Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
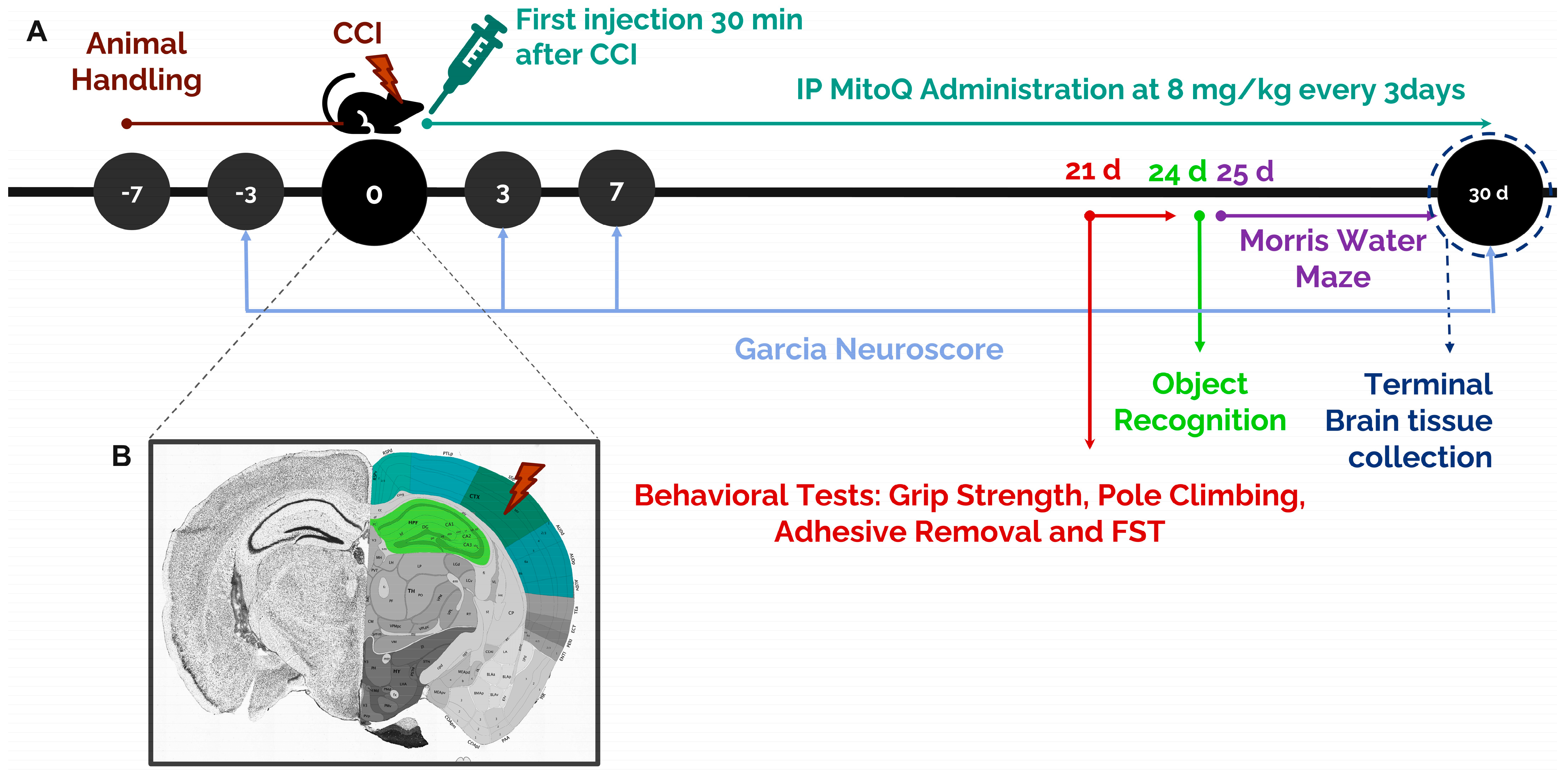
2.2. Stereotaxic Surgery: Controlled Cortical Impact
2.3. Mitoquinone Supplementation
2.4. Garcia Neuroscore
2.5. Grip Strength Test
2.6. Pole Climbing Test
2.7. Adhesive Removal Test
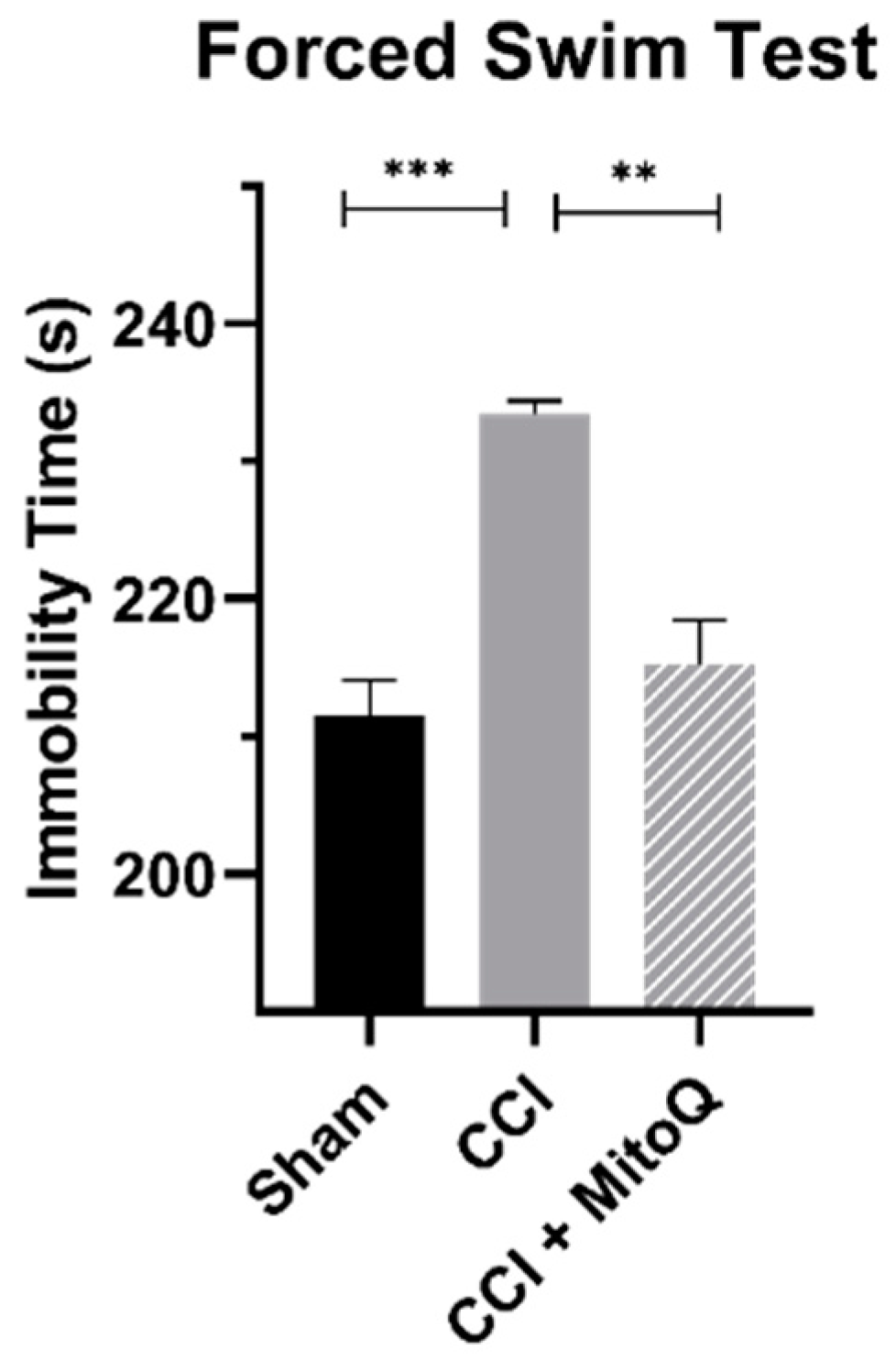
2.8. Forced Swim Test
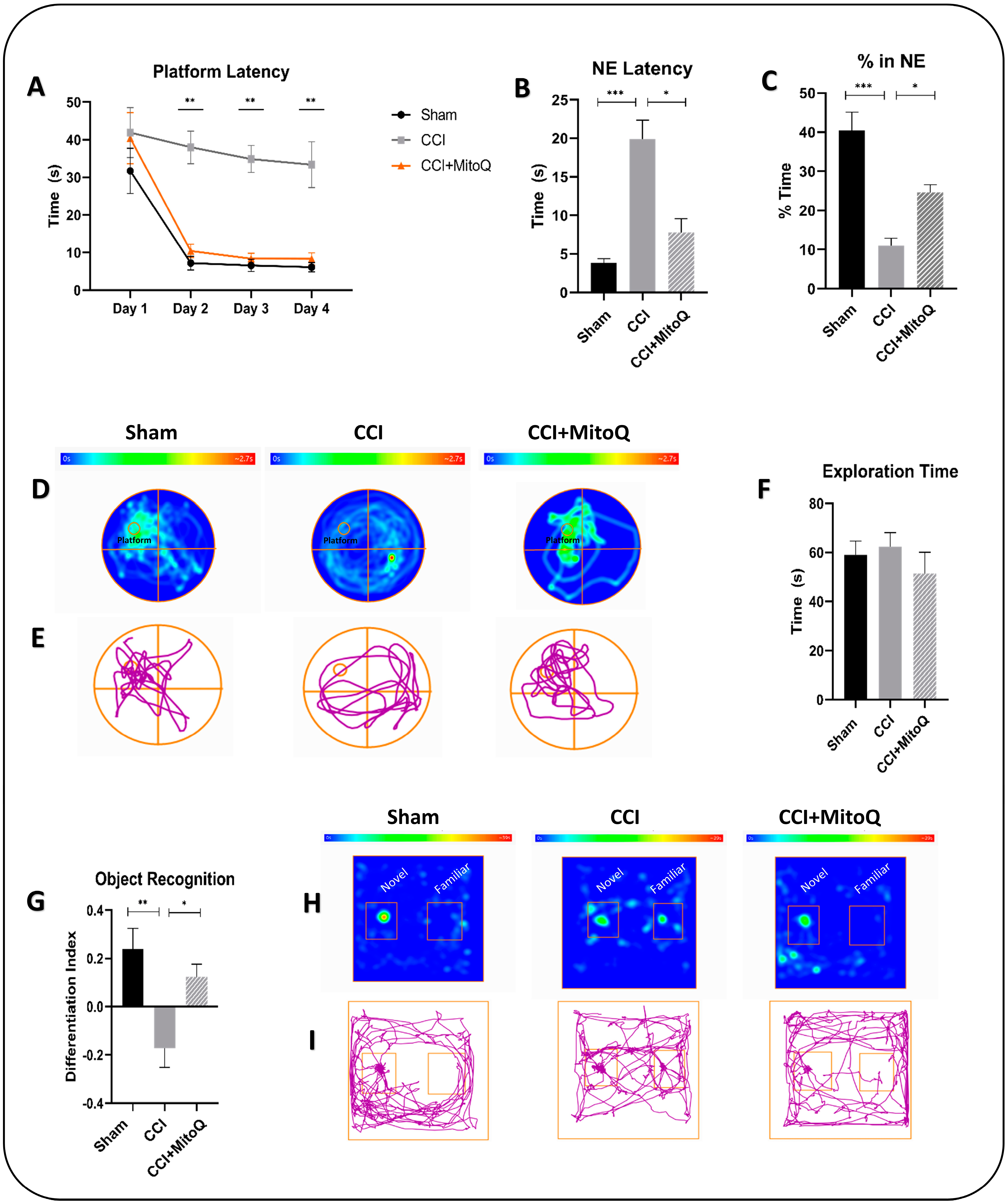
2.9. Morris Water Maze Test
2.10. Novel Object Recognition Test
2.11. RT-qPCR
2.12. Immunofluorescence Staining
2.13. Statistical Analysis
3. Results
3.1. MitoQ Improves General Neurological Function by Ameliorating Motor Coordination, Muscle Strength, and Sensorimotor Function
3.2. Learning Deficits and Recognition Memory Dysfunction Are Alleviated by MitoQ
3.3. MitoQ Decreases Depressive-like Behavior
3.4. MitoQ Upregulates Antioxidative Enzymes
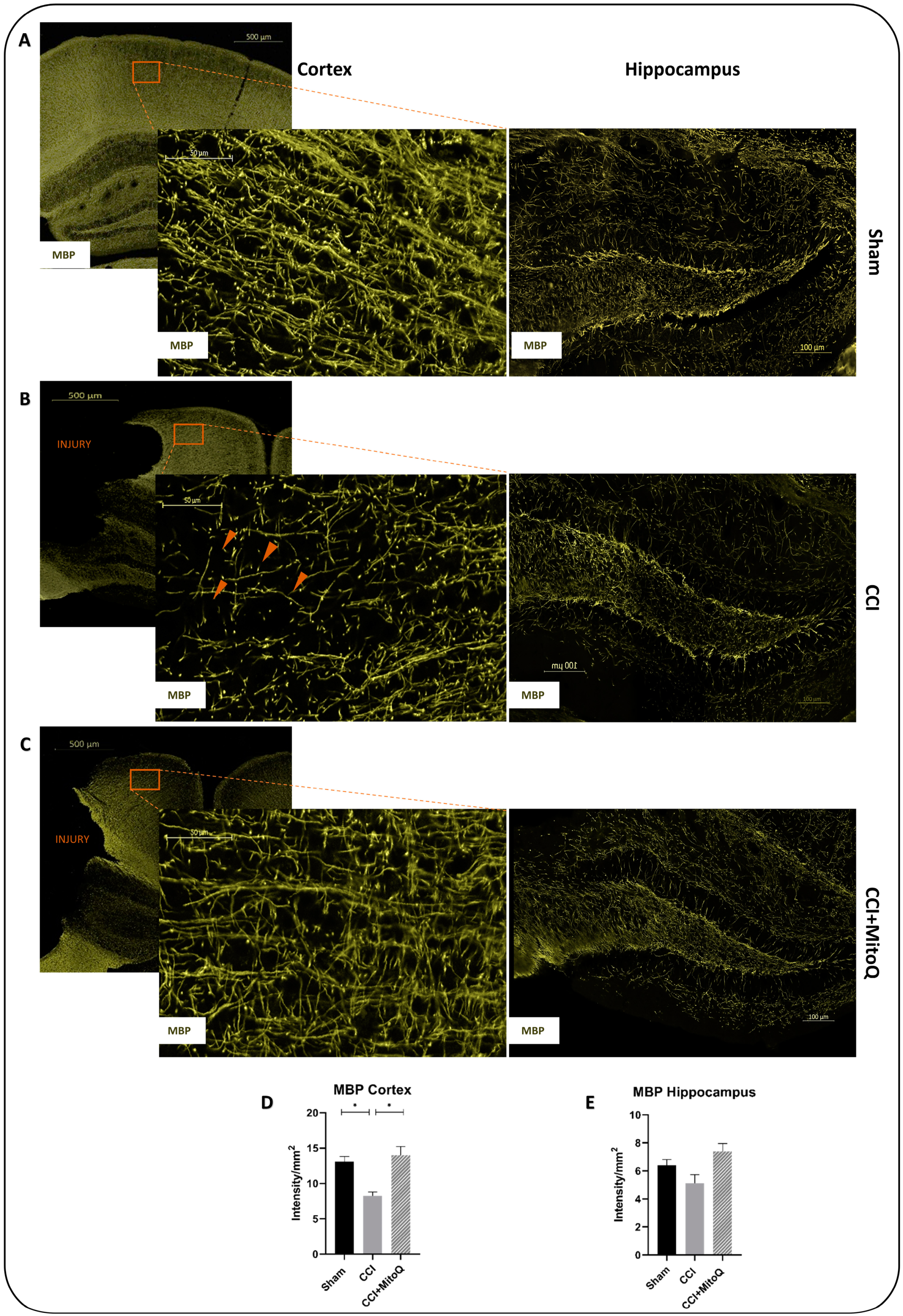
3.5. MitoQ Diminishes Chronic Activation of CNS Inflammatory Cells, Neuronal Death, and Axonal Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Menyar, A.; Mekkodathil, A.; Al-Thani, H.; Consunji, R.; Latifi, R. Incidence, Demographics, and Outcome of Traumatic Brain Injury in The Middle East: A Systematic Review. World Neurosurg. 2017, 107, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, B.; Tofighi, B.; Kufera, J.A.; Hadley, J.; Ahn, E.S.; Cooper, C.; Malik, J.M.; Naff, N.J.; Chang, L.; Radley, M.; et al. Predictors of outcome in civilian gunshot wounds to the head. J. Neurosurg. 2014, 120, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Muneer, P.M.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef]
- Dalgard, C.L.; Cole, J.T.; Kean, W.S.; Lucky, J.J.; Sukumar, G.; McMullen, D.C.; Pollard, H.B.; Watson, W.D. The cytokine temporal profile in rat cortex after controlled cortical impact. Front. Mol. Neurosci. 2012, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Masel, B.E.; DeWitt, D.S. Traumatic brain injury: A disease process, not an event. J. Neurotrauma 2010, 27, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.D.; Hylin, M.J.; Zhao, J.; Moore, A.N.; Waxham, M.N.; Dash, P.K. Altered Mitochondrial Dynamics and TBI Pathophysiology. Front. Syst. Neurosci. 2016, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Torbic, H.; Forni, A.A.; Anger, K.E.; Degrado, J.R.; Greenwood, B.C. Use of antiepileptics for seizure prophylaxis after traumatic brain injury. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharm. 2013, 70, 759–766. [Google Scholar] [CrossRef]
- Greenberg, M. Handbook of Neurosurgery, 6th ed.; Thieme Medical Publishers: New York, NY, USA, 2006; pp. 289–365. [Google Scholar]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic Brain Injury: Current Treatment Strategies and Future Endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Mahmood, A.; Chopp, M. Emerging treatments for traumatic brain injury. Expert Opin. Emerg. Drugs 2009, 14, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; Roos, Y.B. Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: A systematic review. Lancet Neurol. 2006, 5, 1029–1032. [Google Scholar] [CrossRef]
- Deng-Bryant, Y.; Singh, I.N.; Carrico, K.M.; Hall, E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 1114–1126. [Google Scholar] [CrossRef] [Green Version]
- Muizelaar, J.P.; Marmarou, A.; Young, H.F.; Choi, S.C.; Wolf, A.; Schneider, R.L.; Kontos, H.A. Improving the outcome of severe head injury with the oxygen radical scavenger polyethylene glycol-conjugated superoxide dismutase: A phase II trial. J. Neurosurg. 1993, 78, 375–382. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [Green Version]
- Maroz, A.; Anderson, R.F.; Smith, R.A.; Murphy, M.P. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: Implications for in vivo antioxidant activity. Free. Radic. Biol. Med. 2009, 46, 105–109. [Google Scholar] [CrossRef]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.; Murphy, M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 1019–1026. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Shen, R.; Fang, J.; Yang, Y.; Dai, W.; Zhu, Y.; Zhou, M. Mitochondrial-targeted antioxidant MitoQ provides neuroprotection and reduces neuronal apoptosis in experimental traumatic brain injury possibly via the Nrf2-ARE pathway. Am. J. Transl. Res. 2018, 10, 1887–1899. [Google Scholar] [PubMed]
- Sunkin, S.M.; Ng, L.; Lau, C.; Dolbeare, T.; Gilbert, T.L.; Thompson, C.L.; Hawrylycz, M.; Dang, C. Allen Brain Atlas: An integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013, 41, D996–D1008. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–634. [Google Scholar] [CrossRef]
- Smith, J.P.; Hicks, P.S.; Ortiz, L.R.; Martinez, M.J.; Mandler, R.N. Quantitative measurement of muscle strength in the mouse. J. Neurosci. Methods 1995, 62, 15–19. [Google Scholar] [CrossRef]
- Matsuura, K.; Kabuto, H.; Makino, H.; Ogawa, N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J. Neurosci. Methods 1997, 73, 45–48. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.d.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Gusel’nikova, V.V.; Korzhevskiy, D.E. NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Nat. 2015, 7, 42–47. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Shu, H.; Zhang, Z. Disrupted white matter integrity is associated with cognitive deficits in patients with amnestic mild cognitive impairment: An atlas-based study. SAGE Open Med. 2016, 4, 2050312116648812. [Google Scholar] [CrossRef]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. CMLS 2006, 63, 1945–1961. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [Green Version]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Souza, J.M.; Bolatto, C.; Rodríguez-Bottero, S.; Logan, A.; Smith, R.A.; Murphy, M.P.; Barbeito, L.; et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free. Radic. Biol. Med. 2014, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Pinho, B.R.; Duarte, A.I.; Canas, P.M.; Moreira, P.I.; Murphy, M.P.; Oliveira, J.M.A. The interplay between redox signalling and proteostasis in neurodegeneration: In vivo effects of a mitochondria-targeted antioxidant in Huntington’s disease mice. Free. Radic. Biol. Med. 2020, 146, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Rodriguez-Cuenca, S.; Cochemé, H.M.; Logan, A.; Abakumova, I.; Prime, T.A.; Rose, C.; Vidal-Puig, A.; Smith, A.C.; Rubinsztein, D.C.; Fearnley, I.M.; et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free. Radic. Biol. Med. 2010, 48, 161–172. [Google Scholar] [CrossRef]
- Deb, S.; Lyons, I.; Koutzoukis, C.; Ali, I.; McCarthy, G. Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry 1999, 156, 374–378. [Google Scholar] [CrossRef]
- Rogers, J.M.; Read, C.A. Psychiatric comorbidity following traumatic brain injury. Brain Inj. 2007, 21, 1321–1333. [Google Scholar] [CrossRef]
- Meehan, W.P., 3rd; Zhang, J.; Mannix, R.; Whalen, M.J. Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery 2012, 71, 885–891. [Google Scholar] [CrossRef] [Green Version]
- DeFord, S.M.; Wilson, M.S.; Rice, A.C.; Clausen, T.; Rice, L.K.; Barabnova, A.; Bullock, R.; Hamm, R.J. Repeated mild brain injuries result in cognitive impairment in B6C3F1 mice. J. Neurotrauma 2002, 19, 427–438. [Google Scholar] [CrossRef]
- Levine, B.; Kovacevic, N.; Nica, E.I.; Cheung, G.; Gao, F.; Schwartz, M.L.; Black, S.E. The Toronto traumatic brain injury study: Injury severity and quantified MRI. Neurology 2008, 70, 771–778. [Google Scholar] [CrossRef]
- Fox, G.B.; Fan, L.; Levasseur, R.A.; Faden, A.I. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J. Neurotrauma 1998, 15, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Osier, N.D.; Dixon, C.E. The Controlled Cortical Impact Model: Applications, Considerations for Researchers, and Future Directions. Front. Neurol. 2016, 7, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Nguyen, A.; Villeda, S.; Zhang, H.; Ding, Z.; Lindsey, D.; Bieri, G.; Castellano, J.; Beaupre, G.; Wyss-Coray, T. Long-Term Cognitive Impairments and Pathological Alterations in a Mouse Model of Repetitive Mild Traumatic Brain Injury. Front. Neurol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinder, H.A.; Baker, E.W.; Howerth, E.W.; Duberstein, K.J.; West, F.D. Controlled Cortical Impact Leads to Cognitive and Motor Function Deficits that Correspond to Cellular Pathology in a Piglet Traumatic Brain Injury Model. J. Neurotrauma 2019, 36, 2810–2826. [Google Scholar] [CrossRef]
- Atkins, C.M. Decoding hippocampal signaling deficits after traumatic brain injury. Transl. Stroke Res. 2011, 2, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Monti, J.M.; Voss, M.W.; Pence, A.; McAuley, E.; Kramer, A.F.; Cohen, N.J. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front. Aging Neurosci. 2013, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Morrow, E.L.; Dulas, M.R.; Cohen, N.J.; Duff, M.C. Relational Memory at Short and Long Delays in Individuals with Moderate-Severe Traumatic Brain Injury. Front. Hum. Neurosci. 2020, 14, 270. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2020, 17, 446–456. [Google Scholar] [CrossRef]
- Girgis, F.; Pace, J.; Sweet, J.; Miller, J.P. Hippocampal Neurophysiologic Changes after Mild Traumatic Brain Injury and Potential Neuromodulation Treatment Approaches. Front. Syst. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Neary, J.T.; Kang, Y.; Tran, M.; Feld, J. Traumatic injury activates protein kinase B/Akt in cultured astrocytes: Role of extracellular ATP and P2 purinergic receptors. J. Neurotrauma 2005, 22, 491–500. [Google Scholar] [CrossRef]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275 Pt 3, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Fluiter, K.; Opperhuizen, A.L.; Morgan, B.P.; Baas, F.; Ramaglia, V. Inhibition of the membrane attack complex of the complement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J. Immunol. 2014, 192, 2339–2348. [Google Scholar] [CrossRef] [Green Version]
- Morganti, J.M.; Riparip, L.-K.; Rosi, S. Call Off the Dog(ma): M1/M2 Polarization is Concurrent following Traumatic Brain Injury. PLoS ONE 2016, 11, e0148001. [Google Scholar] [CrossRef] [Green Version]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.S.; Chen, J.; Watkins, S.C.; Kochanek, P.M.; Chen, M.; Stetler, R.A.; Loeffert, J.E.; Graham, S.H. Apoptosis-suppressor gene bcl-2 expression after traumatic brain injury in rats. J. Neurosci. Off. J. Soc. Neurosci. 1997, 17, 9172–9182. [Google Scholar] [CrossRef] [Green Version]
- Stoica, B.A.; Faden, A.I. Cell death mechanisms and modulation in traumatic brain injury. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2010, 7, 3–12. [Google Scholar] [CrossRef]
- Kaya, S.S.; Mahmood, A.; Li, Y.; Yavuz, E.; Göksel, M.; Chopp, M. Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res. 1999, 818, 23–33. [Google Scholar] [CrossRef]
- Buki, A.; Povlishock, J.T. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. 2006, 148, 181–193. [Google Scholar] [CrossRef]
- Wright, D.K.; O’Brien, T.J.; Shultz, S.R.; Mychasiuk, R. Sex matters: Repetitive mild traumatic brain injury in adolescent rats. Ann. Clin. Transl. Neurol. 2017, 4, 640–654. [Google Scholar] [CrossRef] [PubMed]






| Criteria | Evaluation |
|---|---|
| Spontaneous Activity | Ability to approach all four walls of the cage |
| Limb Symmetry | Limb symmetry when held by the tail |
| Forepaw Outstretching | Outstretching symmetry of both forelimbs while the hindlimbs are kept in the air |
| Climbing | Ability to climb into and hang onto the cage |
| Body Perception | Reaction to stimulus while the mouse is touched on each side of the body with a stick |
| Vibrissae Touch | Reaction to stimulus while whiskers of the mouse are touched with a stick without entering the visual field |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| mSOD2 | GGCCAAGGGAGATGTTACAA | GAACCTTGGACTCCCACA |
| mCAT | TGAGAAGCCTAAGAACGCAATTC | CCCTTCGCAGCCATGTG |
| mNrf2 | CGAGATATACGCAGGAGAGGTAAGA | GCTCGACAATGTTCTCCAGCTT |
| mβ-actin | CAGCTGAGAGGGAAATCGTG | CGTTGCCAATAGTGATGACC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haidar, M.A.; Shakkour, Z.; Barsa, C.; Tabet, M.; Mekhjian, S.; Darwish, H.; Goli, M.; Shear, D.; Pandya, J.D.; Mechref, Y.; et al. Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines 2022, 10, 250. https://doi.org/10.3390/biomedicines10020250
Haidar MA, Shakkour Z, Barsa C, Tabet M, Mekhjian S, Darwish H, Goli M, Shear D, Pandya JD, Mechref Y, et al. Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines. 2022; 10(2):250. https://doi.org/10.3390/biomedicines10020250
Chicago/Turabian StyleHaidar, Muhammad Ali, Zaynab Shakkour, Chloe Barsa, Maha Tabet, Sarin Mekhjian, Hala Darwish, Mona Goli, Deborah Shear, Jignesh D. Pandya, Yehia Mechref, and et al. 2022. "Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point" Biomedicines 10, no. 2: 250. https://doi.org/10.3390/biomedicines10020250
APA StyleHaidar, M. A., Shakkour, Z., Barsa, C., Tabet, M., Mekhjian, S., Darwish, H., Goli, M., Shear, D., Pandya, J. D., Mechref, Y., El Khoury, R., Wang, K., & Kobeissy, F. (2022). Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines, 10(2), 250. https://doi.org/10.3390/biomedicines10020250









