Abstract
Bronchiolitis obliterans syndrome (BOS) is the most common form of CLAD and is characterized by airflow limitation and an obstructive spirometric pattern without high-resolution computed tomography (HRCT) evidence of parenchymal opacities. Computed tomography and microCT analysis show abundant small airway obstruction, starting from the fifth generation of airway branching and affecting up to 40–70% of airways. The pathogenesis of BOS remains unclear. It is a multifactorial syndrome that leads to pathological tissue changes and clinical manifestations. Because BOS is associated with the worst long-term survival in LTx patients, many studies are focused on the early identification of BOS. Markers may be useful for diagnosis and for understanding the molecular and immunological mechanisms involved in the onset of BOS. Diagnostic and predictive markers of BOS have also been investigated in various biological materials, such as blood, BAL, lung tissue and extracellular vesicles. The aim of this review was to evaluate the scientific literature on markers of BOS after lung transplant. We performed a systematic review to find all available data on potential prognostic and diagnostic markers of BOS.
1. Introduction
A lung transplant is considered the best therapeutic option for patients with end-stage lung disease. Survival after lung transplant continues to improve: median survival is now 6.9 years thanks to the efficacy of prophylaxis, new drugs, and better risk stratification [1]. However, graft failure is responsible for 22.7% of deaths in the interval between 30 days and 1 year after transplant. After the first year, chronic lung allograft dysfunction (CLAD) is the leading cause of death [2]. The international society for heart and lung transplantation (ISHLT) consensus recently re-defined CLAD and related phenotypes [3,4]. CLAD is defined as a persistent decline (≥20%) in the measured forced expiratory volume in the first second (FEV1) from the post-transplant baseline. The date of CLAD onset is defined as the date of the first FEV1 decline ≤80% of baseline [4].
Bronchiolitis obliterans syndrome (BOS), restrictive allograft dysfunction (RAS) and the newly defined mixed-phenotype result are the three major phenotypes of CLAD. BOS is the most common form of CLAD and is characterized by airflow limitation and an obstructive spirometric pattern unexplained by acute rejection, infection or other coexistent condition without high-resolution computed tomography (HRCT) evidence of parenchymal opacities [4,5,6].
Obliterative bronchiolitis (OB) occurs in a number of clinical settings, including infection, inhalational injury, drug toxicity or in the context of a collagen-vascular disease. There are also idiopathic cases [7]. OB is a clinical syndrome marked by progressive dyspnea and cough with the absence of parenchymal lung disease. Among lung transplant recipients, “bronchiolitis obliterans syndrome,” is described as a disorder with clinical and histopathological similarities to OB, representing the leading cause of long-term allograft dysfunction and mortality [8].
Although less frequent, RAS and mixed phenotypes portend a worse prognosis than BOS [9].
Computed tomography and micro-CT analysis show abundant small airway obstruction, starting from the fifth generation of airway branching and affecting up to 40–70% of airways [10]. The pathogenesis of BOS remains unclear. It is a multifactorial syndrome that leads to pathological tissue changes and clinical manifestations [11]. BOS is mediated by several mechanisms, such as alloimmune reactivity, humoral and autoimmunity, activation of innate immune cells and response to nonimmune-related allograft insults, such as infection and aspiration [12]. Employing a systematic approach for the collection of clinical data and the storage of samples can be useful for improving the knowledge of BOS research [13,14].
Because BOS is associated with the worst long-term survival in LTx patients, many studies are focused on the early identification of BOS [15]. Markers may be useful for diagnosis [16,17,18] and for understanding the molecular and immunological mechanisms involved in the onset of BOS [19,20]. Diagnostic and predictive markers of BOS have also been investigated in various biological materials, such as blood, BAL, lung tissue and extracellular vesicles [21,22,23,24].
The aim of this review was to evaluate the scientific literature on markers of BOS after lung transplant. We performed a systematic review to find all available data on potential prognostic and diagnostic markers of BOS. We performed a systematic search of PUBMED scientific papers in the English language. We used the keywords “markers of bronchiolitis obliterans syndrome” and “markers of CLAD” to locate potentially relevant studies. Reference lists of the studies selected were also checked to obtain additional sources. We excluded reviews, case reports, unrelated topics and studies in which BOS was not diagnosed according to the most recent guidelines. One of the 86 articles screened was excluded as it was a duplicate. Of the remaining 85, 14 were excluded because they did not meet the inclusion criteria, 11 were reviews and 3 were case reports. Of the remaining articles, 40 were excluded as unrelated topics, 1 because it concerned the paediatric population, 5 because they were based on guidelines before 2015 and 1 due to an inadequate (very small) cohort. In the end, 31 articles were selected for our study. A flowchart of selected articles is shown in Figure 1.
Figure 1.
Flowchart of the study.
Molecular and Immunological Mechanisms Involved in the Pathogenesis of BOS
The molecular and immunological mechanisms underlying BOS are unclear, and no disease-specific biomarkers have yet been found [25]. From the histopathological point of view, BOS is characterized by the accumulation of a submucosal extracellular matrix, muscle cell hyperplasia and complete obliteration of the airway lumen with partial destruction of the original smooth muscle layer. Chronic inflammation occurs in these patients and leads to excessive recruitment and/or activation of (myo-)fibroblasts in small peripheral airways [26].
Evidence of aberrant angiogenesis has also been reported in bronchiolitis obliterans lesions, consisting of the proliferation and enlargement of the microvasculature [14,15]. This mechanism could explain the flow limitation and dyspnoea typical of patients with BOS [27,28,29].
Antibodies also play a crucial role in the development of BOS. Graft antigen antibodies are closely linked to the development of BOS in lung transplant patients because graft-reactive antibodies induce activation of the complement system and degradation of lung tissue [30].
Concerning immunity, an increase in Th1 cells or cytokine-related Th1 in blood or BAL fluid of BOS patients suggests that Th1 cells play a role in the process of CLAD [16,17]. Th17 seems to be involved in the development of BOS, although the immunological mechanisms are largely unexplored. Th17 supports chronic inflammation that may favour chronic dysfunction through airway fibrosis, neutrophil chemotaxis and/or expansion of autoantibodies [31]. Moreover, an imbalance in Th17 and regulatory T cells, resulting in an increased Th17/Treg ratio, is linked to chronic dysfunction. Inflammatory factors in a BOS microenvironment can favour the differentiation of Treg into Th17 cells through the production of IL-6, modifying the Th17/Treg ratio and facilitating chronic dysfunction [32].
Concerning the role of B cells, no clear molecular mechanisms leading to chronic rejection have been established. An accumulation of B cells is observed in the lung tissue of patients with CLAD [33], and the presence of donor-specific HLA antibodies is related to BOS development [21,22,34,35,36,37].
Toll-like receptors (TLR) have a central role in the pathogenesis of BOS. Infections, ischemia time and ischemia-reperfusion injury can be activated by these receptors. Colonisation by pathogens such as Aspergillus fumigatus and Pseudomonas aeruginosa can also stimulate TLR and chemokine production (CXCL1 and CXCL5) [23,24]. The selected articles are reported in Table 1.

Table 1.
Characteristics of the selected papers.
2. Tissue Markers
The study of tissue from lung transplant patients can be useful for investigating the expression of genes, proteins and molecules specific to BOS lesions. Although biopsy is an invasive procedure, tissue specimens can provide information about molecular and immunological aspects.
2.1. Liver Kinase B1 Gene
Liver kinase B1 (LKB1) is a serine/threonine protein kinase 11 implicated in tumour suppression and in the regulation of cell metabolism [69]. A major function of LKB1 is the activation of 5′ AMP-activated protein kinase (AMPK), a regulator of metabolism and cell growth, which plays an inhibitory role in the epithelial-mesenchymal transition, tissue fibrosis and malignant transformation [70]. LKB1 was originally identified as the product of a loss of function mutation in different genetic syndromes and malignancies [71]. In non-small cell lung cancer, loss of LKB1 is associated with a more aggressive phenotype of this tumour [72,73]. Low expression of LKB1 is associated with a higher risk of organ rejection and with the occurrence and progression of human chronic graft-versus-host disease after bone marrow transplant [74]. A recent study [38] compared LKB1 activities in biopsies from newly diagnosed BOS and stable lung transplant patients, demonstrating significant downregulation of the LKB1 gene in BOS [38]. In line with the literature, the results of the study suggest that the downregulation of LKB1 may lead to tissue fibrosis, facilitating the development of BOS and that this protein could, therefore, be a reliable biomarker of risk of BOS.
2.2. Checkpoint Molecules
Different cell subsets, including dendritic cells, macrophages and T cells, as well as anti-HLA antibodies and interleukins, are implicated in chronic rejection and are involved in the expression and regulation of immune checkpoints [75,76,77]. The role of immune checkpoints in the development of BOS has been partially investigated.
Programmed death 1 (PD1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are proteins expressed on the surface of T and B cells that contribute to the down-regulation of the immune system while promoting self-tolerance by suppressing T cell inflammatory activity [78,79]. The ligand of PD-1, named PDL1, binds PD1, suppressing the effector function of T cells [80]. The role of immune checkpoints has been studied widely as a targeted therapy in cancer [81].
The possible role of the expression and function of immune checkpoint molecules in chronic allograft dysfunction is not clear. Sporadic experiences with immune checkpoint inhibitor treatments of kidney and heart transplant patients with cancer have shown that administration of these molecules results in the rapid development of severe rejection [82]. Righi et al. used immunohistochemistry [46] to detect significantly lower PD-1-, PDL1- and CTLA4 in BOS patients than in those with RAS. The exhausted phenotype of PD-1 cells, expressed in T cells and regulatory T cells (Tregs), proved to be significantly lower in RAS than in BOS patients. The triggers that chronically stimulate Tregs can determine their loss of function. This may contribute to the uncontrolled immune response that characterizes BOS.
2.3. VEGF/VEGFR2
Vascular endothelial growth factor (VEGF) is a strong angiogenic factor essential for angiogenesis. VEGF is active in physiological functions such as bone formation, haematopoiesis and wound healing [83]. It is produced by different cell lines, including macrophages, keratinocytes and fibroblasts. Although VEGF is essential for physiological homeostasis in various cell lines and tissues, it is also important in the pathogenesis of tumour growth and metastasis since it mediates vascular permeability and neo-angiogenesis [84,85,86,87]. VEGF and VEGF receptor 2 (VEGFR2) have been implicated in pulmonary vascular remodelling and in chronic rejection [88,89].
In this regard, a recent study [63] analysed concentrations of VEGF produced by distal-derived lung fibroblasts, before and after stimulation with TGF-β, in LTx patients with BOS and in stable LTx recipients and healthy controls, at baseline and follow-up (3, 6 and 12 months). It emerged that VEGF synthesis from distal-derived lung fibroblasts was significantly lower 3 months after lung transplant than in non-transplanted subjects and was increased at 6 months and 12 months after transplant, achieving the same level as in the healthy controls. These findings demonstrate the processes of the remodelling of pulmonary and bronchial vessels after lung transplant. Stimulation with TGF-β significantly enhanced the synthesis of VEGF in fibroblasts from lung transplant patients and healthy non-transplanted subjects. After TGF-β1 stimulation, levels of VEGF in fibroblasts obtained from patients who developed BOS 12 months after LTx were significantly lower than in patients without chronic rejection. The number of cells expressing markers of collagen production, VEGFR2/p4OH, was higher in patients with BOS, suggesting that BOS is related to chronic inflammation and subsequent fibrosis.
2.4. CXCL9 and CXCL10
C-X-C motif chemokine ligands 9 and 10 (CXCL9 and CXCL10) are chemokines stimulated by IFN-gamma that induce chemotaxis and promote differentiation and multiplication of leukocytes. They have also been associated with a wide spectrum of inflammatory lung diseases through pro- and antifibrotic effects [90]. Common mechanisms of rejection have been reported in different grafted organs, leading researchers to combine data from multiple organs and formulate a common rejection module consisting of 11 genes (CD6, TAP1, CXCL10, CXCL9, INPP5D, ISG20, LCK, NKG7, PSMB9, RUNX3 and BASP1) overexpressed during allograft rejection [91]. In recently published data, chronic rejection has been investigated by comparing the expression of these genes in lung tissue of BOS and RAS patients [66], showing lower expression of TAP1, CXCL9 and CXCL10 in BOS than in RAS patients. The results of the study suggest that the expression of these genes could be a useful marker to identify BOS.
3. Exhaled Breath
Exhaled breath is a useful, easy to obtain and non-invasive method in clinical practice. It is a source of information about mechanisms occurring in the alveoli and small airways. Little is yet known about the role of exhaled biomarkers in the development and pathogenesis of BOS.
3.1. Nitric Oxide
Nitric oxide (NO) is an important mediator involved in chronic inflammation of the lung [92]. At the lung level, NO acts as a vasodilator, bronchodilator, neurotransmitter and inflammatory mediator. High levels of exhaled nitric oxide have been reported in LTX patients with acute rejection, infection and lymphocytic bronchiolitis.
The NO produced by the respiratory system includes alveolar concentrations of NO (CalvNO) and maximum conducting airway wall flux (J’awNO), an expression of bronchial NO [93]. Concentrations of NO in exhaled breath can be investigated by non-invasive measurement of FeNO (fraction of exhaled nitric oxide). FeNO is measured during slow exhalation from total lung capacity against a positive pressure, which may be varied to generate specific exhalation flow rates. Cameli et al. (6) investigated the potential role of FeNO and CalvNO in the diagnosis of CLAD. FeNO was performed in LTx patients, including those with BOS and healthy controls. The study showed higher values of FeNO and CalvNO in LTx patients than in healthy controls. BOS patients showed higher FeNO and CalvNO than non-BOS patients. This suggests that FeNO and CalvNO could be useful markers in the diagnosis of BOS; CalvNO showed higher sensitivity and specificity than FeNO in identifying BOS in LTx patients.
3.2. Exhaled Surfactant Protein A
Surfactant protein A (SP-A) is a member of the collectin family and a component of surfactant; it is mostly produced by alveolar type II cells. The main physiological function consists of reducing the surface tension of the alveoli [94,95]. Several studies have demonstrated the importance of SP-A in respiratory infections [96]. A significant reduction in SP-A in BAL fluid has been demonstrated in lung transplant recipients with BOS compared with stable patients [97]. A recent study [59] compared levels of SP-A in exhaled breath of BOS patients, stable LTx patients and healthy controls. The results indicated that SP-A in exhaled particles and the SP-A/albumin ratio were lower in the BOS group than in the BOS-free group. Low levels of SP-A in exhaled particles were associated with an increased risk of BOS.
4. Circulating Biomarkers
4.1. Serum/Plasma Biomarkers
4.1.1. Lipocalin 2
Lipocalin 2 (LCN2) is a 25 kDa member of the lipocalin protein family, a family sharing a common molecular structure that binds hydrophobic molecules [98,99]. From a pathogenetic point of view, LCN2 has been implicated in modulating apoptosis, showing both pro- and anti-apoptotic activities [100]. In lung transplants, higher serum concentrations of LCN2 have been reported in patients with RAS than in those with BOS. During chronic inflammation of the lung, LCN2 reprograms the immune microenvironment and predisposes tissues to cancer development and progression [101]. Increased serum concentrations of LCN2 and activin-A have also been considered predictors of a lower likelihood of freedom from CLAD in stable LTx patients [42].
4.1.2. Galectins 1 and 3
Galectins are a family of 17 β-galactoside-binding proteins that modulate intracellular signalling through direct interactions with cell adhesion molecules. Galectins are also involved in the regulation of innate and adaptive immune responses [102]. Gal-1 typically functions as an anti-inflammatory and pro-resolving mediator by modulating innate and adaptive immune responses. Serum concentrations of Gal-1 may be dysregulated in various inflammatory scenarios, such as microbial infection, autoimmunity and cancer. Galectin-3 (Gal-3) has been implicated in the development of pulmonary fibrosis. Elevated concentrations of Gal-3 are associated with restrictive interstitial abnormalities, including decreased lung volumes and altered gas exchange [103]. In LTx, a study showed that concentrations of galectin-1 were higher in BOS than in stable LTx patients [45]. According to another study, concentrations of galectin-3 were significantly higher in LTX recipients with airway obstruction than in recipients without any complications [104].
4.1.3. Soluble CD59
CD59 is a small glycosylphosphatidylinositol-anchored protein and the sole membrane regulator of the membrane attack complex (MAC). CD59 has been investigated in different diseases as a predictive biomarker of outcome and disease progression [105]. In sepsis, serum concentrations of soluble CD59 (sCD59) were correlated with the severity of organ damage [106]. Budding et al. were the first to investigate the role of CD59 in lung transplant, showing that in chronic lung allograft dysfunction, BOS patients had higher serum concentrations of sCD59 than non-BOS patients [56].
4.1.4. MMP-3
Matrix metalloproteinase-3 (MMP-3) or stromelysin-3 is a protein expressed by different subsets of cells [107]. MMP-3 has been implicated in a range of pathological processes, including acute lung injury, pulmonary fibrosis and lung cancer. It may also promote the breakdown of alveolar epithelial barriers and acute inflammatory responses, particularly in a setting of ventilator-induced lung injury. Genetic deletion of MMP-3 in mice confers protection against bleomycin-induced fibrosis, while transient overexpression of MMP-3 results in profibrotic responses in rat lungs. This presumably occurs by induction of the Wnt-β-catenin pathway by MMP-3. The protein also mediated the degradation of the ECM, enhancing a profibrotic environment, which may affect the phenotype of fibroblasts and promote further deposition of ECM and fibrosis [107,108].
MMP-3 was consistently identified in patients with BOS, suggesting that this protein may be BOS-specific and linked to the development of the disease. In particular, plasma samples from hematopoietic cell transplant recipients with incipient BOS were compared with those of patients with lung infections, chronic graft-versus-host disease without pulmonary involvement and chronic complications after hematopoietic cell transplant. Plasma concentrations of MMP-3 were not elevated prior to the onset of BOS. MMP-3 was only elevated when FEV1 decreased and could, therefore, be a non-invasive tool for diagnosis rather than a prognostic marker [57,109].
4.1.5. MMP-9
Matrix metalloproteinase-9 (MMP-9), also known as 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B (GELB), belongs to the zinc-metalloproteinase family involved in the degradation of the extracellular matrix [110]. MMPs play key roles in developing and maintaining adequate oxygenation in health and disease. Broadly, except for MMP2 and MMP14, most deletions in MMPs fail to affect lung development; however, their individual absence can alter the pathophysiology of respiratory diseases. Specifically, under stress conditions, such as acute respiratory infection and allergic inflammation, MMP-9 and others can play a protective role through bacterial clearance and production of chemotactic gradients [111].
In plasma, MMP-9 was associated with BOS and predicted the occurrence of CLAD 12 months before it was diagnosed on the basis of lung function [52,112].
In paper [58] included in our review, levels of MMP-9 were investigated via zymography and gelatin degradation. As expected, BAL concentrations of MMP-9 were elevated in BOS patients together with neutrophil percentage. As demonstrated in other studies [52], MMP-9 contributes to the remodelling processes, leading to airway obstruction. In conclusion, MMP-9 can be considered a prognostic and diagnostic biomarker of BOS.
There are many studies that also sustain that the epithelial-mesenchymal transition is the main pathogenetic mechanism in BOS. For example, high levels of MMP-9 have been found in BAL of CLAD patients [112], and TGF-beta (a major inducer of epithelial-mesenchymal transition) has been reported at high concentrations in BAL of BOS patients [113].
4.1.6. Self-Antigens (SAgs): K-Alpha 1 Tubulin and Collagen-V
Antibodies to k-α1 tubulin (K-α1T) and collagen type V (Col V) are both associated with the development of CLAD [114]. K-α1T is a gap junction protein with mostly intracellular functions Col V that is usually hidden in the structure of collagen type I in lung tissue extracellular matrix, but when exposed, it can act as an immunogenic ECM protein [115]. Both neo-antigens can be exposed after graft injury, leading to the induction of an immune response that may be aggravated by the loss of peripheral tolerance through immunosuppression of regulatory T-cells [114]. When bound to alveolar epithelial cells, K-α1T antibodies directly influence the onset of airway obliteration by inducing an increase in fibrogenic growth factor expression and fibroproliferation.
Higher serum concentrations of anti-K-α1T and anti-col V after LTx have been associated with a higher risk of BOS in LTx patients. Saini et al. showed that anti-K-α1T levels in serum and BAL fluid were significantly higher in patients with BOS than in those without BOS, matched for time since transplant. BAL concentrations of anti-Col V were also significantly higher in BOS than in non-BOS patients [116]. The occurrence of antibodies directed against self-antigens after LTx was shown to be linked to the development of donor-specific HLA antibodies in the recipient and could be an interaction between allo- and auto-immunity.
Antibodies to Col V and K-α1T were found in 70% of LTx patients who had antibodies against self-antigens after the transplant. Patients with pre-transplant self-antibodies had shorter BOS-free survival than LTx patients who did not have pre-transplant self-antigen antibodies, suggesting that the measurement of these antibodies may aid risk prediction for BOS after LTx [117]. It was recently demonstrated that concentrations of K-α1T were higher in exosomes from LTx patients with BOS [40,118]. Earlier studies also demonstrated that exosomes isolated from LTx patients with BOS contained lung SAgs (Col-V and K-α1T). Exosomes from LTx contained significantly higher levels of Col-V 6 and 12 months before BOS.
Regarding concentrations of HLA-DR and HLA-DQ, Bansal et al. demonstrated that they were significantly higher in exosomes isolated from LTx patients with RAS than in those of patients with BOS. RAS exosomes also contained higher levels of NfkB, 20S proteasome, PIGR, CIITA and Col-V than BOS exosomes. The concentration of K-α1T was higher in exosomes from LTx patients with BOS [40,118].
4.2. Bronchoalveolar Lavage Fluid Biomarkers
Fibro-bronchoscopy and BAL are procedures currently used in monitoring protocols for LTx recipients. They have provided much data in the search for biomarkers of CLAD in BAL fluid.
4.2.1. Epithelial Cell Death Markers in BAL Fluid
Epithelial cells play an important role in maintaining airflow and in lung defences, both as a physical barrier and through innate and adaptive immune responses [119]. Epithelial injury has been proposed as a mechanism in the pathogenesis of CLAD [120]. Epithelial cell death can be detected by the release of specific intracellular proteins, such as cytokeratins, which are cytoskeletal proteins that maintain the internal organisation and dynamic processes of cells [121].
During necrosis and apoptosis, lung epithelial cells release full-length cytokeratin-18 (CK18). The presence of this protein in BAL fluid may indicate processes of apoptosis and necrosis; M30, obtained from the cleavage of CK18, is an expression of cell apoptosis, while M65, which reflects intact CK18 and caspase-cleaved CK18, is related to cell necrosis.
Levy et al. established possible roles of M30 and M65 in BOS [122]. They collected BAL fluid from these patients routinely for 24 months after transplant. Acute inflammation was indicated in this cohort by >3% neutrophils in the cell count and traces of M30 and M65. The results showed that M30 and M65 were not related to neutrophil count, and that M65 levels were lower in patients with BOS than in those with RAS, while high levels of M65 were associated with worse survival in CLAD patients [67]. These results suggest that M65 could be a useful diagnostic marker of BOS.
4.2.2. Humoral and Cell Immunity Biomarkers in BAL Fluid
Chronic rejection after transplant is predominantly mediated by T cells; however, there is recent evidence that B-cell activation with the production of donor-specific antibodies (DSA) directed against specific human leukocyte antigens (HLA) of the graft is involved [123]. Circulating DSA promotes complement activation, resulting in lung injury [124]. According to the ISHLT definition of antibody-mediated rejection, the presence of these antibodies in BAL fluid is correlated with an increased risk of CLAD [125], especially RAS [126].
In a retrospective study, Vandermeulen et al. [58] investigated DSA in blood in relation to complement activation and immunoglobulins in BAL, as well as the correlation of these humoral markers with airway remodelling.
The association between K-α1T and SAgs that we previously described in the serum of BOS patients was also analysed in BAL fluid in relation to club cell secreted protein (CCSP). CCSP is an anti-inflammatory protein used as a biomarker for respiratory stress in experimental models of acute and chronic lung injury. Lung transplant patients diagnosed with BOS showed a significant decline in BAL fluid concentrations of CCSP 7–9 months before BOS could be diagnosed clinically. Those who developed SAgs at the time of diagnosis of BOS had lower BAL fluid levels of CCSP than did stable LTx patients without SAgs.
Regarding humoral immunity, higher levels of immunoglobulins, without specific distinctions of sub-classes (IgA, IgM, IgG1,2,3,4) and complements (C1q and C4d), were found in the BAL fluid of patients with AMR overlapping with CLAD and a prevalence of RAS phenotype than in patients with BOS and controls, confirming the finding of Roux et al. [126].
The roles of CD4+ and CD8+ cells in solid transplant patients are still unclear. Activation of these cells in acute cell rejection after lung transplant [127] made it necessary to understand their importance in chronic rejection [97]. Hayes et al. [60] described the BAL fluid T cell panel in LTx patients affected with BOS. They demonstrated that LTx patients without BOS did not show changes in CD4+ and CD8+ cells in BAL samples within two years of transplant. On the contrary, recipients who developed BOS showed a decline in CD4+ and an increase in CD8+ cells, with a significant decline in the CD4:CD8 ratio in the same matrix in the same period. Subject to the small population of this study, the T cell profile could be considered a possible biomarker for BOS and probably for other CLAD phenotypes. A recent paper also demonstrated a predominance of Th17 cell subtypes and a depletion of Tregs and Bregs in the BAL fluid of patients with acute rejection with respect to BOS patients [43].
4.2.3. Cytokines and Chemokines in BAL Fluid
In lung transplant patients, cytokine production occurs in two steps: first, in an early antigen-independent cascade triggered by the recipient’s immune system, surgical trauma, donor lung status or ischemic reperfusion injury; second, in a late antigen-dependent cascade that directs activated recipient lymphocytes into the graft [128,129,130]. The balance between the production of pro- and anti-inflammatory cytokines and chemokines is critical for airway repair and, therefore, also for the development of CLAD [131]. Berastegui et al. [54] examined the production of cytokines in lung transplant recipients who developed CLAD. They found that IL-5 seemed to be the most significant biomarker, being more highly expressed in RAS than in BOS. A study by Itabashi et al. also reported that the loss of CCSP may contribute to increased production of IL-8, suggesting that CCSP plays an important role in regulating the proinflammatory cascade [44].
4.2.4. Gene Expression in BAL Fluid
The common rejection module is composed of 11 genes (CD6, TAP1, CXCL10, CXCL9, INPP5D, ISG20, LCK, NKG7, PSMB9, RUNX3 and BASP1) that are overexpressed during allograft dysfunction. It has been studied in many types of solid transplants to quantify inflammation in tissues [132]. Sancreas et al. [66] developed a study to understand how the module could have a biomarker role in acute rejection and CLAD. They performed BAL, trans-bronchial biopsy and histological studies in explant organs, discovering that the expression of these genes is higher in acute rejection than in chronic rejection. The most relevant circulating biomarkers and gene expression are represented in Figure 2.
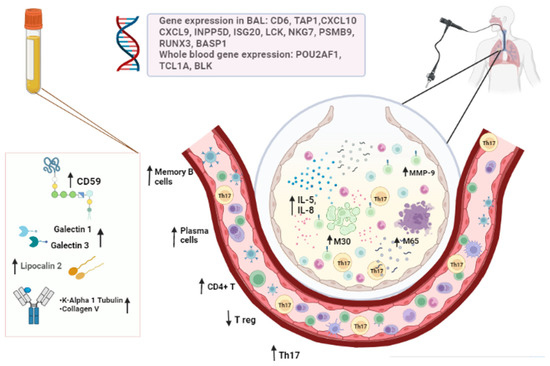
Figure 2.
The most relevant circulating biomarkers and gene expression.
4.2.5. NMR Spectroscopic Detection of Metabolites in BAL Fluid
There have been few studies on the possible utility of NMR spectroscopy in respiratory diseases. Some have focused on the mouse respiratory system [133], on preterm infants with respiratory distress syndrome [134] and on paediatric patients with cystic fibrosis, in search of a correlation between the degree of airway inflammation and the number of metabolites in BAL fluid [135]. Although little information is available about the metabolic profile of BAL fluid in adults, and even less in BOS patients, Ciaramelli et al. developed a pilot study to assess the suitability of using NMR spectroscopy to explore the metabolic profile of the BAL fluid of lung transplant recipients with or without BOS [55]. If this method could predict BOS, early intervention to prevent irreversible lung damage may be possible.
NMR is able to detect different metabolites, such as amino acids, Krebs cycle intermediates, mono- and disaccharides, nucleotides and phospholipid precursors. The authors tried to understand whether some of these molecules could be early biomarkers of BOS. They found high levels of some branched-chain amino acids (valine, leucine, isoleucine) in the BAL fluid of BOS patients, and these levels were related to the severity of BOS.
Another potential role is played by the taurine/hypotaurine pathway. Taurine is a regulator of cell volume via membrane stabilisation and has antioxidative, anti-inflammatory and anti-apoptotic properties [136]. Taurine is a weak agonist of chloride-permeable gamma-aminobutyric acid type A receptors (GABA-A R) and glycine receptors (GlyR) that are located not only in neural synapses but also in the central nervous system and the lungs. This suggests that taurine plays a role in the lungs, potentiating the relaxation of airway smooth muscle cells through binding to GABA-A R and secretion of mucus by goblet cells [137]. The release of ROS by neutrophils and macrophages during inflammatory states could stimulate a decrease in the uptake of intracellular taurine and an increase in the taurine release into extracellular fluids, with a consequent increase in mucus secretion. Increased levels of taurine in the BAL fluid of BOS patients may, therefore, reflect inflammation-induced osmotic stress or epithelial cell damage. Since taurine accumulates in the cytoplasm of neutrophils and other leukocytes, its presence may also suggest high levels of leukocytes in the airways (due to a state of inflammation) [138]. Moreover, levels of lactate in BAL fluid are related to an inflammatory state. BOS patients with increased BAL fluid levels of lactate could have an inflammatory status that exacerbates the disease. Together these results suggest that there are possible markers for early diagnosis of BOS.
5. Extracellular Vesicles
5.1. LKB1 from Tissue
Downregulation of LKB1 has been demonstrated in circulating exosomes prior to clinical diagnosis of BOS, suggesting that this enzyme may have a role in the pathogenesis of the syndrome. In particular, incubation of BOS-exosomes also decreased LKB1 expression and induced epithelial-mesenchymal transition markers in an air–liquid interface culture method [38]. This study provided new evidence that exosomes released from transplanted lungs undergoing chronic rejection are associated with an inactivated tumour suppressor gene, LKB1, and this loss induces epithelial-mesenchymal transition, leading to CLAD in humans.
5.2. miRNAs
Recent studies suggest that epigenetic regulation of microRNAs might play a role in the development of BOS. Di Carlo S. et al. found, through in situ hybridisation, the dysregulation of two candidates, miR-34a and miR-21, as pathogenetic factors of BOS [139]. Xu Z. et al. identified miR-144 as the most significant altered miRNA. miR-144 is a principal critical regulator of TGF-β signalling, involving an increase in Smad-2, Smad-4, FGF and VEGF, leading to diminished fibrogenesis [140].
Recently, the alteration of Smad-4 has been found in association with miR-155-5p and miR-23b-3p, which resulted in an altered expression in responders to extracorporeal photopheresis [141].
Budding et al. showed that miR-21, miR-29a, miR-103 and miR-191 levels were significantly higher in BOS+ patients prior to clinical BOS diagnosis [142]. Most studies have focused on extracellular miRNAs as potential biomarkers since they are stable and can be detected in blood, urine and other body fluids by simple, sensitive and relatively cheap assays, even after years of sample storage. miR-21 and miR-29a are the most commonly observed aberrant miRNAs in human cancers. Post-transcriptionally, miR-21 downregulates the expression of the tumour-suppressor gene PTEN and stimulates growth and invasion in non-small cell lung cancer. Inhibition of miR-21 reduces cancer cell proliferation, migration and invasion. miR-103 has recently been associated with the metastatic capacity of primary lung tumours [143].
Bozzini S. et al. suggested that miR-21-3p has been identified in the context of OB fibrogenesismiR-21-3p playing a role as a profibrotic effector in CLAD fibro-obliterative processes [144].
6. Whole Blood and BAL Cell Subsets
To avoid the onset of BOS, it is important to define the cause of inflammation and to understand how immune cells are implicated in inflammation and/or repair. Immunosuppression therapy is the major management strategy for preventing the rejection of the new organ by activation of the immune system [145]. Patients receive a regimen of immunosuppressants that reduces the differentiation and proliferation of lymphocytes, including B and T cells [146].
6.1. B Cells
Many recent studies have focused on the role and association between long-term allograft survival and B cells. B lymphocytes are a cell population that expresses clonally different cell-surface immunoglobulin (Ig) receptors that recognize specific antigen epitopes [147]. Phenotypically, B cells are differentiated on the basis of the expression of specific cell-surface markers [148]. Transitional B cells are the most immature peripheral B cells and express CD24 and CD38. Whereas human memory B cells can be divided into different populations depending on the expression of markers IgD and CD27: naïve (IgD+CD27-), non-switched memory (IgD+CD27+), switched memory (IgD-CD27+) and CD27-negative (IgD-CD27-) B cells, also known as double-negative B cells (DN B cells). According to the literature, memory B cells and plasmablasts are correlated with early acute antibody-mediated rejection [149], while naïve and transitional B cells are associated with tolerance in kidney and liver transplants [150].
The peripheral B cell subset has been investigated in lung transplant recipients with and without BOS and in healthy controls. An increase in DN B cells was recorded in patients with BOS, along with a decrease in transitional and naïve B cells in BOS patients with respect to healthy controls [151]. The medication used to prevent the development of allograft dysfunction usually prevents the proliferation of B cells.
During BOS, the immune system is exposed to abundant antigens due to chronic inflammation and repair processes [152]. It is probable that naïve and transitional B cells differentiate, and their peripheral frequency decreases while DN B cell numbers increase.
Regulatory B cells (Bregs) function as immunosuppressors through the production of anti-inflammatory cytokines, such as IL-10, IL-35 and transforming growth factor β (TGF-β) [153]. Regarding B cells, CD19+CD24hiCD38hi are reported to be elevated in the BAL of acute rejection patients [154]. They suppress immunopathology by prohibiting the expansion of pathogenic T cells and other proinflammatory lymphocytes. Regulatory B cells support immunological tolerance and may be involved in maintaining long-term allograft function.
CD9 is a cell surface glycoprotein of the tetraspanin family, characterized by four transmembrane-spanning domains and two extracellular domains [155]. Several studies implicate CD9 in the immunosuppressive activity of Bregs [156].
Brosseau et al. [68] demonstrated the importance of Bregs and the CD9 marker, a powerful predictive marker of stability and long-term BOS-free survival after lung transplant. Twenty-four months after transplant, plasma concentrations of CD9 were higher in stable patients than in those who would develop BOS. CD9 B-cell frequencies allow excellent discrimination between stable patients and those destined to develop BOS at 24 months.
6.2. T Cells
CD8+ and CD4+ T cells are two subpopulations of the adaptive immune system: CD4+ T cells are helper T cells that assist other lymphocytes in evoking an immune response [157]. CD8+ T cells are cytotoxic T cells that kill virus-infected cells and tumour cells [158]. They are also involved in transplant rejection. Durand et al. [48] demonstrated that the CD4+ and CD8+ compartments are not biased in BOS patients. An increased proportion of circulating CD4+CD25hiFoxP3+ T cells was reported one month after LTx in patients who proceeded to develop BOS within 3 years. These cells may act as negative regulators of BOS development. Although this result is encouraging, the underlying mechanisms leading to the increased proportion of regulatory CD4+CD25hiFoxP3+ T cells in BOS patients remain unclear.
Regulatory T cells modulate the activation and proliferation of other immune cells and are crucial for maintaining T-cell tolerance to self-antigens. They secrete different immunosuppressive cytokines, such as IL-10 and TGF-β [134,135].
Piloni et al. [50] analysed the long-term peripheral kinetics of Tregs in lung transplant patients and assessed their association with different clinical variables. Previous studies demonstrated that peripheral Tregs may be a major regulatory subset in lung transplant. The latest results confirmed the role of Tregs in lung graft acceptance/rejection. Peripheral Treg counts fell significantly in CLAD patients. The degree of this decrease was correlated with the severity of BOS.
Th17 cells secrete IL-17, IL-22 and IL-23, and recruit neutrophils to sites of infection. In this way, they cause inflammation and autoimmunity [159,160,161].
In order to determine the phenotype of Tregs, Bregs and Th17 cells, samples of peripheral blood from stable lung transplant patients were analysed [43]. An increase in Th17 subtype percentages in PBMCs and BAL fluid and a reduction in Tregs and hence in the Treg/th17 ratio were observed in acute rejection patients. It was demonstrated that cytotoxic and proinflammatory lymphocyte percentages increased after transplant [162]. To explore their role, Hodge et al. [65] measured intracellular cytotoxic mediator granzyme B, interferon-gamma, tumour necrosis factor (TNF), alpha proinflammatory cytokines and CD28 in blood, BAL fluid and large airways in patients with BOS. The results indicated a significant decrease in T cells, an increase in NKT-like cells and an increase in CD8+ T and NKT-like cells in BOS patients with respect to stable patients and healthy controls. In detail, the percentages of senescent CD28null CD8+ T and CD8- T and NKT-like cells increased in BAL fluid and in large and small airways in patients with BOS. Loss of CD28 was associated with more T and NKT-like cells expressing granzyme B, IFN-γ and TNF-α. Loss of CD28 seemed to be associated with repeated antigen stimulation, as demonstrated in chronic obstructive pulmonary disease [163]. A good idea may be to find some treatment options targeting the proinflammatory nature of these cells.
6.3. Monocyte-Macrophage Lineage
The monocyte-macrophage lineage plays an important role in the immunopathology of chronic rejection [164]. Monocytes recognize “danger signals” via pattern recognition receptors. They conduct phagocytosis, present antigens, secrete chemokines and proliferate in response to infection and injury.
Human blood monocytes can be divided into three subsets, classical, intermediate and non-classical, based on CD16 and CD14 expression [165]. To distinguish the non-classical and intermediate subsets, another marker, 6-sulfo LacNAc (SLAN), has been identified. Described for the first time as a cell-surface marker of a certain type of dendritic cell in human blood, SLAN is a carbohydrate residue associated with the non-classical monocyte subset [166]. The functions of monocyte subsets are different and also depend on the inflammatory pathology. In the lungs, the classical subset can differentiate into pulmonary dendritic cells and macrophages, whereas the functions of the other two subsets are still unknown [167].
Schreurs et al. analysed peripheral monocyte subsets in LTx patients with and without BOS [61] and found a reduction in the number of non-classical monocytes, i.e., those expressing SLAN-positivity, in the former, whereas the expression of SLAN-positivity was higher in BOS patients. To highlight the activity of T cell co-stimulation and antigen presentation, they also stained blood monocytes for surface markers HLA-DR and CD86. The only monocyte marker that proved significant in BOS was HLA-DR, which showed a significant increase in expression on non-classical monocytes. Although BOS is an outcome of chronic inflammation, it does not lead to increased production of monocytes. Further studies will be necessary to validate these results.
6.4. Whole Blood Gene Expression Profiles
Performing a non-invasive microarray gene expression profiling of whole blood, Danger et al. [49] identified and validated POU2AF1 (POU Class 2 Homeobox Associating Factor 1), TCL1A (T-cell leukaemia/lymphoma protein 1A) and BLK (B-lymphoid tyrosine kinase) as three predictive biomarkers of BOS more than 6 months before diagnosis. By monitoring these three genes, it proved possible to stratify patients on the basis of BOS risk.
POU2AF1 is a B cell transcriptional coactivator implicated in B cell development and function [168]. It is also expressed in T cells and functions as a T-cell-dependent B cell response. BLK encodes a non-receptor protein, tyrosine kinase, and is involved in the regulation of B cell receptor signalling [169]. TCL1A, known as an oncogene, acts as a coactivator of serine-threonine kinase Akt and promotes cell survival, growth and proliferation [170]. TCL1A has been reported to be downregulated in the peripheral blood of patients with BOS before disease onset. This is in line with the findings of another study [171], where the gene was downregulated at the time of acute allograft rejection of transplanted kidneys but overexpressed in tolerant patients. The exact contribution of these genes to the development of BOS remains to be investigated.
7. Cell Culture Supernatants
Matrix Metalloproteinase 9
The pathogenesis of BOS remains largely unknown. We can observe the histological characteristics of the lung tissue of LTX patients. BOS is characterized by the infiltration of lymphocytes into the bronchiole walls, followed by a fibrotic process. This leads to the deposition of the extracellular matrix (ECM) that obliterates the small airways [172]. It has been postulated that this fibrotic process begins with epithelial damage caused by alloimmune and non-alloimmune mechanisms, followed by unsuccessful repair. Fibroblasts play a central role in this process, although it is unclear whether they are activated in situ or recruited from circulation. Another role in fibrosis is played by bronchial epithelial cells (BECs) and the epithelial-mesenchymal transition, which leads to the downregulation of epithelial markers (e.g., E-cadherin) and upregulation of mesenchymal markers (e.g., vimentin) [173]. Bronchial epithelial cells, in the process of mesenchymal differentiation, produce matrix metalloproteinases and ECM proteins (including type-I collagen and fibronectin). Matrix metalloproteinase (MMP)-9 plays an important role in all diseases based on air-remodelling processes. According to this pathogenetic explanation of BOS, the allogeneic immune response is the start of the process, but the effect of T cells on BECs is unclear. To demonstrate the role of allogenic immunity via epithelial-mesenchymal transition in BOS, Pain et al. analysed the response of BECs stimulated by T-cells in synergy with TGF-beta [52]. They set out to detect MMP-9 in the cell culture supernatant of LTx patients. Concentrations of MMP-9 were high 12 months before lung function diagnosis, suggesting the role of this metalloproteinase as an early biomarker of CLAD. However, MMP-9 did not distinguish RAS and BOS.
8. Radiological Markers
8.1. HRCT Quantitative Image Analysis Score
Quantitative image analysis (QIA) of the lung parenchyma could be a useful tool for phenotyping CLAD patients. It could also have prognostic value in terms of survival [39]. The QIA score is based on the proportion of lung volume affected by interstitial disease or air-trapping. It is an imaging scoring system that can be performed on lung CT images by estimating the percentage of Quantitative Ground Glass (QGG), Quantitative Lung Fibrosis (QLF) and Quantitative Honey Combing (QHC). QHC+QLF+QGG is a measure of total interstitial disease (QILD). Air-trapping is then scored by analysing CT scans during end-expiration or residual volume.
Weigt et al. used the QIA score in LTx patients to verify its possible utility for phenotyping CLAD [39]. They analysed chest HRCT images of bilateral LTx patients within 90 days of CLAD onset and in a control (no-CLAD) group. They found that BOS cases had a lower score for interstitial disease than patients with RAS or mixed CLAD phenotypes. However, BOS showed more air-trapping. These results were also compared with the standard-phenotyping system (2019 ISHLT consensus). There was quite a good concordance between the two methods. Another important result was that the risk of death was higher in RAS and mixed phenotypes (with higher QILD) than in BOS. The QIA score on HRCT images can, therefore, be considered a diagnostic as well as a prognostic marker.
8.2. Pleural Thickening Evaluated by Lung Ultrasound
Pleural thickening may be focal or diffuse, and it may have different causes, both benign and malignant [174]. In interstitial lung disease, the lung parenchyma and pleura show increased tissue density, which can be demonstrated by lung ultrasonography as a significant number of B-line reverberation artefacts indicative of interstitial syndrome and pleural thickening. RAS is characterized by typical radiological alterations of interstitial lung disease that are absent in BOS, namely pleural thickening and an upper lobe-dominant fibrotic pattern [155].
By virtue of its sensitivity and specificity, lung ultrasonography can be a supplementary tool for identifying pleural thickening on upper lobes useful for phenotyping CLAD patients.
In 2015, Davidsen et al. demonstrated the utility of lung ultrasonography for identifying pleural thickening in CLAD patients, assuming pleural thickening to be a marker of RAS, absent in BOS [41]. They studied BOS and RAS prospectively by chest HRCT and lung ultrasonography. Pleural thickening was more pronounced in RAS, as were chest HRCT features, demonstrating the potential utility of ultrasonographic features and radiological markers to discriminate BOS from RAS.
8.3. Parametric Response Mapping of Functional Small Airway Disease by CT
Parametric response mapping (PRM) is a CT method that can be used to quantify functional small airway disease and parenchymal disease. In BOS, the predominant pathogenetic mechanism is associated with small airway disease and, consequently, with airflow limitation [175,176]. Belloli et al. compared the concordance between spirometry data of bilateral lung transplant recipients and PRM results [53]. Patients with isolated FEV1 decline had significantly worse functional small airway disease than their control subjects, whereas patients with declines in FEV1 and FVC (less than 80% at baseline) had worse parenchymal disease. PRM could be useful for phenotyping CLAD patients. Moreover, functional small airway disease >30% proved to be a strong predictor of survival. Patients with these radiological characteristics lived, on average, 2.6 years less than patients with functional small airway disease <30%, suggesting a prognostic role for this marker. Figure 3 represents the radiological and tissue markers of BOS.
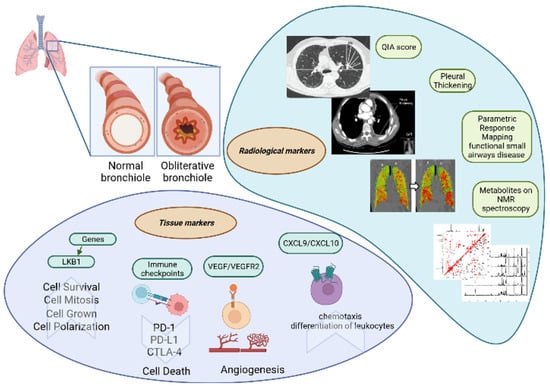
Figure 3.
The radiological and tissue markers of BOS.
9. Future Directions
Several markers have already been investigated in CLAD in different specimens, including serum, plasma, cells and tissue. Recently, clinical, functional and radiological scores have been made for the prediction of BOS. Considering that a single biomarker with appropriate accuracy and reliability able to predict the diagnosis and prognosis of BOS is not available, the purpose will be implementing a panel of biomarkers from the biological origin with radiological and/or clinic-functional findings. Furthermore, other biomarkers that showed a good correlation with the pathogenesis of BOS (such as miRNA and gene expression profile) are largely unexplored. Moreover, the idea of using biomarkers of other fibrotic lung diseases (such as IPF) may help to find a novel set of proteins with diagnostic and prognostic features.
Author Contributions
Conceptualization, D.C. and M.G.; methodology, L.B., S.C. (Stefano Cattelan), G.F., S.C. (Sara Croce) and M.A.; formal analysis, D.B. and A.F.; investigation, L.V., L.L., A.S., P.P. and A.P.; data curation M.d., P.C.; writing—original draft preparation, L.B., D.C., M.G., S.C. (Stefano Cattelan), G.F., S.C. (Sara Croce) and M.A.; writing—review and editing, E.B.; supervision D.B., A.F., L.V., L.L., A.S., P.P. and A.P.; project administration, L.B. The Tuscany Transplant Group contribute for the conceptualization of the study and the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed at Siena University without any funding sponsors.
Acknowledgments
The Tuscany Transplant Group comprises: Gonfiotti Alessandro, Viggiano Domenico, Borgianni Sara Gatteschi Lavinia, Salimbene Ottavia and Mugnaini Giovanni.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Idiopathic pulmonary fibrosis (IPF), tumour growth factor-β (TGF-β), interleukin (IL-), lung function tests (LFT), high-resolution computed tomography (HRCT), pulmonary functional test (PFT), bronchoalveolar lavage (BAL), antithrombin III (ATIII), platelet-derived growth factor (PDGF), fibroblast growth factor receptor (FGFR), vascular endothelial growth factor receptor (VEGFR), multidisciplinary discussion (MTD).
References
- Chambers, D.C.; Cherikh, W.S.; Harhay, M.O.; Hayes, D.; Hsich, E.; Khush, K.K.; Meiser, B.; Potena, L.; Rossano, J.W.; Toll, A.E. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart–lung transplantation Report—2019, Focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1042–1055. [Google Scholar] [CrossRef]
- Martinu, T.; Chen, D.-F.; Palmer, S.M. Acute rejection and humoral sensitization in lung transplant recipients. Proc. Am. Thorac. Soc. 2009, 6, 54–65. [Google Scholar] [CrossRef]
- Meyer, K.C.; Raghu, G.; Verleden, G.M.; Corris, P.A.; Aurora, P.; Wilson, K.C.; Brozek, J.; Glanville, A.R.; the ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: Diagnosis and management of bronchiolitis obliterans syndrome. Eur. Respir. J. 2014, 44, 1479–1503. [Google Scholar] [CrossRef]
- Levy, L.; Huszti, E.; Renaud-Picard, B.; Berra, G.; Kawashima, M.; Takahagi, A.; Fuchs, E.; Ghany, R.; Moshkelgosha, S.; Keshavjee, S. Risk assessment of chronic lung allograft dysfunction phenotypes: Validation and proposed refinement of the 2019 International Society for Heart and Lung Transplantation classification system. J. Heart Lung Transplant. 2020, 39, 761–770. [Google Scholar] [CrossRef]
- Verleden, G.M.; Vos, R.; Verleden, S.E.; De Wever, W.; De Vleeschauwer, S.I.; Willems-Widyastuti, A.; Scheers, H.; Dupont, L.J.; Van Raemdonck, D.E.; Vanaudenaerde, B.M. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation 2011, 92, 703–708. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Perillo, F.; Metella Refini, R.; Bergantini, L.; Bellisai, F.; Selvi, E.; Cameli, P.; Manganelli, S.; Conticini, E.; Cantarini, L.; et al. Efficacy of baricitinib in treating rheumatoid arthritis: Modulatory effects on fibrotic and inflammatory biomarkers in a real-life setting. Int. Immunopharmacol. 2020, 86, 106748. [Google Scholar] [CrossRef]
- Markopoulou, K.D.; Cool, C.D.; Elliot, T.L.; Lynch, D.A.; Newell, J.; Hale, V.A.; Brownz, K.K.; Schwarz, M.I.; Tuder, R.M. Obliterative bronchiolitis: Varying presentations and clinicopathological correlation. Eur. Respir. J. Eur. Respir. Soc. 2002, 19, 20–30. [Google Scholar] [CrossRef]
- Aguilar, P.R.; Michelson, A.P.; Isakow, W. Obliterative Bronchiolitis. Transplantation 2016, 100, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.L.; Palmer, S.M. Bronchiolitis Obliterans Syndrome: The Final Frontier for Lung Transplantation. Chest 2011, 140, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; von der Thusen, J.; Roux, A.; Brouwers, E.S.; Braubach, P.; Kuehnel, M.; Laenger, F.; Jonigk, D. When tissue is the issue: A histological review of chronic lung allograft dysfunction. Am. J. Transplant. 2020, 20, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- King, T.E. Bronchiolitis obliterans. Lung 1989, 167, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; d’Alessandro, M.; Otranto, A.; Cavallaro, D.; Gangi, S.; Fossi, A.; Perillo, F.; Luzzi, L.; Zanfrini, E.; Paladini, P.; et al. Characterization of NKG2-A/-C, Kir and CD57 on NK Cells Stimulated with pp65 and IE-1 Antigens in Patients Awaiting Lung Transplant. Life 2022, 12, 1081. [Google Scholar] [CrossRef] [PubMed]
- Daga, S.; Fallerini, C.; Baldassarri, M.; Fava, F.; Valentino, F.; Doddato, G.; Benetti, E.; Furini, S.; Giliberti, A.; Tita, R.; et al. Employing a systematic approach to biobanking and analyzing clinical and genetic data for advancing COVID-19 research. Eur. J. Hum. Genet. 2021, 29, 745–759. [Google Scholar] [CrossRef]
- Hanif, Z.; Sufiyan, N.; Patel, M.; Akhtar, M.Z. Role of biobanks in transplantation. Ann. Med. Surg. 2018, 28, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Arjuna, A.; Olson, M.T.; Walia, R.; Bremner, R.M.; Smith, M.A.; Mohanakumar, T. An update on current treatment strategies for managing bronchiolitis obliterans syndrome after lung transplantation. Expert Rev. Respir. Med. 2021, 15, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; Bargagli, E.; d’Alessandro, M.; Refini, R.M.; Cameli, P.; Galasso, L.; Scapellato, C.; Montagnani, F.; Scolletta, S.; Franchi, F.; et al. Prognostic bioindicators in severe COVID-19 patients. Cytokine. 2021, 141, 155455. [Google Scholar] [CrossRef] [PubMed]
- Vietri, L.; Cameli, P.; Perruzza, M.; Cekorja, B.; Bergantini, L.; d’Alessandro, M.; Refini, R.M.; Pieroni, M.; Fossi, A.; Bennett, D.; et al. Pirfenidone in idiopathic pulmonary fibrosis: Real-life experience in the referral centre of Siena. Ther. Adv. Respir. Dis. 2020, 14, 1753466620906326. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Bergantini, L.; Cameli, P.; Lanzarone, N.; Antonietta Mazzei, M.; Alonzi, V.; Sestini, P.; Bargagli, E. Serum KL-6 levels in pulmonary Langerhans’ cell histiocytosis. Eur. J. Clin. Investig. 2020, 50, e13242. [Google Scholar] [CrossRef]
- Vietri, L.; Bargagli, E.; Bennett, D.; Fossi, A.; Cameli, P.; Bergantini, L.; d’Alessandro, M.; Paladini, P.; Luzzi, L.; Gentili, F.; et al. Serum Amyloid A in lung transplantation. Sarcoidosis Vasc. Diffuse. Lung Dis. 2020, 37, 2–7. [Google Scholar]
- Vietri, L.; Bennett, D.; Cameli, P.; Bergantini, L.; Cillis, G.; Sestini, P.; Bargagli, E.; Rottoli, P. Serum amyloid A in patients with idiopathic pulmonary fibrosis. Respir. Investig. 2019, 57, 430–434. [Google Scholar] [CrossRef]
- d’Alessandro, M.; Carleo, A.; Cameli, P.; Bergantini, L.; Perrone, A.; Vietri, L.; Lanzarone, N.; Vagaggini, C.; Sestini, P.; Bargagli, E. BAL biomarkers’ panel for differential diagnosis of interstitial lung diseases. Clin. Exp. Med. 2020, 20, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Salvini, M.; Fui, A.; Cillis, G.; Cameli, P.; Mazzei, M.A.; Fossi, A.; Refini, R.M.; Rottoli, P. Calgranulin B and KL-6 in Bronchoalveolar Lavage of Patients with IPF and NSIP. Inflammation 2019, 42, 463–470. [Google Scholar] [CrossRef]
- Bargagli, E.; Olivieri, C.; Nikiforakis, N.; Cintorino, M.; Magi, B.; Perari, M.G.; Vagaggini, C.; Spina, D.; Prasse, A.; Rottoli, P. Analysis of macrophage migration inhibitory factor (MIF) in patients with idiopathic pulmonary fibrosis. Respir. Physiol. Neurobiol. 2009, 167, 261–267. [Google Scholar] [CrossRef]
- Rottoli, P.; Bargagli, E. Is bronchoalveolar lavage obsolete in the diagnosis of interstitial lung disease? Curr. Opin. Pulm. Med. 2003, 9, 418–425. [Google Scholar] [CrossRef]
- Hardison, M.T.; Galin, F.S.; Calderon, C.E.; Djekic, U.V.; Parker, S.B.; Wille, K.M.; Jackson, P.L.; Oster, R.A.; Young, K.R.; Blalock, J.E.; et al. The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation. J. Immunol. 2009, 182, 4423–4431. [Google Scholar] [CrossRef]
- Laohaburanakit, P.; Chan, A.; Allen, R.P. Bronchiolitis obliterans. Clin. Rev. Allergy Immunol. 2003, 25, 259–274. [Google Scholar] [CrossRef]
- Verleden, S.E.; Vasilescu, D.M.; Willems, S.; Ruttens, D.; Vos, R.; Vandermeulen, E.; Hostens, J.; McDonough, J.E.; Verbeken, E.K.; Verschakelen, J.; et al. The site and nature of airway obstruction after lung transplantation. Am. J. Respir. Crit. Care Med. 2014, 189, 292–300. [Google Scholar] [CrossRef]
- Kuehnel, M.; Maegel, L.; Vogel-Claussen, J.; Robertus, J.L.; Jonigk, D. Airway remodelling in the transplanted lung. Cell Tissue Res. 2017, 367, 663–675. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Holmes-Liew, C.-L.; Reynolds, P.N.; Holmes, M. Bronchiolitis obliterans syndrome is associated with increased peripheral blood natural killer and natural killer T-like granzymes, perforin, and T-helper-type 1 pro-inflammatory cytokines. J. Heart Lung Transplant. 2012, 31, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hodge, G.; Ahern, J.; Liew, C.; Hopkins, P.; Chambers, D.C.; Reynolds, P.N.; Holmes, M. Increased levels of T cell granzyme b in bronchiolitis obliterans syndrome are not suppressed adequately by current immunosuppressive regimens. Clin. Exp. Immunol. 2009, 158, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Wilkes, D.S. The role of autoimmunity in obliterative bronchiolitis after lung transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L307–L311. [Google Scholar] [CrossRef] [PubMed]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Ruttens, D.; Vandermeulen, E.; Bellon, H.; Van Raemdonck, D.E.; Dupont, L.J.; Vanaudenaerde, B.M.; Verleden, G.; Vos, R. Restrictive chronic lung allograft dysfunction: Where are we now? J. Heart Lung Transplant. 2015, 34, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.; Smith, M.A.; Phelan, D.; Sundaresan, S.; Trulock, E.P.; Lynch, J.P.; Cooper, J.D.; Patterson, G.A.; Mohanakumar, T. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation 1999, 67, 1155–1161. [Google Scholar] [CrossRef]
- Kauke, T.; Kneidinger, N.; Martin, B.; Dick, A.; Schneider, C.; Schramm, R.; Meimarakis, G.; Preissler, G.; Eickelberg, O.; Von Dossow, V.; et al. Bronchiolitis obliterans syndrome due to donor-specific HLA-antibodies. Tissue Antigens 2015, 86, 178–185. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Sunny, S.S.; Oliphant, V.; Perry, J.; Brodlie, M.; Johnson, G.E.; Ward, C.; Gould, K.; Corris, P.A.; De Soyza, A.; et al. Pseudomonas aeruginosa accentuates epithelial-to-mesenchymal transition in the airway. Eur. Respir. J. 2011, 37, 1237–1247. [Google Scholar] [CrossRef]
- Gregson, A.L.; Wang, X.; Weigt, S.S.; Palchevskiy, V.; Lynch, J.P.; Ross, D.J.; Kubak, B.M.; Saggar, R.; Fishbein, M.C.; Ardehali, A.; et al. Interaction between Pseudomonas and CXC chemokines increases risk of bronchiolitis obliterans syndrome and death in lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 518–526. [Google Scholar] [CrossRef]
- Rahman, M.; Ravichandran, R.; Bansal, S.; Sanborn, K.; Bowen, S.; Eschbacher, J.; Sureshbabu, A.; Fleming, T.; Bharat, A.; Walia, R.; et al. Novel role for tumor suppressor gene, liver kinase B1, in epithelial-mesenchymal transition leading to chronic lung allograft dysfunction. Am. J. Transplant. 2022, 22, 843–852. [Google Scholar] [CrossRef]
- Weigt, S.S.; Kim, G.-H.J.; Jones, H.D.; Ramsey, A.L.; Amubieya, O.; Abtin, F.; Pourzand, L.; Lee, J.; Shino, M.Y.; DerHovanessian, A.; et al. Quantitative Image Analysis at Chronic Lung Allograft Dysfunction Onset Predicts Mortality. Transplantation 2021, 106, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Arjuna, A.; Perincheri, S.; Poulson, C.; Bremner, R.M.; Smith, M.A.; Tokman, S.; Mohanakumar, T. Restrictive allograft syndrome vs bronchiolitis obliterans syndrome: Immunological and molecular characterization of circulating exosomes. J. Heart Lung Transplant. 2022, 41, 24–33. [Google Scholar] [CrossRef]
- Davidsen, J.R.; Laursen, C.B.; Højlund, M.; Lund, T.K.; Jeschke, K.N.; Iversen, M.; Kalhauge, A.; Bendstrup, E.; Carlsen, J.; Perch, M.; et al. Lung Ultrasound to Phenotype Chronic Lung Allograft Dysfunction in Lung Transplant Recipients. A Prospective Observational Study. J. Clin. Med. 2021, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Veraar, C.; Kliman, J.; Benazzo, A.; Oberndorfer, F.; Laggner, M.; Hacker, P.; Raunegger, T.; Janik, S.; Jaksch, P.; Klepetko, W.; et al. Potential novel biomarkers for chronic lung allograft dysfunction and azithromycin responsive allograft dysfunction. Sci. Rep. 2021, 11, 6799. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; D’Alessandro, M.; De Vita, E.; Perillo, F.; Fossi, A.; Luzzi, L.; Paladini, P.; Perrone, A.; Rottoli, P.; Sestini, P.; et al. Regulatory and Effector Cell Disequilibrium in Patients with Acute Cellular Rejection and Chronic Lung Allograft Dysfunction after Lung Transplantation: Comparison of Peripheral and Alveolar Distribution. Cells 2021, 10, 780. [Google Scholar] [CrossRef]
- Itabashi, Y.; Ravichandran, R.; Bansal, S.; Bharat, A.; Hachem, R.; Bremner, R.; Smith, M.; Mohanakumar, T. Decline in Club Cell Secretory Proteins, Exosomes Induction and Immune Responses to Lung Self-antigens, Kα1 Tubulin and Collagen V, Leading to Chronic Rejection After Human Lung Transplantation. Transplantation 2021, 105, 1337–1346. [Google Scholar] [CrossRef]
- D’alessandro, M.; Bergantini, L.; Fossi, A.; De Vita, E.; Perillo, F.; Luzzi, L.; Paladini, P.; Sestini, P.; Rottoli, P.; Bargagli, E.; et al. The Role of Galectins in Chronic Lung Allograft Dysfunction. Lung 2021, 199, 281–288. [Google Scholar] [CrossRef]
- Righi, I.; Vaira, V.; Morlacchi, L.C.; Croci, G.A.; Rossetti, V.; Blasi, F.; Ferrero, S.; Nosotti, M.; Rosso, L.; Clerici, M. Immune Checkpoints Expression in Chronic Lung Allograft Rejection. Front. Immunol. 2021, 12, 714132. [Google Scholar] [CrossRef]
- Cameli, P.; Bargagli, E.; Fossi, A.; Bennett, D.; Voltolini, L.; Refini, R.M.; Gotti, G.; Rottoli, P. Exhaled nitric oxide and carbon monoxide in lung transplanted patients. Respir. Med. 2015, 109, 1224–1229. [Google Scholar] [CrossRef][Green Version]
- Durand, M.; Lacoste, P.; Danger, R.; Jacquemont, L.; Brosseau, C.; Durand, E.; Tilly, G.; Loy, J.; Foureau, A.; Royer, P.-J.; et al. High circulating CD4+CD25hiFOXP3+ T-cell sub-population early after lung transplantation is associated with development of bronchiolitis obliterans syndrome. J. Heart Lung Transplant. 2018, 37, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Danger, R.; Royer, P.-J.; Reboulleau, D.; Durand, E.; Loy, J.; Tissot, A.; Lacoste, P.; Roux, A.; Reynaud-Gaubert, M.; Gomez, C.; et al. Blood Gene Expression Predicts Bronchiolitis Obliterans Syndrome. Front. Immunol. 2017, 8, 1841. [Google Scholar] [CrossRef] [PubMed]
- Piloni, D.; Morosini, M.; Magni, S.; Balderacchi, A.; Scudeller, L.; Cova, E.; Oggionni, T.; Stella, G.; Tinelli, C.; Antonacci, F.; et al. Analysis of long term CD4+CD25highCD127- T-reg cells kinetics in peripheral blood of lung transplant recipients. BMC Pulm. Med. 2017, 17, 102. [Google Scholar] [CrossRef]
- Budding, K.; Rossato, M.; van de Graaf, E.A.; Kwakkel-van Erp, J.M.; Radstake TR, D.J.; Otten, H.G. Serum miRNAs as potential biomarkers for the bronchiolitis obliterans syndrome after lung transplantation. Transpl. Immunol. 2017, 42, 1–4. [Google Scholar] [CrossRef]
- Pain, M.; Royer, P.-J.; Loy, J.; Girardeau, A.; Tissot, A.; Lacoste, P.; Roux, A.; Reynaud-Gaubert, M.; Kessler, R.; Mussot, S.; et al. T Cells Promote Bronchial Epithelial Cell Secretion of Matrix Metalloproteinase-9 via a C-C Chemokine Receptor Type 2 Pathway: Implications for Chronic Lung Allograft Dysfunction. Am. J. Transplant. 2017, 17, 1502–1514. [Google Scholar] [CrossRef]
- Belloli, E.A.; Gu, T.; Wang, Y.; Vummidi, D.; Lyu, D.M.; Combs, M.P.; Chughtai, A.; Murray, S.; Galbán, C.J.; Lama, V.N. Radiographic Graft Surveillance in Lung Transplantation: Prognostic Role of Parametric Response Mapping. Am. J. Respir. Crit. Care Med. 2021, 204, 967–976. [Google Scholar] [CrossRef]
- Berastegui, C.; Gómez-Ollés, S.; Sánchez-Vidaurre, S.; Culebras, M.; Monforte, V.; López-Meseguer, M.; Bravo, C.; Ramon, M.-A.; Romero, L.; Sole, J.; et al. BALF cytokines in different phenotypes of chronic lung allograft dysfunction in lung transplant patients. Clin. Transplant. 2017, 31, e12898. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Fumagalli, M.; Viglio, S.; Bardoni, A.M.; Piloni, D.; Meloni, F.; Iadarola, P.; Airoldi, C. 1H NMR To Evaluate the Metabolome of Bronchoalveolar Lavage Fluid (BALf) in Bronchiolitis Obliterans Syndrome (BOS): Toward the Development of a New Approach for Biomarker Identification. J. Proteome Res. 2017, 16, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Budding, K.; Van De Graaf, E.A.; Kardol-Hoefnagel, T.; Erp, J.M.K.-V.; Luijk, B.D.; Oudijk, E.-J.D.; Van Kessel, D.A.; Grutters, J.C.; Hack, C.E.; Otten, H.G. Soluble CD59 is a Novel Biomarker for the Prediction of Obstructive Chronic Lung Allograft Dysfunction After Lung Transplantation. Sci. Rep. 2016, 6, 26274. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, Z.; Yu, J.; Daguindau, E.; Kushekhar, K.; Zhang, Q.; Ogata, Y.; Gafken, P.R.; Inamoto, Y.; Gracon, A.; et al. Proteomic Characterization Reveals That MMP-3 Correlates with Bronchiolitis Obliterans Syndrome Following Allogeneic Hematopoietic Cell and Lung Transplantation. Am. J. Transplant. 2016, 16, 2342–2351. [Google Scholar] [CrossRef]
- Vandermeulen, E.; Verleden, S.E.; Bellon, H.; Ruttens, D.; Lammertyn, E.; Claes, S.; Vandooren, J.; Ugarte-Berzal, E.; Schols, D.; Emonds, M.-P.; et al. Humoral immunity in phenotypes of chronic lung allograft dysfunction: A broncho-alveolar lavage fluid analysis. Transpl. Immunol. 2016, 38, 27–32. [Google Scholar] [CrossRef]
- Ericson, P.A.; Mirgorodskaya, E.; Hammar, O.S.; Viklund, E.A.; Almstrand, A.-C.R.; Larsson, P.J.-W.; Riise, G.; Olin, A.-C. Low Levels of Exhaled Surfactant Protein A Associated with BOS After Lung Transplantation. Transplant. Direct. 2016, 2, e103. [Google Scholar] [CrossRef]
- Hayes, D.; Harhay, M.O.; Nicol, K.K.; Liyanage, N.P.M.; Keller, B.C.; Robinson, R.T. Lung T-Cell Profile Alterations are Associated with Bronchiolitis Obliterans Syndrome in Cystic Fibrosis Lung Transplant Recipients. Lung 2020, 198, 157–161. [Google Scholar] [CrossRef]
- Schreurs, I.; Meek, B.; Hijdra, D.; van Moorsel, C.; Luijk, H.; Erp, J.K.-V.; Oudijk, E.; van Kessel, D.; Grutters, J. Lung Transplantation Has a Strong Impact on the Distribution and Phenotype of Monocyte Subsets. Transplant. Proc. 2020, 52, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, I.; Meek, B.; Hijdra, D.; Rodriguez Gomez, M.; van Moorsel CH, M.; Luijk, H.D.; Kwakkel-van Erp, J.M.; Oudijk, E.J.; van Kessel, D.A.; Grutters, J.C. Lung Transplant Patients Show a Dissimilar Peripheral B-Cell Subset Ratio Compared With Healthy Controls. Exp. Clin. Transplant. 2020, 18, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Larsson-Callerfelt, A.-K.; Müller, C.; Andersson-Sjöland, A.; Thiman, L.; Larsson, H.; Hallgren, O.; Bjermer, L.; Eriksson, L.; Westergren-Thorsson, G. VEGF synthesis and VEGF receptor 2 expression in patients with bronchiolitis obliterans syndrome after lung transplantation. Respir. Med. 2020, 166, 105944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gunasekaran, M.; Ravichandran, R.; Fisher, C.E.; Limaye, A.P.; Hu, C.; McDyer, J.; Kaza, V.; Bharat, A.; Tokman, S.; et al. Circulating exosomes with lung self-antigens as a biomarker for chronic lung allograft dysfunction: A retrospective analysis. J. Heart Lung Transplant. 2020, 39, 1210–1219. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Liu, H.; Nguyen, P.; Holmes-Liew, C.-L.; Holmes, M. Bronchiolitis obliterans syndrome is associated with increased senescent lymphocytes in the small airways. J. Heart Lung Transplant. 2021, 40, 108–119. [Google Scholar] [CrossRef]
- Sacreas, A.; Yang, J.Y.C.; Vanaudenaerde, B.M.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; Verleden, G.M.; Vos, R.; Verleden, S.E.; Sarwal, M.M. The common rejection module in chronic rejection post lung transplantation. PLoS ONE. 2018, 13, e0205107. [Google Scholar] [CrossRef]
- Levy, L.; Tigert, A.; Huszti, E.; Saito, T.; Mitsakakis, N.; Moshkelgosha, S.; Joe, B.; Boonstra, K.M.; Tikkanen, J.M.; Keshavjee, S.; et al. Epithelial cell death markers in bronchoalveolar lavage correlate with chronic lung allograft dysfunction subtypes and survival in lung transplant recipients—A single-center retrospective cohort study. Transpl. Int. 2019, 32, 965–973. [Google Scholar] [CrossRef]
- Brosseau, C.; Danger, R.; Durand, M.; Durand, E.; Foureau, A.; Lacoste, P.; Tissot, A.; Roux, A.; Reynaud-Gaubert, M.; Kessler, R.; et al. Blood CD9+ B cell, a biomarker of bronchiolitis obliterans syndrome after lung transplantation. Am. J. Transplant. 2019, 19, 3162–3175. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Li, H.-B. Recent progress on liver kinase B1 (LKB1): Expression, regulation, downstream signaling and cancer suppressive function. Int. J. Mol. Sci. 2014, 15, 16698–16718. [Google Scholar] [CrossRef]
- Saxena, M.; Balaji, S.A.; Deshpande, N.; Ranganathan, S.; Pillai, D.M.; Hindupur, S.K.; Rangarajan, A. AMP-activated protein kinase promotes epithelial-mesenchymal transition in cancer cells through Twist1 upregulation. J. Cell Sci. 2018, 131, jcs208314. [Google Scholar] [CrossRef]
- Bonanno, S.; Zulato, E.; Pavan, A.; Attili, I.; Pasello, G.; Conte, P.; Indraccolo, S. LKB1 and Tumor Metabolism: The Interplay of Immune and Angiogenic Microenvironment in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1874. [Google Scholar] [CrossRef]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Skoulidis, F.; Byers, L.A.; Diao, L.; Papadimitrakopoulou, V.A.; Tong, P.; Izzo, J.; Behrens, C.; Kadara, H.; Parra, E.R.; Canales, J.R.; et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015, 5, 860–877. [Google Scholar] [CrossRef]
- Su, X.; Wang, Q.; Guo, W.; Pei, X.; Niu, Q.; Liu, M.; Liu, Y.; Chen, S.; Feng, S.; He, Y.; et al. Loss of Lkb1 impairs Treg function and stability to aggravate graft-versus-host disease after bone marrow transplantation. Cell Mol. Immunol. 2020, 17, 483–495. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Angaswamy, N.; Brand, D.; Weber, J.; Gelman, A.G.; Hachem, R.; Trulock, E.P.; Meyers, B.; Patterson, G.; Mohanakumar, T. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin. Exp. Immunol. 2012, 167, 158–168. [Google Scholar] [CrossRef]
- Shastri, N.; Yewdell, J.W. Editorial overview: Antigen processing and presentation: Where cellular immunity begins. Curr. Opin. Immunol. 2015, 34, v–vii. [Google Scholar] [CrossRef]
- Sacreas, A.; Taupin, J.-L.; Emonds, M.-P.; Daniëls, L.; Van Raemdonck, D.E.; Vos, R.; Verleden, G.M.; Vanaudenaerde, B.M.; Roux, A.; Verleden, S.E. Intragraft donor-specific anti-HLA antibodies in phenotypes of chronic lung allograft dysfunction. Eur. Respir. J. 2019, 54, 1900847. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Immune Checkpoint Inhibitors in Heart or Lung Transplantation: Early Results from a Registry Initiative—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32265077/ (accessed on 11 April 2022).
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Shijubo, N.; Kojima, H.; Nagata, M.; Ohchi, T.; Suzuki, A.; Abe, S.; Sato, N. Tumor angiogenesis of non-small cell lung cancer. Microsc. Res. Tech. 2003, 60, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Volm, M.; Koomägi, R.; Mattern, J.; Stammler, G. Angiogenic growth factors and their receptors in non-small cell lung carcinomas and their relationships to drug response in vitro. Anticancer Res. 1997, 17, 99–103. [Google Scholar]
- Volm, M.; Koomägi, R.; Mattern, J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int. J. Cancer 1997, 74, 64–68. [Google Scholar] [CrossRef]
- Vascular Endothelial Growth Factor in Human Lung Transplantation—CHEST. Available online: https://journal.chestnet.org/article/S0012-3692(15)37575-9/ppt (accessed on 11 April 2022).
- Kastelijn, E.A.; Rijkers, G.T.; Van Moorsel, C.H.; Zanen, P.; Erp, J.M.K.-V.; Van De Graaf, E.A.; Van Kessel, D.A.; Grutters, J.C.; Bosch, J.M.V.D. Systemic and exhaled cytokine and chemokine profiles are associated with the development of bronchiolitis obliterans syndrome. J. Heart Lung Transplant. 2010, 29, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Roedder, S.; Kimura, N.; de Vusser, K.; Morgan, A.A.; Gong, Y.; Fischbein, M.P.; Robbins, R.C.; Naesens, M.; Butte, A.J.; et al. A Common Rejection Module (CRM) for Acute Rejection Across Multiple Organs Identifies Novel Therapeutics for Organ Transplantation. J. Exp. Med. 2013, 210, 2205–2221. Available online: https://rupress.org/jem/article/210/11/2205/41536/A-common-rejection-module-CRM-for-acute-rejection (accessed on 11 April 2022). [CrossRef]
- American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005, 171, 912–930. [Google Scholar] [CrossRef]
- Tsoukias, N.M.; George, S.C. A two-compartment model of pulmonary nitric oxide exchange dynamics. J. Appl. Physiol. 1998, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1998, 1408, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.; Tornoe, I.; Nielsen, O.; Koch, C.; Steinhilber, W.; Holmskov, U. Expression and localization of lung surfactant protein A in human tissues. Am. J. Respir. Cell Mol. Biol. 2003, 29, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Barreira, E.R.; Precioso, A.R.; Bousso, A. Pulmonary surfactant in respiratory syncytial virus bronchiolitis: The role in pathogenesis and clinical implications. Pediatr. Pulmonol. 2011, 46, 415–420. [Google Scholar] [CrossRef]
- Meloni, F.; Salvini, R.; Bardoni, A.M.; Passadore, I.; Solari, N.; Vitulo, P.; Oggionni, T.; Viganò, M.; Pozzi, E.; Fietta, A.M. Bronchoalveolar lavage fluid proteome in bronchiolitis obliterans syndrome: Possible role for surfactant protein A in disease onset. J. Heart Lung Transplant. 2007, 26, 1135–1143. [Google Scholar] [CrossRef]
- Dekens, D.W.; Eisel, U.L.M.; Gouweleeuw, L.; Schoemaker, R.G.; De Deyn, P.P.; Naudé, P.J.W. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res. Rev. 2021, 70, 101414. [Google Scholar] [CrossRef]
- Redl, B.; Habeler, M. The diversity of lipocalin receptors. Biochimie 2022, 192, 22–29. [Google Scholar] [CrossRef]
- Lipocalin-2, Pro- or Anti-Apoptotic?—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19160065/ (accessed on 11 April 2022).
- Treekitkarnmongkol, W.; Hassane, M.; Sinjab, A.; Chang, K.; Hara, K.; Rahal, Z.; Zhang, J.; Lu, W.; Sivakumar, S.; McDowell, T.L.; et al. Augmented Lipocalin-2 Is Associated with Chronic Obstructive Pulmonary Disease and Counteracts Lung Adenocarcinoma Development. Am. J. Respir. Crit. Care Med. 2021, 203, 90–101. [Google Scholar] [CrossRef]
- Vasta, G.R. Galectins in Host-Pathogen Interactions: Structural, Functional and Evolutionary Aspects. Adv. Exp. Med. Biol. 2020, 1204, 169–196. [Google Scholar]
- Ho, J.E.; Gao, W.; Levy, D.; Santhanakrishnan, R.; Araki, T.; Rosas, I.O.; Hatabu, H.; Latourelle, J.C.; Nishino, M.; Dupuis, J.; et al. Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am. J. Respir. Crit. Care Med. 2016, 194, 77–83. [Google Scholar] [CrossRef]
- Shevchenko, O.; Tsirulnikova, O.; Sharapchenko, S.; Pashkov, I.; Bekov, M.; Shigaev, E.; Gichkun, O.; Velikiy, D.; Gautier, S. MiR-339 and galectin-3, diagnostic value in patients with airway obstruction after lung transplantation. Transpl. Int. 2021, 34, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.A.; Harris, C.L.; Williams, A.S.; Mizuno, M.; Gallagher, S.; Smith, R.A.G.; Morgan, B.P. Generation of a recombinant, membrane-targeted form of the complement regulator CD59, activity in vitro and in vivo. J. Biol. Chem. 2003, 278, 48921–48927. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.M.; AAl-Binni, M.; Bani Hani, A.; Abu Abeeleh, M.; Abu-Humaidan, A.H.A. Complement Terminal Pathway Activation is Associated with Organ Failure in Sepsis Patients. J. Inflamm. Res. 2022, 15, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, C.M.; Radisky, D.C.; Aschner, Y.; Downey, G.P. The importance of matrix metalloproteinase-3 in respiratory disorders. Expert Rev. Respir. Med. 2014, 8, 411–421. [Google Scholar] [CrossRef]
- Inamoto, Y.; Martin, P.J.; Onstad, L.E.; Cheng, G.S.; Williams, K.M.; Pusic, I.; Ho, V.T.; Arora, M.; Pidala, J.; Flowers, M.E.D.; et al. Relevance of Plasma Matrix Metalloproteinase-9 for Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2021, 27, 759. [Google Scholar] [CrossRef]
- Nagase, H.; Woessner, J.F. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Hendrix, A.Y.; Kheradmand, F. The Role of Matrix Metalloproteinases in Development, Repair, and Destruction of the Lungs. Prog. Mol. Biol. Transl. Sci. 2017, 148, 1–29. [Google Scholar]
- Kennedy, V.E.; Todd, J.L.; Palmer, S.M. Bronchoalveolar Lavage as a Tool to Predict, Diagnose and Understand Bronchiolitis Obliterans Syndrome. Am. J. Transplant. 2013, 13, 552–561. [Google Scholar] [CrossRef]
- McWilliams, T.; Zheng, L.; Orsida, B.; Levvey, B.; Walters, E.; Whitford, H.; Snell, G.; Williams, T. The profibrotic cytokine transforming growth factor beta (TGFβ) is elevated early in the development of bronchiolitis obliterans syndrome (BOS) in lung transplant recipients (LTR). J. Heart Lung Transplant. 2004, 23, S126. [Google Scholar] [CrossRef]
- Hachem, R.R.; Tiriveedhi, V.; Patterson, G.A.; Aloush, A.; Trulock, E.P.; Mohanakumar, T. Antibodies to K-α 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am. J. Transplant. 2012, 12, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Sumpter, T.L.; Wilkes, D.S. Role of autoimmunity in organ allograft rejection: A focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L1129–L1139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saini, D.; Weber, J.; Ramachandran, S.; Phelan, D.; Tiriveedhi, V.; Liu, M.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.; et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J. Heart Lung Transplant. 2011, 30, 624–631. [Google Scholar] [CrossRef]
- Rao, U.; Sharma, M.; Mohanakumar, T.; Ahn, C.; Gao, A.; Kaza, V. Prevalence of antibodies to lung self-antigens (Kα1 tubulin and collagen V) and donor specific antibodies to HLA in lung transplant recipients and implications for lung transplant outcomes: Single center experience. Transpl. Immunol. 2019, 54, 65–72. [Google Scholar] [CrossRef]
- Tiriveedhi, V.; Gelman, A.E.; Mohanakumar, T. HIF-1α signaling by airway epithelial cell K-α1-tubulin: Role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012, 273, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Saenz, S.A.; Taylor, B.C.; Artis, D. Welcome to the neighborhood: Epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008, 226, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A.; Parker, S.M.; A Brougham, K.; Johnson, G.E.; Gorowiec, M.R.; Ward, C.; Lordan, J.L.; Corris, P.A.; Kirby, J.A.; Fisher, A.J. Epithelial to mesenchymal transition (EMT) and airway remodelling after human lung transplantation. Thorax 2009, 64, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Waddell, T.K.; Wagnetz, U.; Roberts, H.C.; Hwang, D.M.; Haroon, A.; Wagnetz, D.; Chaparro, C.; Singer, L.; Hutcheon, M.A.; et al. Restrictive allograft syndrome (RAS): A novel form of chronic lung allograft dysfunction. J. Heart Lung Transplant. 2011, 30, 735–742. [Google Scholar] [CrossRef]
- Verleden, G.M.; Raghu, G.; Meyer, K.C.; Glanville, A.R.; Corris, P. A new classification system for chronic lung allograft dysfunction. J. Heart Lung Transplant. 2014, 33, 127–133. [Google Scholar] [CrossRef]
- Colvin, R.B.; Smith, R.N. Antibody-mediated organ-allograft rejection. Nat. Rev. Immunol. 2005, 5, 807–817. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.D.; Wang, Z.; Chen, D.-F.; Reinsmoen, N.L.; Finlen-Copeland, C.A.; Davis, W.A.; Zaas, D.W.; Palmer, S.M. Implications for human leukocyte antigen antibodies after lung transplantation: A 10-year experience in 441 patients. Chest 2013, 144, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Roux, A.; Le Lan, I.B.; Holifanjaniaina, S.; Thomas, K.A.; Hamid, A.M.; Picard, C.; Grenet, D.; De Miranda, S.; Douvry, B.; Beaumont-Azuar, L.; et al. Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am. J. Transplant. 2016, 16, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Gregson, A.L.; Hoji, A.; Saggar, R.; Ross, D.J.; Kubak, B.M.; Jamieson, B.D.; Weigt, S.S.; Lynch, J.P.; Ardehali, A.; Belperio, J.A.; et al. Bronchoalveolar immunologic profile of acute human lung transplant allograft rejection. Transplantation 2008, 85, 1056–1059. [Google Scholar] [CrossRef]
- Zheng, L.; Orsida, B.; Whitford, H.; Levvey, B.; Ward, C.; Walters, E.; Williams, T.J.; Snell, G.I. Longitudinal comparisons of lymphocytes and subtypes between airway wall and bronchoalveolar lavage after human lung transplantation. Transplantation 2005, 80, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, E.; Lammertyn, E.; Verleden, S.E.; Ruttens, D.; Bellon, H.; Ricciardi, M.; Somers, J.; Bracke, K.R.; Eynde, K.V.D.; Tousseyn, T.; et al. Immunological diversity in phenotypes of chronic lung allograft dysfunction: A comprehensive immunohistochemical analysis. Transpl. Int. 2017, 30, 134–143. [Google Scholar] [CrossRef]
- Bharat, A.; Narayanan, K.; Street, T.; Fields, R.C.; Steward, N.; Aloush, A.; Meyers, B.; Schuessler, R.; Trulock, E.P.; Patterson, G.A.; et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation 2007, 83, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Belperio, J.A.; Weigt, S.S.; Fishbein, M.C.; Lynch, J.P. Chronic lung allograft rejection: Mechanisms and therapy. Proc. Am. Thorac. Soc. 2009, 6, 108–121. [Google Scholar] [CrossRef]
- Sigdel, T.K.; Bestard, O.; Tran, T.Q.; Hsieh, S.-C.; Roedder, S.; Damm, I.; Vincenti, F.; Sarwal, M.M. A Computational Gene Expression Score for Predicting Immune Injury in Renal Allografts. PLoS ONE 2015, 10, e0138133. [Google Scholar] [CrossRef]
- Hong, J.-H.; Lee, W.-C.; Hsu, Y.-M.; Liang, H.-J.; Wan, C.-H.; Chien, C.-L.; Lin, C.-Y. Characterization of the biochemical effects of naphthalene on the mouse respiratory system using NMR-based metabolomics. J. Appl. Toxicol. 2014, 34, 1379–1388. [Google Scholar] [CrossRef]
- Fabiano, A.; Gazzolo, D.; Zimmermann, L.J.; Gavilanes, A.W.; Paolillo, P.; Fanos, V.; Caboni, P.; Barberini, L.; Noto, A.; Atzori, L. Metabolomic analysis of bronchoalveolar lavage fluid in preterm infants complicated by respiratory distress syndrome: Preliminary results. J. Matern. Fetal. Neonatal Med. 2011, 24 (Suppl. 2), 55–58. [Google Scholar] [CrossRef]
- Wolak, J.E.; Esther, C.R.; O’Connell, T.M. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers 2009, 14, 55–60. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Zhang, L.; Duan, Y.; Guo, Q.; Wang, W.; He, S.; Li, J.; Yin, Y. Taurine is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019, 63, e1800536. [Google Scholar] [CrossRef]
- Chen, J.; Xue, X.; Cai, J.; Jia, L.; Sun, B.; Zhao, W. Protective effect of taurine on sepsis-induced lung injury via inhibiting the p38/MAPK signaling pathway. Mol. Med. Rep. 2021, 24, 653. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Wei, B.K.; Dong, G.; Wang, X. Protective Effect of Taurine on Paraquat-Induced Lung Epithelial Cell Injury. Adv. Exp. Med. Biol. 2019, 1155, 739–746. [Google Scholar] [PubMed]
- Di Carlo, S.; Rossi, E.; Politano, G.; Inghilleri, S.; Morbini, P.; Calabrese, F.; Benso, A.; Savino, A.; Cova, E.; Zampieri, D.; et al. Identification of miRNAs Potentially Involved in Bronchiolitis Obliterans Syndrome: A Computational Study. PLoS ONE 2016, 11, e0161771. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ramachandran, S.; Gunasekaran, M.; Zhou, F.; Trulock, E.; Kreisel, D.; Hachem, R.; Mohanakumar, T. MicroRNA-144 dysregulates the transforming growth factor-β signaling cascade and contributes to the development of bronchiolitis obliterans syndrome after human lung transplantation. J. Heart Lung Transplant. 2015, 34, 1154–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bozzini, S.; Del Fante, C.; Morosini, M.; Berezhinskiy, H.O.; Auner, S.; Cattaneo, E.; Della Zoppa, M.; Pandolfi, L.; Cacciatore, R.; Perotti, C.; et al. Mechanisms of Action of Extracorporeal Photopheresis in the Control of Bronchiolitis Obliterans Syndrome (BOS): Involvement of Circulating miRNAs. Cells 2022, 11, 1117. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Yang, D.; Wang, J.-J.; Li, J.-S.; Xu, Q.-Y. miR-103 Functions as a Tumor Suppressor by Directly Targeting Programmed Cell Death 10 in NSCLC. Oncol. Res. 2018, 26, 519–528. [Google Scholar] [CrossRef]
- Bozzini, S.; Pandolfi, L.; Rossi, E.; Inghilleri, S.; Zorzetto, M.; Ferrario, G.; Di Carlo, S.; Politano, G.; De Silvestri, A.; Frangipane, V.; et al. miRNAs Potentially Involved in Post Lung Transplant-Obliterative Bronchiolitis: The Role of miR-21-5p. Cells 2021, 10, 688. [Google Scholar] [CrossRef] [PubMed]
- Melissa, L.T.; Jeremy, D.F.; Timothy, M.C. Pharmacotherapy of Lung Transplantation: An Overview. 2013. Available online: https://journals.sagepub.com/doi/abs/10.1177/0897190012466048 (accessed on 11 April 2022).
- Traitanon, O.; Mathew, J.M.; La Monica, G.; Xu, L.; Mas, V.; Gallon, L. Differential Effects of Tacrolimus versus Sirolimus on the Proliferation, Activation and Differentiation of Human B Cells. PLoS ONE 2015, 10, e0129658. [Google Scholar] [CrossRef] [PubMed]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Eibel, H.; Kraus, H.; Sic, H.; Kienzler, A.-K.; Rizzi, M. B cell Biology: An Overview. Curr. Allergy Asthma Rep. 2014, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Stegall, M.D.; Raghavaiah, S.; Gloor, J.M. The (re)emergence of B cells in organ transplantation. Curr. Opin. Organ Transplant. 2010, 15, 451–455. [Google Scholar] [CrossRef]
- Girmanova, E.; Hruba, P.; Viklicky, O. Circulating biomarkers of tolerance. Transplant. Rev. 2015, 29, 68–72. [Google Scholar] [CrossRef]
- Lung Transplant Patients Show a Dissimilar Peripheral B—Cell Subset Ratio Compared with Healthy Controls—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32279657/ (accessed on 11 April 2022).
- Gauthier, J.M.; Li, W.; Hsiao, H.-M.; Takahashi, T.; Arefanian, S.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Mechanisms of Graft Rejection and Immune Regulation after Lung Transplant. Ann. Am. Thorac. Soc. 2017, 14, S216–S219. [Google Scholar] [CrossRef]
- B Cell Development and Maturation | SpringerLink. Available online: https://link.springer.com/chapter/10.1007/978-981-15-3532-1_1 (accessed on 11 April 2022).
- Liu, Y.; Duan, W.-R.; Liu, S.; Liu, T.; Chang, Y.-J.; Fan, X.-M. Correlation of CD19+CD24hiCD38hi B cells in coronary artery disease with severity of atherosclerosis. Chin. Med. J. 2020, 133, 1257–1258. [Google Scholar] [CrossRef]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Pefanis, E.; Chao, J.; Rothschild, G.; Tachibana, I.; Chen, J.K.; Ivanov, I.I.; Rabadan, R.; Takeda, Y.; et al. Transcriptomics Identify CD9 as a Marker of Murine IL-10-Competent Regulatory B Cells. Cell Rep. 2015, 13, 1110–1117. [Google Scholar] [CrossRef]
- Read, K.A.; Powell, M.D.; Sreekumar, B.K.; Oestreich, K.J. In Vitro Differentiation of Effector CD4+ T Helper Cell Subsets. Methods Mol. Biol. 2019, 1960, 75–84. [Google Scholar] [PubMed]
- Kurachi, M. CD8+ T cell exhaustion. Semin Immunopathol. 2019, 41, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, P.; Du, X.; Liu, Y.; Zhang, B.; Qi, F. Regulatory T cells induce transplant immune tolerance. Transpl. Immunol. 2021, 67, 101411. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Li-Liew, C.; Chambers, D.; Hopkins, P.; Reynolds, P.N.; Holmes, M. Time post-lung transplant correlates with increasing peripheral blood T cell granzyme B and proinflammatory cytokines. Clin. Exp. Immunol. 2010, 161, 584–590. [Google Scholar] [CrossRef]
- Hodge, G.; Mukaro, V.; Reynolds, P.N.; Hodge, S. Role of increased CD8/CD28(null) T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clin. Exp. Immunol. 2011, 166, 94–102. [Google Scholar] [CrossRef]
- Bosch, T.P.P.V.D.; Caliskan, K.; Kraaij, M.D.; Constantinescu, A.A.; Manintveld, O.C.; Leenen, P.J.M.; von der Thüsen, J.H.; Groningen, M.C.C.-V.; Baan, C.C.; Rowshani, A.T. CD16+ Monocytes and Skewed Macrophage Polarization toward M2 Type Hallmark Heart Transplant Acute Cellular Rejection. Front. Immunol. 2017, 8, 346. Available online: https://www.frontiersin.org/article/10.3389/fimmu.2017.00346 (accessed on 11 April 2022).
- The Three Human Monocyte Subsets: Implications for Health and Disease | SpringerLink. Available online: https://link.springer.com/article/10.1007/s12026-012-8297-3 (accessed on 11 April 2022).
- 6-Sulfo LacNAc (Slan) as a Marker for Non-classical Monocytes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31572354/ (accessed on 11 April 2022).
- Chiu, S.; Bharat, A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr. Opin. Organ Transplant. 2016, 21, 239–245. [Google Scholar] [CrossRef]
- Luo, Y.; Fujii, H.; Gerster, T.; Roeder, R.G. A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell 1992, 71, 231–241. [Google Scholar] [CrossRef]
- Dymecki, S.M.; Niederhuber, J.E.; Desiderio, S.V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science 1990, 247, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.; Künstle, G.; Obata, T.; Sha, M.; Noguchi, M. The protooncogene TCL1 is an Akt kinase coactivator. Mol. Cell 2000, 6, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Brinas, F.; Danger, R.; Brouard, S. TCL1A, B Cell Regulation and Tolerance in Renal Transplantation. Cells 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- White, E.S. Lung extracellular matrix and fibroblast function. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Zhou, Y.; Han, W.; Fu, X. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp. Biol. Med. 2016, 241, 1–13. [Google Scholar] [CrossRef]
- Downer, N.J.; Ali, N.J.; Au-Yong, I.T.H. Investigating pleural thickening. BMJ 2013, 346, e8376. [Google Scholar] [CrossRef] [PubMed]
- Morshid, A.; Moshksar, A.; Das, A.; Duarte, A.G.; Palacio, D.; Villanueva-Meyer, J. HRCT Diagnosis of Pleuroparenchymal fibroelastosis: Report of two cases. Radiol. Case Rep. 2021, 16, 1564–1569. [Google Scholar] [CrossRef]
- Hakim, A.; Cooke, K.R.; Pavletic, S.Z.; Khalid, M.; Williams, K.M.; Hashmi, S.K. Diagnosis and treatment of bronchiolitis obliterans syndrome accessible universally. Bone Marrow Transplant. 2019, 54, 383–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).