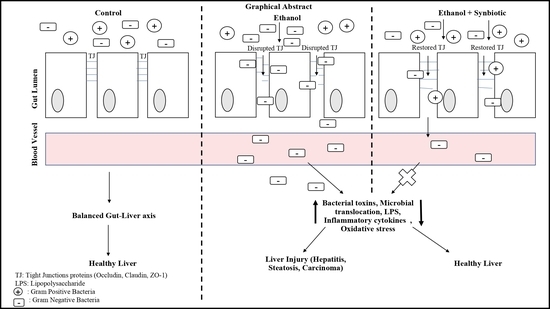
Synbiotic Intervention Ameliorates Oxidative Stress and Gut Permeability in an In Vitro and In Vivo Model of Ethanol-Induced Intestinal Dysbiosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Culture Collection and Chemicals
2.2. Culturing of Caco-2 Cell Line
2.3. Caco-2 Cells Experimental Dosing for Chronic Ethanol Model
2.4. Animals Housing and Chronic Ethanol Feeding of Male Wistar Rats
2.5. Establishment of Intestinal Barrier Dysfunction with Ethanol and Analysis of the Preventive Effect of Probiotics and AGE Administration
2.5.1. In Vitro and In Vivo Experimentation
2.6. Caco-2 Transepithelial Electrical Resistance (TEER) Evaluation
2.7. Hematoxylin and Eosin (H/E) Colon Staining
2.8. Lipogenesis Analysis by Oil Red O Staining Confocal Imaging
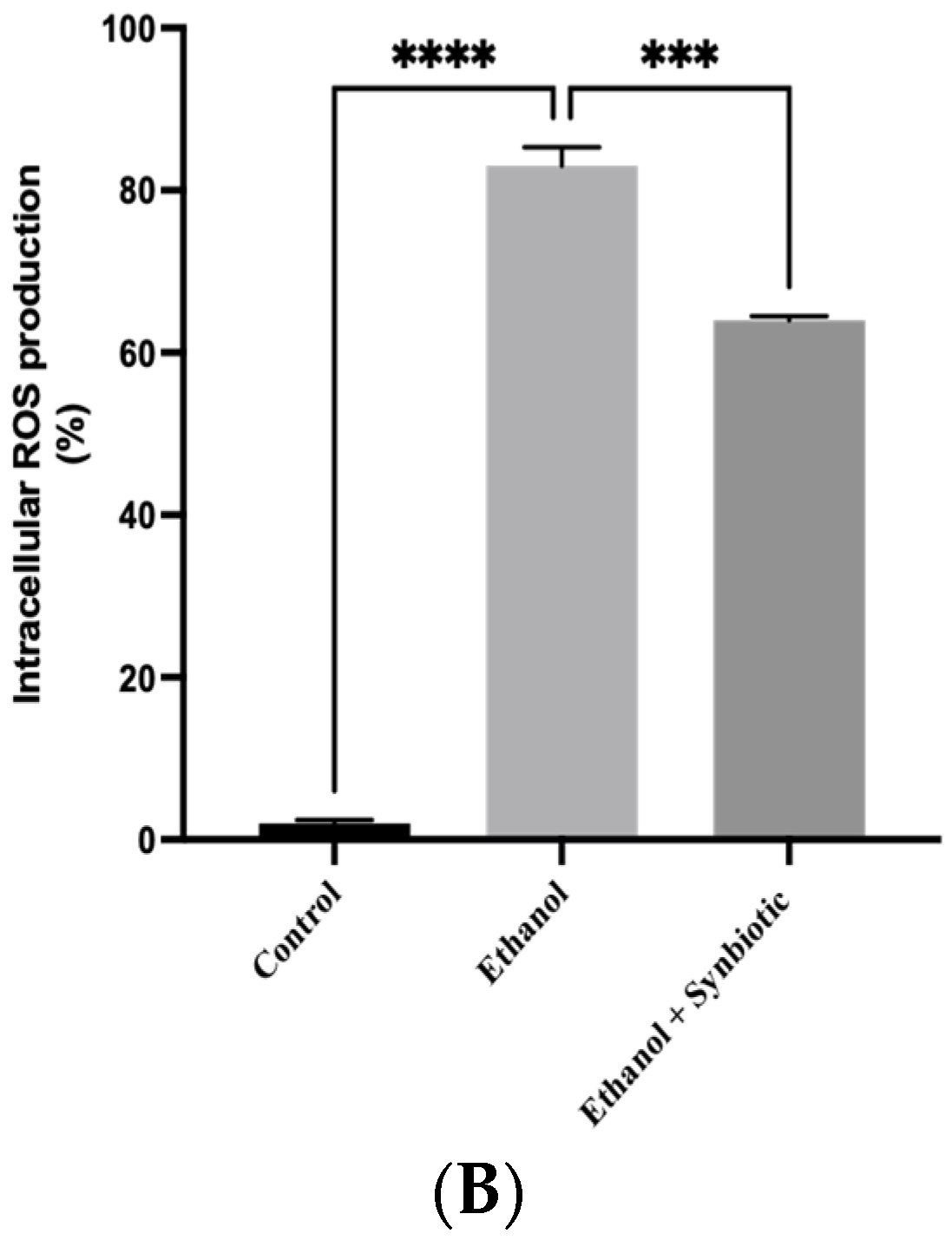
2.9. Caco-2 Cells ROS Estimation
2.10. Colon Oxidative Stress Assessment
2.10.1. Colonic Malondialdehyde (MDA) by TBA Method
2.10.2. HPLC Method for Serum MDA Content Evaluation
2.10.3. RNA Extraction and qRT-PCR
2.11. Analytical Statistics
2.12. Feces Collection
High-Throughput 16S rRNA Gene Amplicon Sequencing
3. Results
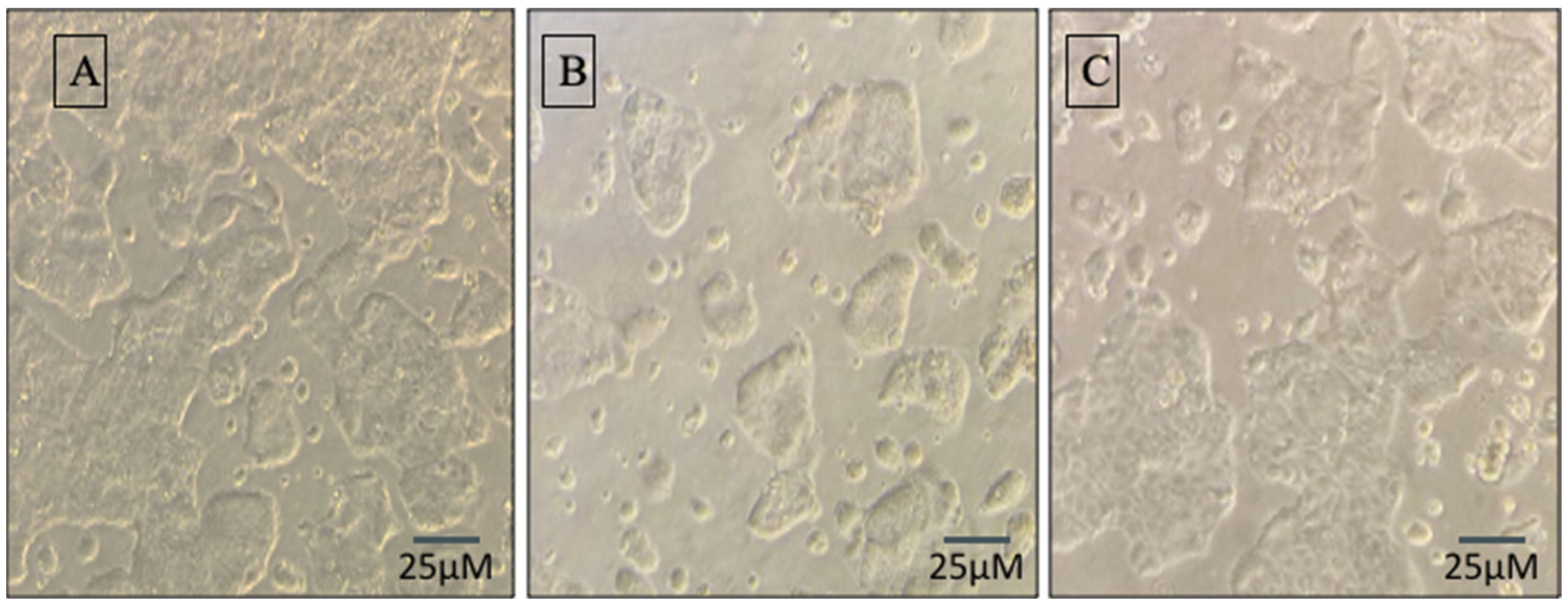
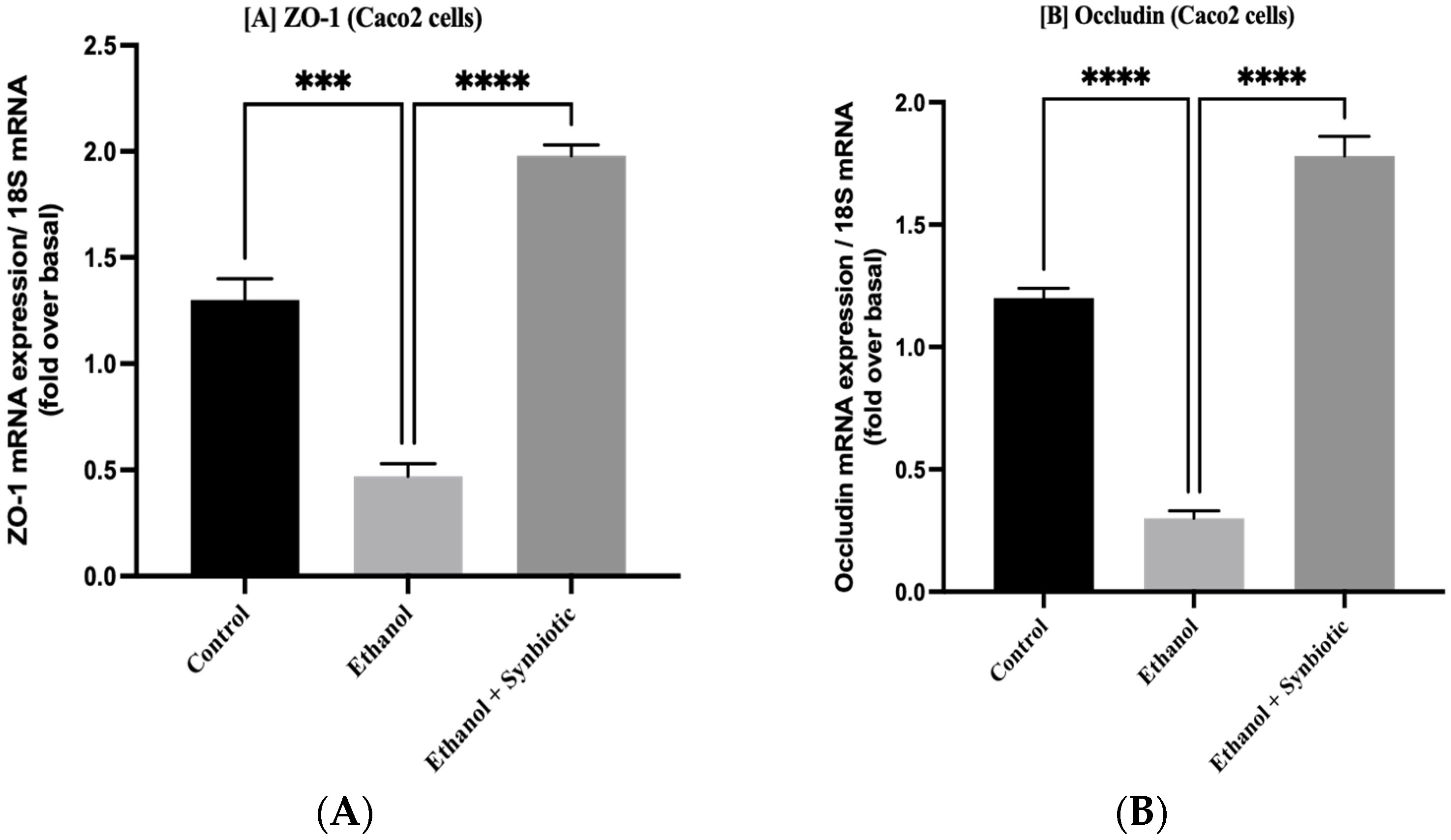
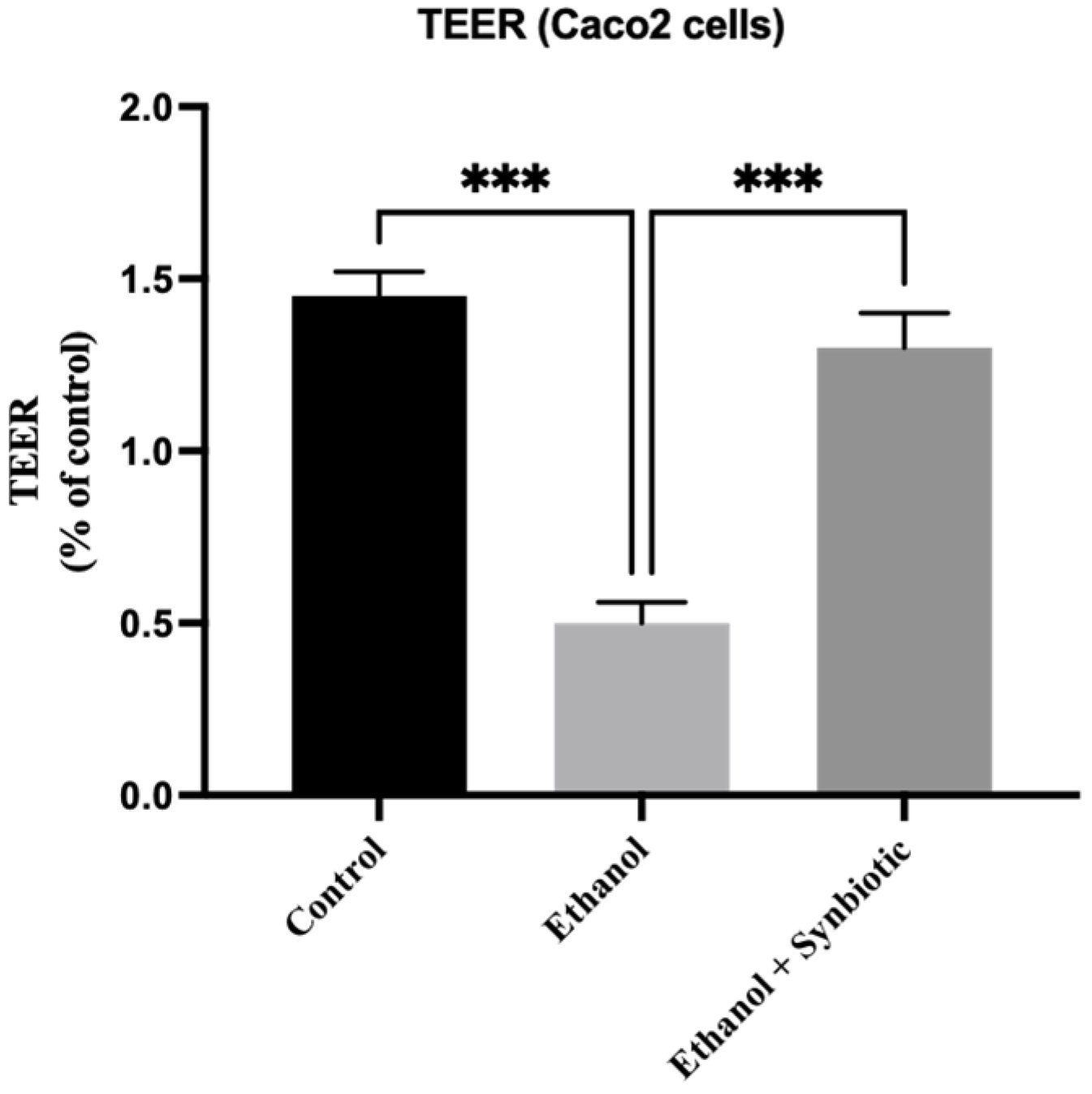
3.1. Synbiotic Intervention Lowers Morphological and Intestinal Barrier Alterations in Caco-2 Cells
TEER Analysis
3.2. Synbiotic Improves Weight and Length Proportion of Colon in ALD Rats
3.3. Synbiotic Enhances Rat Colon Tissue Integrity
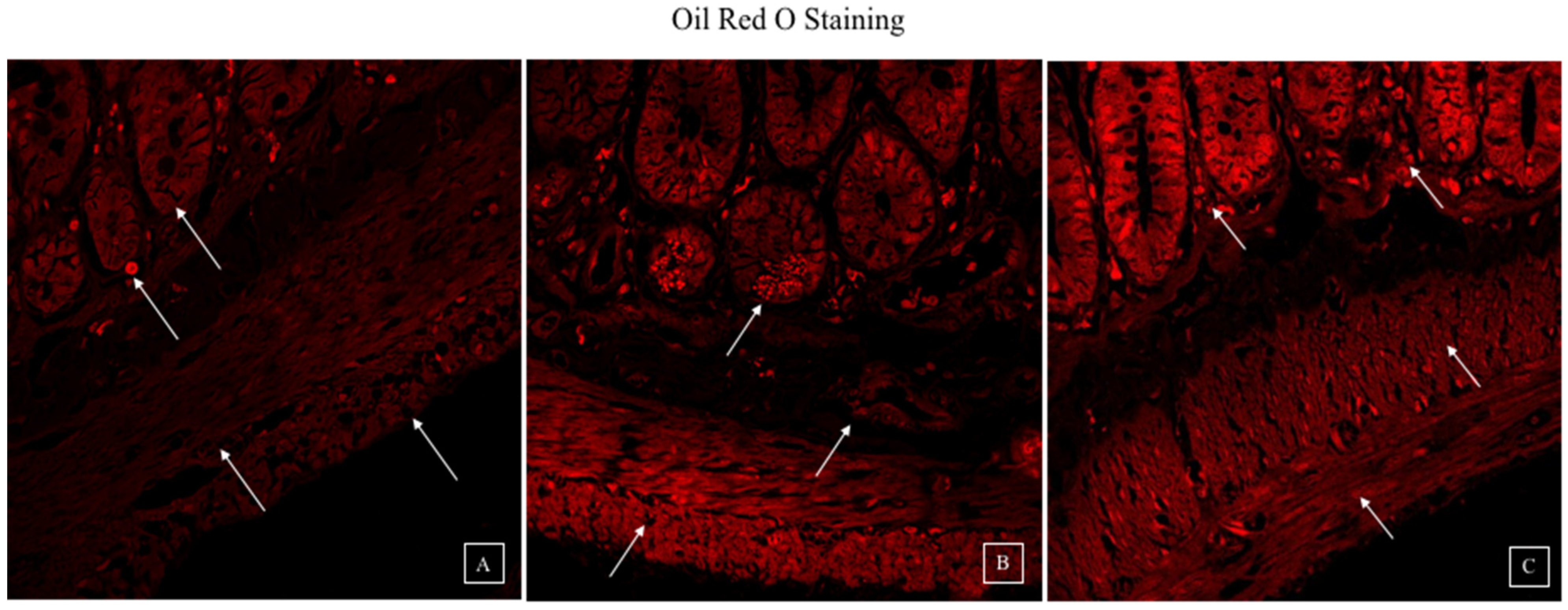
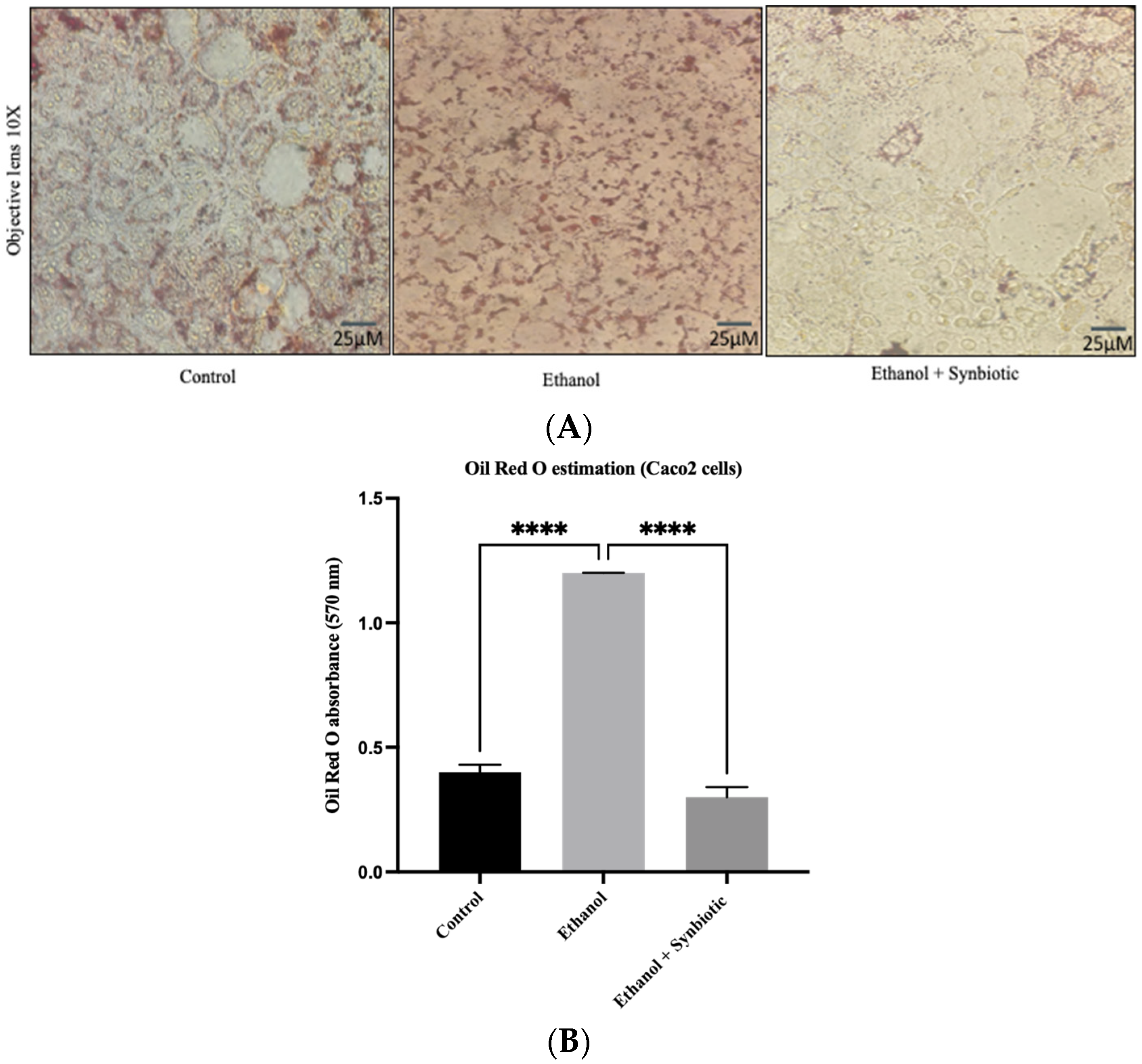
3.4. Synbiotic Intervention Reduced Lipogenesis in the Rat Colonic Epithelium
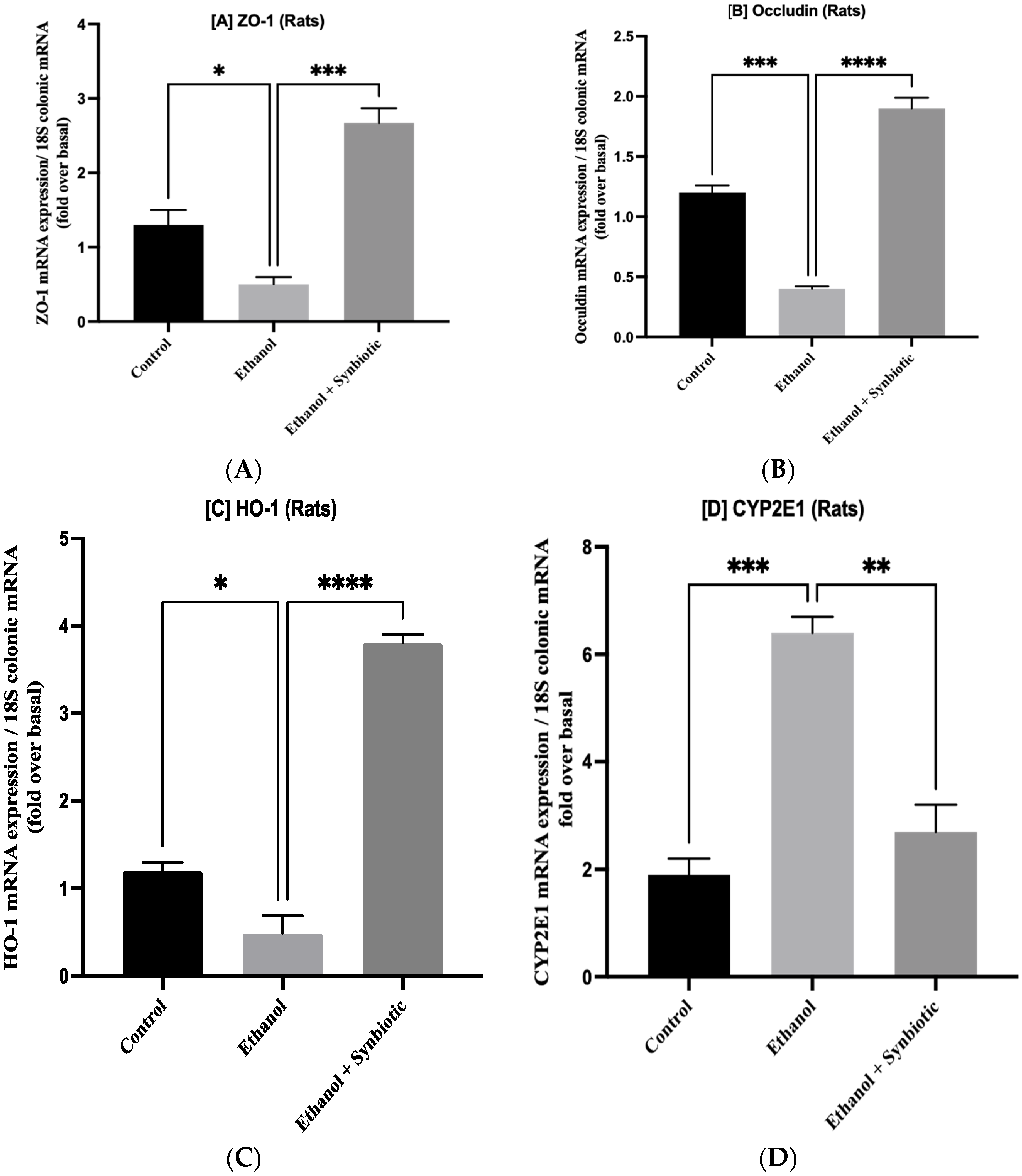
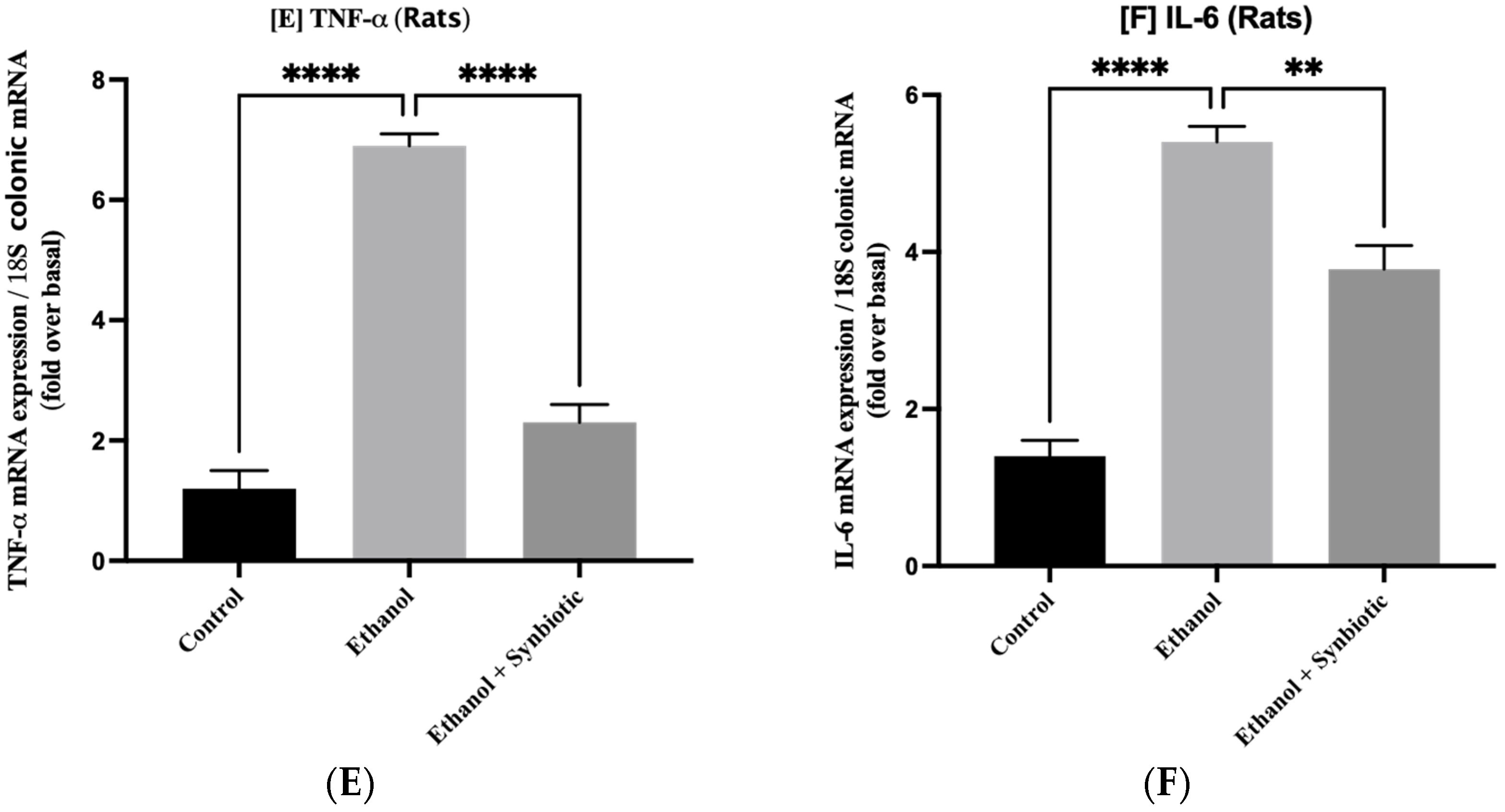
3.5. Synbiotic Treatment Reduces the Effects of Alcohol on TJ Expression and Permeability in the Colon
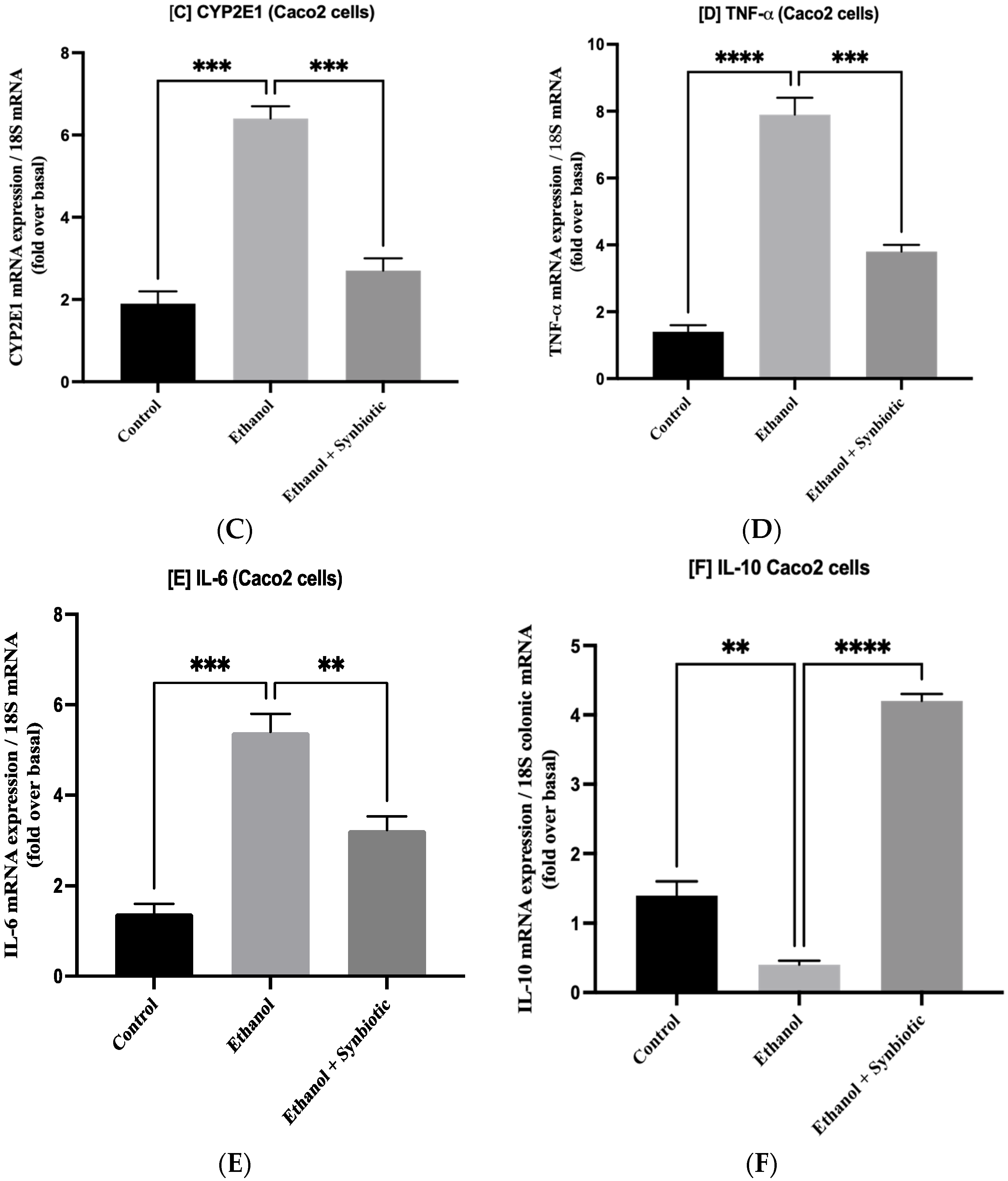
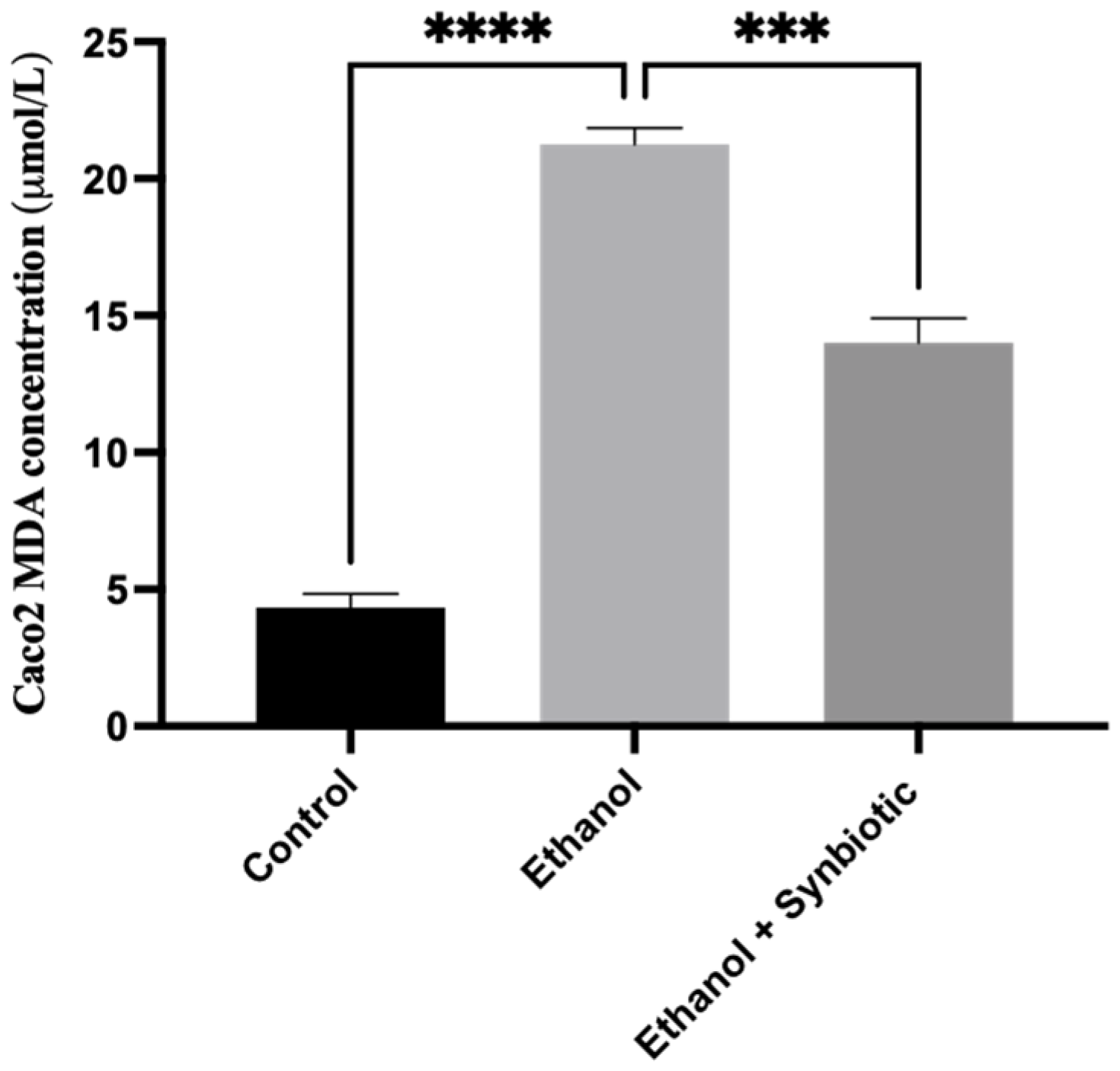
3.6. Preventative Therapy Synbiotic Reduces Ethanol-Mediated Intestinal Oxidative Stress
3.6.1. High-Performance Liquid Chromatography (HPLC) for MDA Analysis of Caco-2 Cells
3.6.2. HPLC Analysis of Rat Serum Samples
3.6.3. MDA Concentration of Rat Colon Tissue by HPLC Method
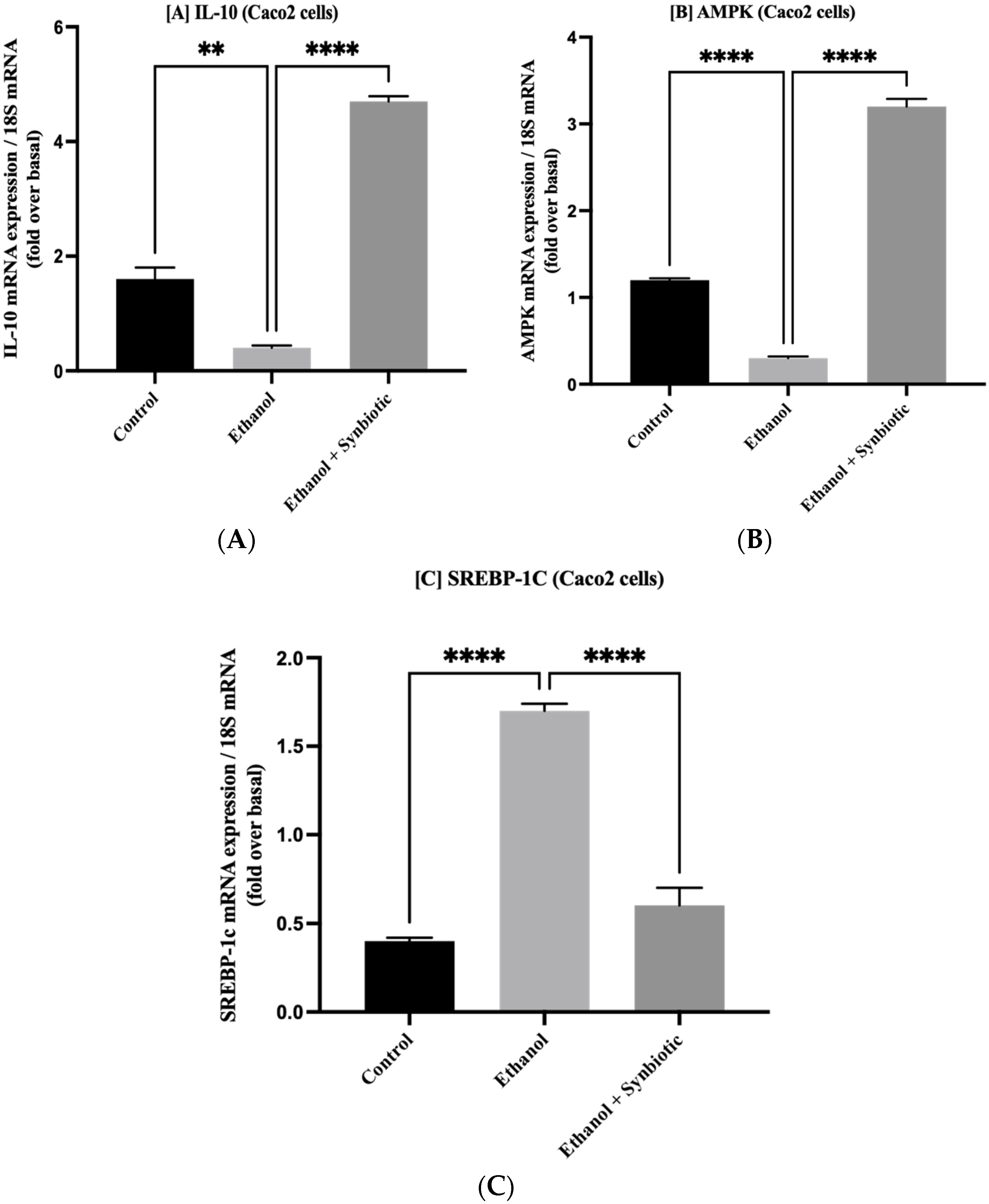
3.7. Synbiotic Administration Reduces Rat Colonic Metabolism of Lipids
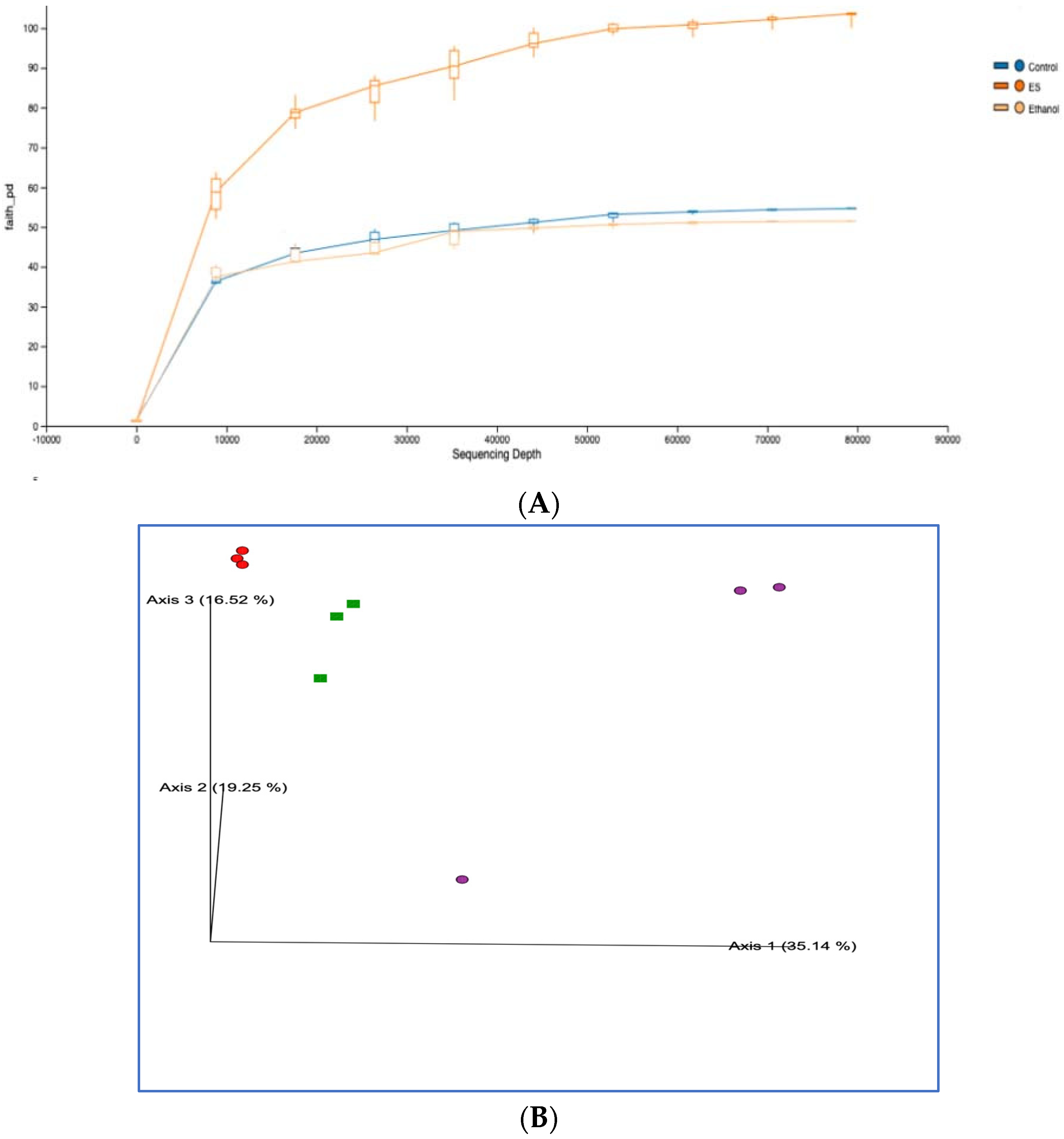
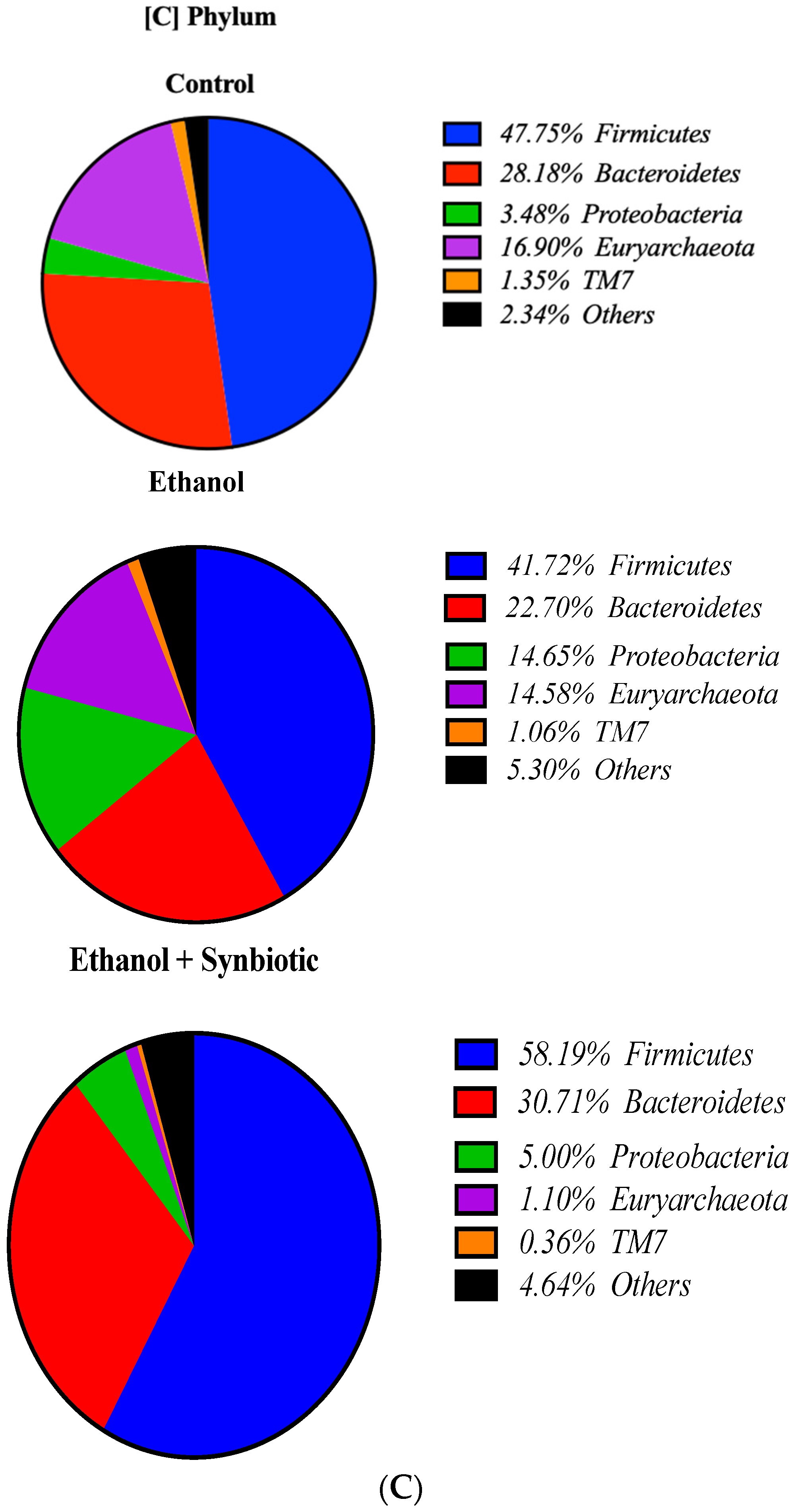
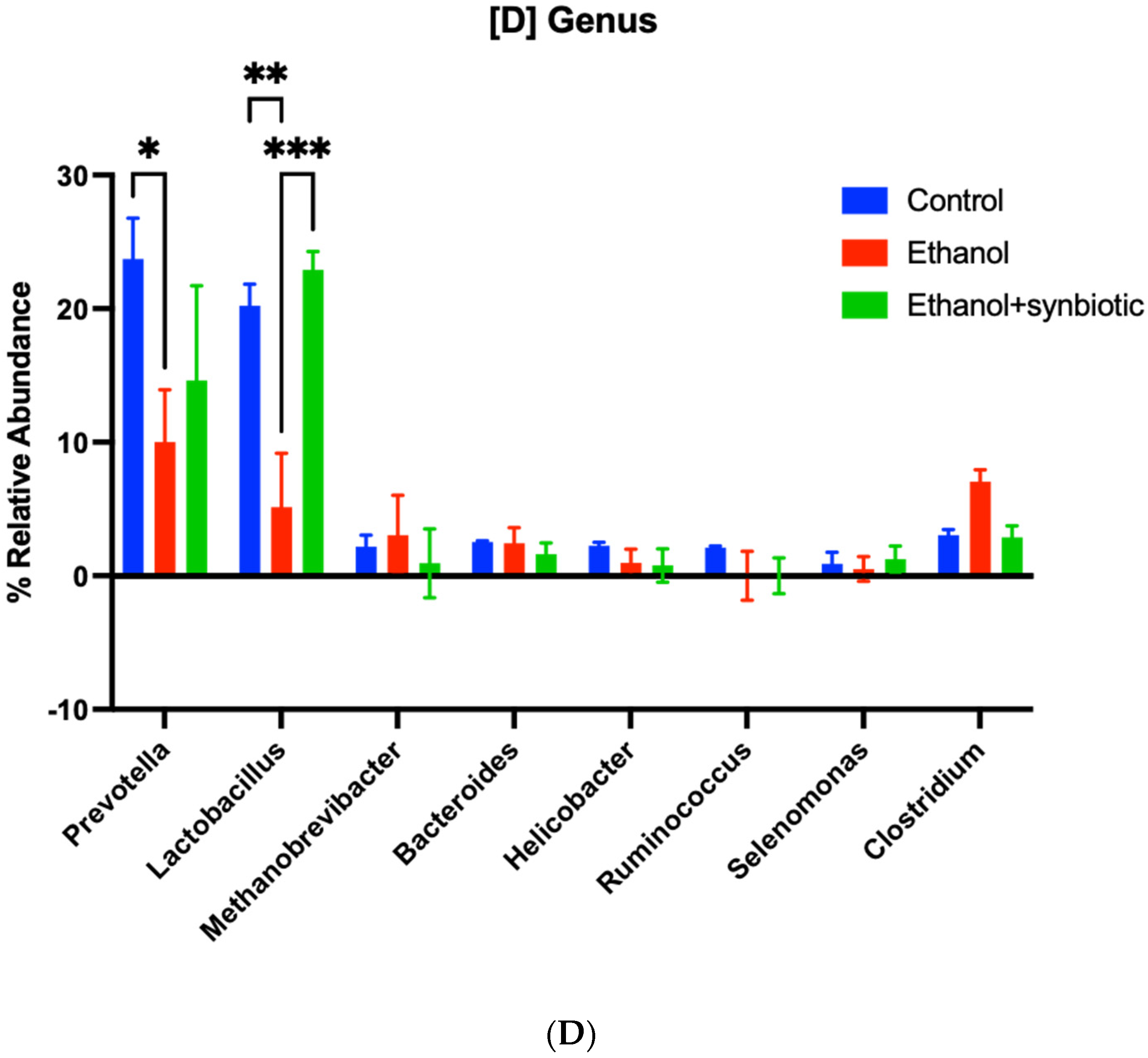
3.8. High-Throughput 16S rRNA Gene Sequencing of Rat Feces Demonstrates That Synbiotics Restore Gut Flora
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Status Report on Alcohol and Health 2018; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Jung, J.H.; Kim, S.-E.; Suk, K.T.; Kim, D.J. Gut microbiota-modulating agents in alcoholic liver disease: Links between host metabolism and gut microbiota. Front. Med. 2022, 9, 913842. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Wang, M.; Zhang, J.; Barve, S.S.; McClain, C.J.; Joshi-Barve, S. Acrolein Disrupts Tight Junction Proteins and Causes Endoplasmic Reticulum Stress-Mediated Epithelial Cell Death Leading to Intestinal Barrier Dysfunction and Permeability. Am. J. Pathol. 2017, 187, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Zhong, W.; McClain, C.J.; Cave, M.; Kang, Y.J.; Zhou, Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 298, G625. [Google Scholar] [CrossRef]
- Purohit, V.; Bode, J.C.; Bode, C.; Brenner, D.A.; Choudhry, M.A.; Hamilton, F.; Kang, Y.J.; Keshavarzian, A.; Rao, R.; Sartor, R.B.; et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol 2008, 42, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pritchard, M.T.; Nagy, L.E. Anti-inflammatory pathways and alcoholic liver disease: Role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J. Gastroenterol. 2010, 16, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol. Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Abdelmegeed, M.A.; Jang, S.; Song, B.-J. Increased Sensitivity to Binge Alcohol-Induced Gut Leakiness and Inflammatory Liver Disease in HIV Transgenic Rats. PLoS ONE 2015, 10, e0140498. [Google Scholar] [CrossRef]
- Brown, J.; Wang, H.; Hajishengallis, G.N.; Martin, M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 2011, 90, 417–427. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Choudhary, S.; Holmes, E.W.; Yong, S.; Banan, A.; Jakate, S.; Fields, J.Z. Preventing Gut Leakiness by Oats Supplementation Ameliorates Alcohol-Induced Liver Damage in Rats. J. Pharmacol. Exp. Ther. 2001, 299, 442–448. [Google Scholar]
- Nitric Oxide and Its Metabolites Mediate Ethanol-Induced Microtubule Disruption and Intestinal Barrier Dysfunction. Available online: https://pubmed.ncbi.nlm.nih.gov/10945852/ (accessed on 8 May 2022).
- Forsyth, C.B.; Voigt, R.M.; Shaikh, M.; Tang, Y.; Cederbaum, A.I.; Turek, F.W.; Keshavarzian, A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G185–G195. [Google Scholar] [CrossRef] [PubMed]
- Elamin, E.E.; Masclee, A.A.; Dekker, J.; Jonkers, D.M. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr. Rev. 2013, 71, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Leclercq, S.; Stärkel, P.; Delzenne, N.M.; de Timary, P. The gut microbiota: A new target in the management of alcohol dependence? Alcohol 2019, 74, 105–111. [Google Scholar] [CrossRef]
- Hartmann, P.; Seebauer, C.T.; Schnabl, B. Alcoholic Liver Disease: The Gut Microbiome and Liver Cross Talk. Alcohol. Clin. Exp. Res. 2015, 39, 763–775. [Google Scholar] [CrossRef]
- Shukla, P.K.; Meena, A.S.; Manda, B.; Gomes-Solecki, M.; Dietrich, P.; Dragatsis, I.; Rao, R.K. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor-dependent mechanism. FASEB J. 2018, 32, 6274–6292. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Farhadi, A.; Jakate, S.M.; Tang, Y.; Shaikh, M.; Keshavarzian, A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009, 43, 163–172. [Google Scholar] [CrossRef]
- Bergheim, I.; Guo, L.; Davis, M.; Lambert, J.; Beier, J.I.; Duveau, I.; Luyendyk, J.P.; Roth, R.A.; Arteel, G.E. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology 2006, 130, 2099–2112. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Krogh Pedersen, H.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, M.J.; Lee, J.H.; Han, J., II; Kim, J.H.; Sok, D.E.; Kim, M.R. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J. Med. Food 2011, 14, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Colín-González, A.L.; Santamaría, A. Garlic, Gastrointestinal Protection and Oxidative Stress. In Gastrointestinal Tissue Oxidative; Elsevier: Amsterdam, The Netherlands, 2017; pp. 275–288. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.-H.; Deepthi Gadde, U.; Oh, S.-T.; Lee, S.-J.; Lillehoj, H.S. Dietary Allium hookeri reduces inflammatory response and increases expression of intestinal tight junction proteins in LPS-induced young broiler chicken. Res. Vet. Sci. 2017, 112, 149–155. [Google Scholar] [CrossRef]
- Patel, F.; Parwani, K.; Patel, D.; Mandal, P. Metformin and Probiotics Interplay in Amelioration of Ethanol-Induced Oxidative Stress and Inflammatory Response in an in Vitro and in Vivo Model of Hepatic Injury. Mediat. Inflamm. 2021, 2021, 6636152. [Google Scholar] [CrossRef] [PubMed]
- Kuri-Harcuch, W.; Green, H. Adipose conversion of 3T3 cells depends on a serum factor. Proc. Natl. Acad. Sci. USA 1978, 75, 6107–6109. [Google Scholar] [CrossRef]
- Kema, V.H.; Khan, I.; Jamal, R.; Vishwakarma, S.K.; Lakki Reddy, C.; Parwani, K.; Patel, F.; Patel, D.; Khan, A.A.; Mandal, P. Protective Effects of Diallyl Sulfide Against Ethanol-Induced Injury in Rat Adipose Tissue and Primary Human Adipocytes. Alcohol. Clin. Exp. Res. 2017, 41, 1078–1092. [Google Scholar] [CrossRef]
- Tüközkan, N.; Erdamar, H.; Seven, I. Measurement of Total Malondialdehyde in Plasma and Tissues by High-Performance Liquid Chromatography and Thiobarbituric Acid Assay. Firat Tip Dergisi 2006, 11, 88–92. [Google Scholar]
- Mateos, R.; Goya, L.; Bravo, L. Determination of malondialdehyde by liquid chromatography as the 2,4-dinitrophenylhydrazone derivative: A marker for oxidative stress in cell cultures of human. J. Chromatogr. B 2004, 805, 33–39. [Google Scholar] [CrossRef]
- Patel, F.; Parwani, K.; Rao, P.; Patel, D.; Rawal, R.; Mandal, P. Prophylactic Treatment of Probiotic and Metformin Mitigates Ethanol-Induced Intestinal Barrier Injury: In Vitro, in Vivo, and in Silico Approaches. Mediat. Inflamm. 2021, 2021, 5245197. [Google Scholar] [CrossRef]
- Patel, D.; Bapodra, S.L.; Madamwar, D.; Desai, C. Electroactive bacterial community augmentation enhances the performance of a pilot scale constructed wetland microbial fuel cell for treatment of textile dye wastewater. Bioresour. Technol. 2021, 332, 125088. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 302, G966–G978. [Google Scholar] [CrossRef]
- Singh, S.; Osna, N.A.; Kharbanda, K.K. Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. World J. Gastroenterol. 2017, 23, 6549. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Parlesak, A.; Schütt, C.; Bode, J.C.; Bode, C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol. Alcohol. 2002, 37, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Bode, J.C. Activation of the innate immune system and alcoholic liver disease: Effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol. Clin. Exp. Res. 2005, 29, 166S–171S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, J.; Chang, B.; Wang, B.; Zhang, D.; Wang, B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol. Med. Rep. 2014, 9, 2352–2356. [Google Scholar] [CrossRef]
- Chaudhry, K.K.; Shukla, P.K.; Mir, H.; Manda, B.; Gangwar, R.; Yadav, N.; McMullen, M.; Nagy, L.E.; Rao, R.K. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J. Nutr. Biochem. 2016, 27, 16–26. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The Protective Effects of Estrogen on the Cardiovascular System. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Rao, R.K. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol. Biol. 2008, 447, 171–183. [Google Scholar] [CrossRef]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G1280–G1288. [Google Scholar] [CrossRef]
- Meena, A.S.; Shukla, P.K.; Sheth, P.; Rao, R.K. EGF receptor plays a role in the mechanism of glutamine-mediated prevention of alcohol-induced gut barrier dysfunction and liver injury. J. Nutr. Biochem. 2019, 64, 128–143. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 103. [Google Scholar] [CrossRef] [PubMed]
- Bellos, D.A.; Sharma, D.; McMullen, M.R.; Wat, J.; Saikia, P.; de la Motte, C.A.; Nagy, L.E. Specifically-sized hyaluronan (35 kDa) prevents ethanol-induced disruption of epithelial tight junctions through a Layilin-dependent mechanism in Caco-2 cells. Alcohol. Clin. Exp. Res. 2019, 43, 1848. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Zheng, X.; Li, Q.; Qiu, Y.; Li, H.; Chen, H.; Zhou, Z.; Jia, W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; You, Q.; He, S.; Meng, Q.; Gao, J.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; et al. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 2018, 9, 761. [Google Scholar] [CrossRef]
- Banan, A.; Choudhary, S.; Zhang, Y.; Fields, J.Z.; Keshavarzian, A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: Evidence for oxidative and cytoskeletal mechanisms. J. Pharmacol. Exp. Ther. Dec. 1999, 291, 1075–1085. [Google Scholar]
- Al Kattar, S.; Jurjus, R.; Pinon, A.; Leger, D.Y.; Jurjus, A.; Boukarim, C.; Diab-Assaf, M.; Liagre, B. Metformin and Probiotics in the Crosstalk between Colitis-Associated Colorectal Cancer and Diabetes in Mice. Cancers 2020, 12, 1857. [Google Scholar] [CrossRef]
- Dolpady, J.; Sorini, C.; Di Pietro, C.; Cosorich, I.; Ferrarese, R.; Saita, D.; Clementi, M.; Canducci, F.; Falcone, M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J. Diabetes Res. 2016, 2016, 7569431. [Google Scholar] [CrossRef]
- del Carmen, S.; de Moreno de LeBlanc, A.; Levit, R.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G.; LeBlanc, J.G. Anti-cancer effect of lactic acid bacteria expressing antioxidant enzymes or IL-10 in a colorectal cancer mouse model. Int. Immunopharmacol. 2017, 42, 122–129. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Role of CYP2E1 in Ethanol-Induced Oxidant Stress, Fatty Liver and Hepatotoxicity. Dig. Dis. 2010, 28, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, H.; Xu, Y.; Wang, X.; Qiu, Z.; Jiang, L. Allicin Decreases Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells through Suppression of Mitochondrial Dysfunction and Activation of Nrf2. Cell. Physiol. Biochem. 2017, 41, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, H.; Kwon, E.; Han, M.; Kim, D. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Hermiston, M.L.; Gordon, J.I. Inflammatory Bowel Disease and Adenomas in Mice Expressing a Dominant Negative N-Cadherin. Science 1995, 270, 1203–1207. [Google Scholar] [CrossRef]
- Wick, E.; Sears, C.L. Bacteroides spp. and diarrhea. Curr. Opin. Infect. Dis. 2010, 23, 470–474. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Hu, S.; Zhang, J.; Wu, J.; Shao, N.; Bo, X.; Ni, M.; Ying, X. Gut metagenomes of type 2 diabetic patients have characteristic singlenucleotide polymorphism distribution in Bacteroides Coprocola. Microbiome 2017, 5, 15. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; de Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef]
- Medellin-Peña, M.J.; Griffiths, M.W. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl. Environ. Microbiol. 2009, 75, 1165–1172. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.C.; Yang, J.; Wang, J.F.; Zhu, Y.H. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Appl. Environ. Microbiol. 2016, 82, 1173–1182. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Puri, P.; Liangpunsakul, S.; Christensen, J.E.; Shah, V.H.; Kamath, P.S.; Gores, G.J.; Walker, S.; Comerford, M.; Katz, B.; Borst, A.; et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology 2018, 67, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Vasques-Monteiro, I.M.L.; Silva-Veiga, F.M.; Miranda, C.S.; de Andrade Gonçalves, É.C.B.; Daleprane, J.B.; Souza-Mello, V. A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr. Res. 2021, 91, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; De Vos, W.M.; Pakarinen, M.P. Intestinal Microbiota Signatures Associated With Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. J. Parenter. Enter. Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Sali, A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front. Nutr. 2018, 5, 122. [Google Scholar] [CrossRef]





| Sr. no. | Name of the Gene | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| 1 | 18S | ACGGAAGGGCACCACCAGGA | CACCACCACCCACGGAATCG |
| 2 | ZO-1 | TATTATGGCACATCAGCACG | TGGGCAAACAGACCAAGC |
| 3 | Occludin | CTCGAGAAAGTGCTGAGTGCCTGGAC | AAGCTTTCGGTGACCAATTCACCTGA |
| 4 | CYP2E1 | AGGGTACCATGTCTGCCCTCGGAGTGA | ACAATTTGAAAGCTTGTTTGAAAGCGG |
| 5 | NOX | GTACAAATTCCAGTGTGCAGACCAC | GTACAAATTCCAGTGTGCAGACCAC |
| 6 | TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| 7 | IL-6 | GACAACTTTGGCATTGTGG | ATGCAGGGATGATGTTCTG |
| 8 | IL-10 | ACTGCTAACCGACTCCTTA | TAAGGAGTCGGTTAGCAGT |
| Sr. no. | Name of the Gene | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| 1 | 18S | ACGGAAGGGCACCACCAGGA | CACCACCACCCACGGAATCG |
| 2 | ZO-1 | TATTATGGCACATCAGCACG | TGGGCAAACAGACCAAGC |
| 3 | Occludin | CTCGAGAAAGTGCTGAGTGCCTGGAC | AAGCTTTCGGTGACCAATTCACCTGA |
| 4 | CYP2E1 | AGGGTACCATGTCTGCCCTCGGAGTGA | ACAATTTGAAAGCTTGTTTGAAAGCGG |
| 5 | NOX | GTACAAATTCCAGTGTGCAGACCAC | GTACAAATTCCAGTGTGCAGACCAC |
| 6 | TNF-α | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| 7 | IL-6 | GACAACTTTGGCATTGTGG | ATGCAGGGATGATGTTCTG |
| 8 | IL-10 | ACTGCTAACCGACTCCTTA | TAAGGAGTCGGTTAGCAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, D.; Desai, C.; Singh, D.; Soppina, V.; Parwani, K.; Patel, F.; Mandal, P. Synbiotic Intervention Ameliorates Oxidative Stress and Gut Permeability in an In Vitro and In Vivo Model of Ethanol-Induced Intestinal Dysbiosis. Biomedicines 2022, 10, 3285. https://doi.org/10.3390/biomedicines10123285
Patel D, Desai C, Singh D, Soppina V, Parwani K, Patel F, Mandal P. Synbiotic Intervention Ameliorates Oxidative Stress and Gut Permeability in an In Vitro and In Vivo Model of Ethanol-Induced Intestinal Dysbiosis. Biomedicines. 2022; 10(12):3285. https://doi.org/10.3390/biomedicines10123285
Chicago/Turabian StylePatel, Dhara, Chirayu Desai, Deepmala Singh, Virupakshi Soppina, Kirti Parwani, Farhin Patel, and Palash Mandal. 2022. "Synbiotic Intervention Ameliorates Oxidative Stress and Gut Permeability in an In Vitro and In Vivo Model of Ethanol-Induced Intestinal Dysbiosis" Biomedicines 10, no. 12: 3285. https://doi.org/10.3390/biomedicines10123285
APA StylePatel, D., Desai, C., Singh, D., Soppina, V., Parwani, K., Patel, F., & Mandal, P. (2022). Synbiotic Intervention Ameliorates Oxidative Stress and Gut Permeability in an In Vitro and In Vivo Model of Ethanol-Induced Intestinal Dysbiosis. Biomedicines, 10(12), 3285. https://doi.org/10.3390/biomedicines10123285