 2% |
| Betablockers | 27% |
| Statins | 26% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Befekadu, R.; Grenegård, M.; Larsson, A.; Christensen, K.; Ramström, S. Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction. Biomedicines 2022, 10, 275. https://doi.org/10.3390/biomedicines10020275
Befekadu R, Grenegård M, Larsson A, Christensen K, Ramström S. Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction. Biomedicines. 2022; 10(2):275. https://doi.org/10.3390/biomedicines10020275
Chicago/Turabian StyleBefekadu, Rahel, Magnus Grenegård, Anders Larsson, Kjeld Christensen, and Sofia Ramström. 2022. "Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction" Biomedicines 10, no. 2: 275. https://doi.org/10.3390/biomedicines10020275
APA StyleBefekadu, R., Grenegård, M., Larsson, A., Christensen, K., & Ramström, S. (2022). Dynamic Changes in Pentraxin-3 and Neprilysin in ST Segment Elevation Myocardial Infarction. Biomedicines, 10(2), 275. https://doi.org/10.3390/biomedicines10020275







