Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence
Abstract
1. Introduction
2. Materials and Methods
Detection of Peripheral Blood sjTRECs
3. Results
3.1. Study Population
3.2. sjTREC as a Marker of Early Immunosenescence in Autoimmune and COVID 19 Patients
3.3. Peripheral sjTRECs Levels of the Studied Groups Differ According to the Age Subgroup and Gender
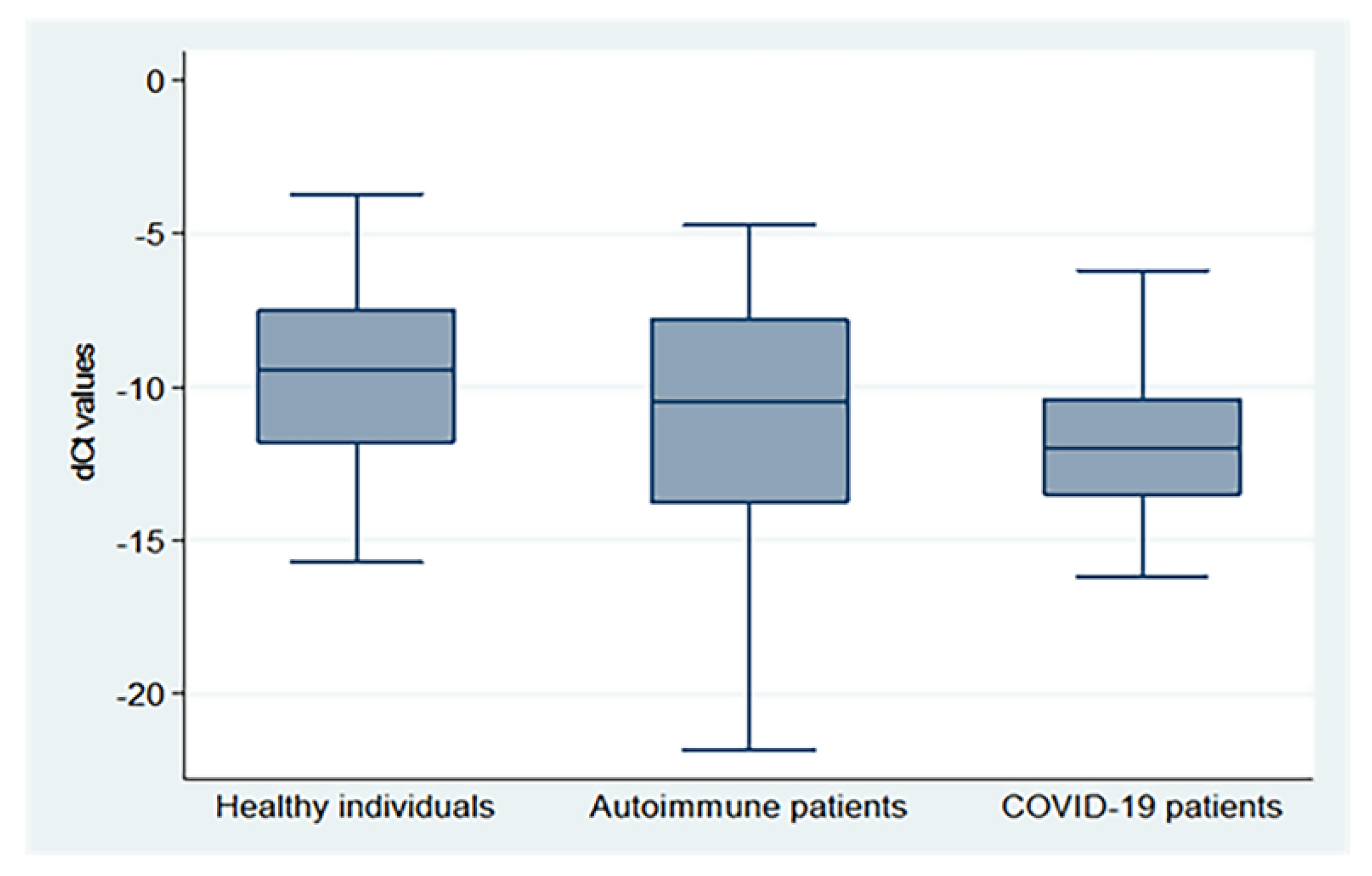
3.4. Peripheral sjTRECs Levels Are Insignificantly Decreased in Severe COVID-19 and Active Auto Immune Cases
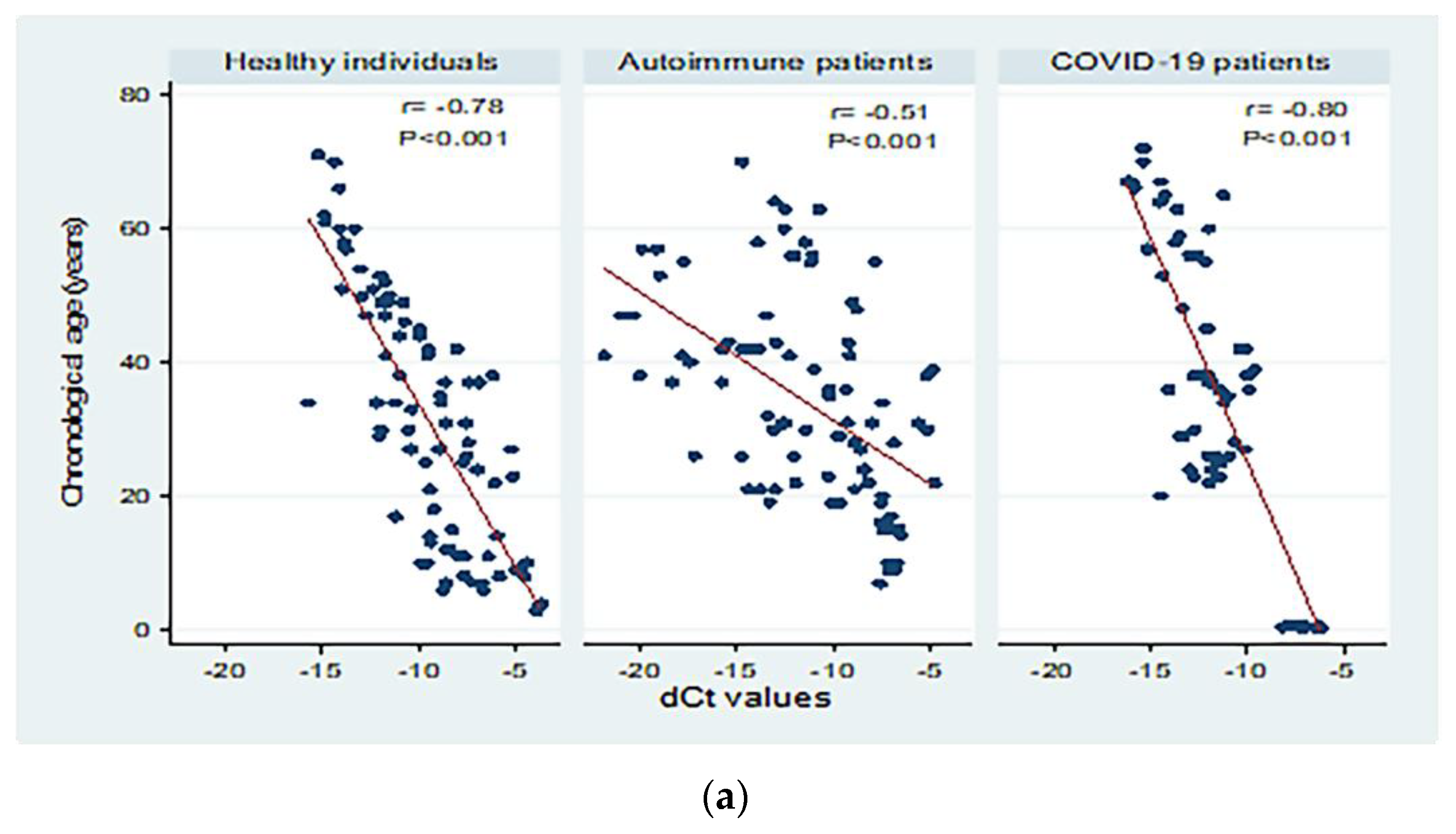
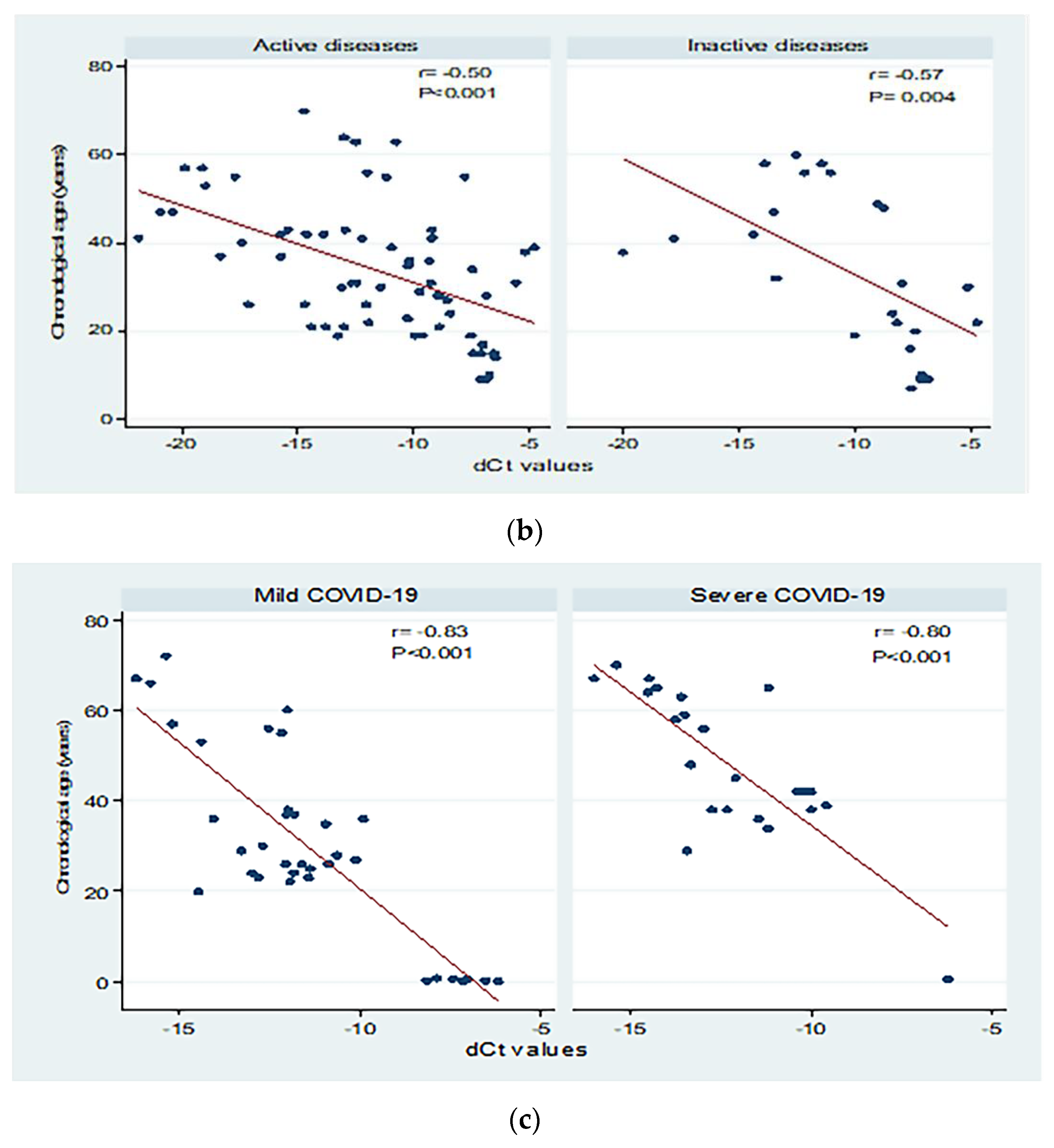
3.5. Peripheral sjTRECs Levels Had Significant Negative Correlation with Chronological Age in All Studied Groups
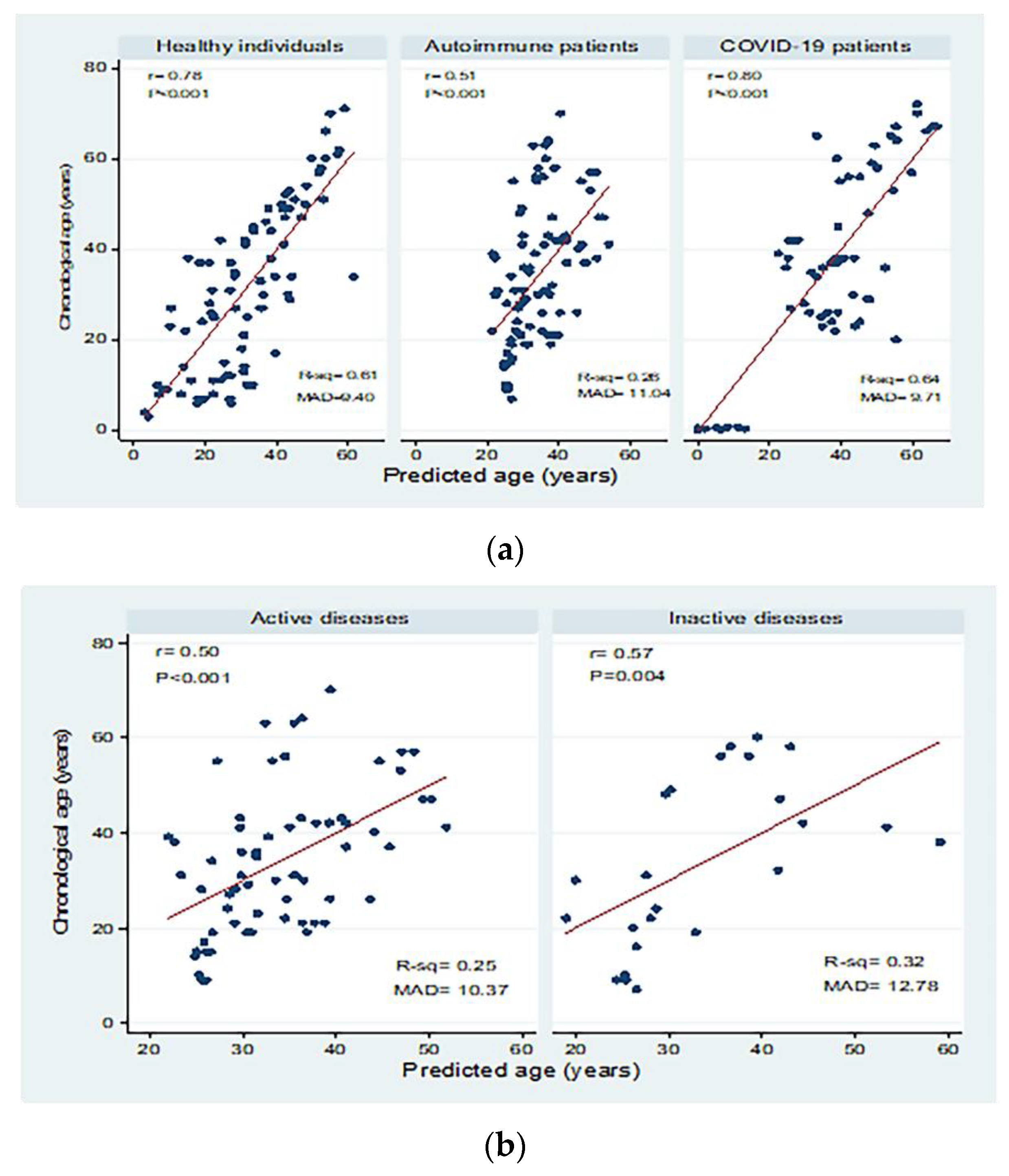
3.6. Peripheral sjTRECs Levels as a Biomarker for Age Prediction in All Studied Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freire-Aradas, A.; Phillips, C.; Lareu, M.V. Forensic individual age estimation with DNA: From initial approaches to methylation tests. Forensic Sci. Rev. 2017, 2, 121–144. [Google Scholar]
- Davis, P.J.; Hägg, U. The accuracy and precision of the “Demirjian system” when used for age determination in Chinese children. Swed. Dent. J. 1994, 18, 113–116. [Google Scholar] [PubMed]
- Zubakov, D.; Liu, F.; van Zelm, M.C.; Vermeulen, J.; Oostra, B.A.; van Duijn, C.M.; Driessen, G.J.; van Dongen, J.J.; Kayser, M.; Langerak, A.W. Estimating human age from T-cell DNA rearrangements. Curr. Biol. 2010, 20, R970–R971. [Google Scholar] [CrossRef]
- Li, Y. Recent thymic output function in patients with hematological malignancy. Hematology 2005, 10, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chinn, I.K.; Blackburn, C.C.; Manley, N.R.; Sempowski, G.D. Changes in primary lymphoid organs with aging. Semin. Immunol. 2012, 24, 309–320. [Google Scholar] [CrossRef]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- van den Dool, C.; de Boer, R.J. The effects of age, thymectomy, and HIV Infection on alpha and beta TCR excision circles in naive T cells. J. Immunol. 2006, 177, 4391–4401. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Gaballah, I.F.; Rashed, L.A. Age Estimation in Living Egyptians Using Signal Joint T-cell Receptor Excision Circle Rearrangement. J. Forensic Sci. 2016, 61, 1107–1111. [Google Scholar] [CrossRef]
- Pai, A.A.; Bell, J.T.; Marioni, J.C.; Pritchard, J.K.; Gilad, Y. A genome-wide study of DNA methylation patterns and gene expression levels in multiple human and chimpanzee tissues. PLoS Genet. 2011, 7, e1001316. [Google Scholar] [CrossRef]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Fransen, J.; Stucki, G.; van Riel, P.L.C.M. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis Care Res. 2003, 49, S214–S224. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.; Gladman, D.D.; Urowitz, M.B.; Caron, D.; Chang, C.H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992, 35, 630–640. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet 1990, 335, 1078–1080. [Google Scholar]
- Helliwell, P.S.; FitzGerald, O.; Fransen, J.; Gladman, D.D.; Kreuger, G.G.; Callis-Duffin, K.; McHugh, N.; Mease, P.J.; Strand, V.; Waxman, R.; et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann. Rheum. Dis. 2013, 72, 986–991. [Google Scholar] [CrossRef]
- Bhakta, B.B.; Brennan, P.; James, T.E.; Chamberlain, M.A.; Noble, B.A.; Silman, A.J. Behçet’s disease: Evaluation of a new instrument to measure clinical activity. Rheumatology 1999, 38, 728–733. [Google Scholar] [CrossRef]
- Consolaro, A.; Ruperto, N.; Bracciolini, G.; Frisina, A.; Gallo, M.C.; Pistorio, A.; Verazza, S.; Negro, G.; Gerloni, V.; Goldenstein-Schainberg, C.; et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann. Rheum. Dis. 2014, 73, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.L.; Gao, J.; Wang, H.; Wang, H.S.; Lu, H.L.; Sun, H.Y. Predicting human age with bloodstains by sjTREC quantification. PLoS ONE 2012, 7, e42412. [Google Scholar] [CrossRef][Green Version]
- Yamanoi, E.; Uchiyama, S.; Sakurada, M.; Ueno, Y. sjTREC quantification using SYBR quantitative PCR for age estimation of bloodstains in a Japanese population. Leg. Med. 2018, 32, 71–74. [Google Scholar] [CrossRef]
- Spólnicka, M.; Pośpiech, E.; Pepłońska, B.; Zbieć-Piekarska, R.; Makowska, Ż.; Pięta, A.; Karłowska-Pik, J.; Ziemkiewicz, B.; Wężyk, M.; Gasperowicz, P.; et al. DNA methylation in ELOVL2 and C1orf132 correctly predicted chronological age of individuals from three disease groups. Int. J. Leg. Med. 2018, 132, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Aljafary, M.A.; Alkhulaifi, F.M.; Aldossary, H.A.; Alshammari, T.; Al-Qaaneh, A.; Aldahhan, R.; Alkhalifah, Z.; Gaymalov, Z.Z.; Shehzad, A.; et al. Thymus Gland: A Double Edge Sword for Coronaviruses. Vaccines 2021, 9, 1119. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, A.R.; Morgan, T.A.; Anderson, A.; Catterall, J.; Patterson, A.M.; Foster, H.E.; Isaacs, J.D. Thymic function in juvenile idiopathic arthritis. Ann. Rheum. Dis. 2009, 68, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Güneş, H.; Dinçer, S.; Acıpayam, C.; Yurttutan, S.; Özkars, M.Y. What chances do children have against COVID-19? Is the answer hidden within the thymus? Eur. J. Pediatr. 2021, 180, 983–986. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, Y.C.; Chen, C.L.; Chiu, C.H. SARS-CoV-2 infection in children: Transmission dynamics and clinical characteristics. J. Formos. Med. Assoc. Taiwan Yi Zhi 2020, 119, 670–673. [Google Scholar] [CrossRef]
- Kayser, C.; Alberto, F.L.; da Silva, N.P.; Andrade, L.E. Decreased number of T cells bearing TCR rearrangement excision circles (TREC) in active recent onset systemic lupus erythematosus. Lupus 2004, 13, 906–911. [Google Scholar] [CrossRef]
- Koetz, K.; Bryl, E.; Spickschen, K.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9203–9208. [Google Scholar] [CrossRef]
- Thewissen, M.; Somers, V.; Venken, K.; Linsen, L.; van Paassen, P.; Geusens, P.; Damoiseaux, J.; Stinissen, P. Analyses of immunosenescent markers in patients with autoimmune disease. Clin. Immunol. 2007, 123, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Aili, A.; Sun, X.; Pang, X.; Ge, Q.; Zhang, Y.; Jin, R. Th1 Biased Progressive Autoimmunity in Aged Aire-Deficient Mice Accelerated Thymic Epithelial Cell Senescence. Aging Dis. 2019, 10, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Mok, C.; Chen, Z.; Feng, L.; Li, Z.; Huang, J.; Ke, C.-W.; Deng, X.; Ling, Y.; Wu, S.; et al. Characteristics of Traveler with Middle East Respiratory Syndrome, China, 2015. Emerg. Infect. Dis. 2015, 21, 2278–2280. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, C.; Dong, Q.; Zhuang, H.; Song, S.; Peng, G.; Dwyer, D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005, 9, 323–330. [Google Scholar] [CrossRef]
- Cuvelier, P.; Roux, H.; Couëdel-Courteille, A.; Dutrieux, J.; Naudin, C.; Charmeteau de Muylder, B.; Cheynier, R.; Squara, P.; Marullo, S. Protective reactive thymus hyperplasia in COVID-19 acute respiratory distress syndrome. Crit. Care 2021, 25, 4. [Google Scholar] [CrossRef]
- Ferrando-Martínez, S.; Franco, J.M.; Hernandez, A.; Ordoñez, A.; Gutierrez, E.; Abad, A.; Leal, M. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age 2009, 31, 87–97. [Google Scholar] [CrossRef]
- Cho, S.; Ge, J.; Seo, S.B.; Kim, K.; Lee, H.Y.; Lee, S.D. Age estimation via quantification of signal-joint T cell receptor excision circles in Koreans. Leg. Med. 2014, 16, 135–138. [Google Scholar] [CrossRef]
- Lang, P.O.; Mitchell, W.A.; Govind, S.; Aspinall, R. Real time-PCR assay estimating the naive T-cell pool in whole blood and dried blood spot samples: Pilot study in young adults. J. Immunol. Methods 2011, 369, 133–140. [Google Scholar] [CrossRef]
- Lorenzi, A.R.; Patterson, A.M.; Pratt, A.; Jefferson, M.; Chapman, C.E.; Ponchel, F.; Isaacs, J.D. Determination of thymic function directly from peripheral blood: A validated modification to an established method. J. Immunol. Methods 2008, 339, 185–194. [Google Scholar] [CrossRef]
- Pido-Lopez, J.; Imami, N.; Aspinall, R. Both age and gender affect thymic output: More recent thymic migrants in females than males as they age. Clin. Exp. Immunol. 2001, 125, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2011, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Just, H.L.; Deleuran, M.; Vestergaard, C.; Deleuran, B.; Thestrup-Pedersen, K. T-cell receptor excision circles (TREC) in CD4+ and CD8+ T-cell subpopulations in atopic dermatitis and psoriasis show major differences in the emission of recent thymic emigrants. Acta Derm.-Venereol. 2008, 88, 566–572. [Google Scholar] [CrossRef]
- Yap, H.Y.; Tee, S.Z.; Wong, M.M.; Chow, S.K.; Peh, S.C.; Teow, S.Y. Pathogenic Role of Immune Cells in Rheumatoid Arthritis: Implications in Clinical Treatment and Biomarker Development. Cells 2018, 7, 161. [Google Scholar] [CrossRef]
- Yuan, S.; Zeng, Y.; Li, J.; Wang, C.; Li, W.; He, Z.; Ye, J.; Li, F.; Chen, Y.; Lin, X.; et al. Phenotypical changes and clinical significance of CD4(+)/CD8(+) T cells in SLE. Lupus Sci. Med. 2022, 9, e000660. [Google Scholar] [CrossRef] [PubMed]
- Omoyinmi, E.; Hamaoui, R.; Pesenacker, A.; Nistala, K.; Moncrieffe, H.; Ursu, S.; Wedderburn, L.R.; Woo, P. Th1 and Th17 cell subpopulations are enriched in the peripheral blood of patients with systemic juvenile idiopathic arthritis. Rheumatology 2012, 51, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, D.; Yasuda, J.; Ikeshima-Kataoka, H.; Ozawa, Y.; Yoshida, K.; Yasuda, C.; Kingetsu, I.; Saito, S.; Yamada, A. Decreased numbers of signal-joint T cell receptor excision circle-containing CD4+ and CD8+ cells in systemic lupus erythematosus patients. Mod. Rheumatol. 2007, 17, 296–300. [Google Scholar] [CrossRef]
- Vieira, Q.F.; Kayser, C.; Kallas, E.G.; Andrade, L.E. Decreased recent thymus emigrant number is associated with disease activity in systemic lupus erythematosus. J. Rheumatol. 2008, 35, 1762–1767. [Google Scholar]
- Kong, F.K.; Chen, C.L.; Cooper, M.D. Reversible disruption of thymic function by steroid treatment. J. Immunol. 2002, 168, 6500–6505. [Google Scholar] [CrossRef]
- Crispin, J.C.; Alcocer-Varela, J.; de Pablo, P.; Martínez, A.; Richaud-Patin, Y.; Alarcón-Segovia, D. Immunoregulatory defects in patients with systemic lupus erythematosus in clinical remission. Lupus 2003, 12, 386–393. [Google Scholar] [CrossRef]
- Osborne, A.; Carrier, N.; de Brum-Fernandes, A.J.; Liang, P.; Masetto, A.; Boire, G. Quantification of T-Cell Receptor Excision Circles (TREC) from Peripheral Blood in Patients with Inflammatory Polyarthritis of Recent Onset (EPA): Association with Radiographic Joint Damage. In Arthritis & Rheumatology; Wiley-Blackwell: Hoboken, NJ, USA, 2015. [Google Scholar]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet. Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Zhou, J.; Wong, B.H.; Li, C.; Cheng, Z.S.; Lin, X.; Poon, V.K.; Sun, T.; Lau, C.C.; Chan, J.F.; et al. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology 2014, 454–455, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.-Y.; Cheng, Z.-S.; Poon, V.K.-M.; Sun, T.; Lau, C.C.-Y.; Wong, K.K.-Y.; Chan, J.Y.-W.; et al. Active Replication of Middle East Respiratory Syndrome Coronavirus and Aberrant Induction of Inflammatory Cytokines and Chemokines in Human Macrophages: Implications for Pathogenesis. J. Infect. Dis. 2013, 209, 1331–1342. [Google Scholar] [CrossRef]
- Chen, J.; Kelley, W.J.; Goldstein, D.R. Role of Aging and the Immune Response to Respiratory Viral Infections: Potential Implications for COVID-19. J. Immunol. 2020, 205, 313–320. [Google Scholar] [CrossRef]
- Wang, W.; Thomas, R.; Oh, J.; Su, D.M. Thymic Aging May Be Associated with COVID-19 Pathophysiology in the Elderly. Cells 2021, 10, 628. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R.; Tsokos, G.C. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 207. [Google Scholar] [CrossRef] [PubMed]

| Healthy Individuals (n = 85) | Autoimmune Patients (n = 90) | COVID-19 Patients (n = 58) | Test | p | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||||
| Gender | Females | 49 (57.65) | 54 (60.00) | 27 (46.55) | X2 = 2.77 | 0.25 |
| Males | 36 (42.35) | 36 (40.00) | 31 (53.45) | |||
| Age (years) | Subadults (<18) | 25 (29.41) | 13 (14.44) | 8 (13.79) | X2 = 12.31 | 0.06 |
| Young adults (18–34) | 23 (27.06) | 35 (38.89) | 16 (27.59) | |||
| Middle age (35–49) | 21 (24.71) | 26 (28.89) | 16 (27.59) | |||
| Older age ≥ 50 | 16 (18.82) | 16 (17.78) | 18 (31.03) | |||
| Mean ± SD Range | 31.79 ± 18.19 3–71 | 33.88 ± 15.57 7–70 | 37.35 ± 20.97 0.11–72 | F = 1.65 | 0.19 | |
| Healthy Individuals | Autoimmune Patients | COVID-19 Patients | F | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | ||||
| Age group (years) | Subadults (<18) | 25 | −7.23 ± 2.06 | 13 | −7.04 ± 0.36 | 8 | −7.07 ± 0.73 | 0.08 | 0.93 |
| Young adults (18–34) | 23 | −9.26 ± 2.48 ! | 35 | −10.14 ± 2.91 ! | 16 | −12.04 ± 1.16 ab! | 5.85 | 0.004 | |
| Middle age (35–49) | 21 | −9.86 ± 1.92 ! | 26 | −13.54 ± 4.73 a!¶ | 16 | −11.42 ± 1.34 ! | 7.24 | 0.001 | |
| Older age ≥ 50 | 16 | −13.52 ± 1.13 !¶‡ | 16 | −13.67 ± 3.49 !¶ | 18 | −14.07 ± 1.47 !¶‡ | 0.13 | 0.88 | |
| F | 31.79 | 13.97 | 56.54 | ||||||
| p | <0.001 | <0.001 | <0.001 | ||||||
| Gender | Females | 49 | −9.39 ± 2.68 | 54 | −12.07 ± 4.33 a | 27 | −11.66 ± 2.91 a | 8.18 | 0.0005 |
| Males | 36 | −9.93 ± 3.25 | 36 | −10.15 ± 3.64 | 31 | −11.95 ± 2.16 a | 4.09 | 0.02 | |
| t | 0.84 | 2.20 | 0.42 | ||||||
| p | 0.40 | 0.03 | 0.67 | ||||||
| Group | N | p | R2 | Adj. R2 | SE | MAD | B (95% CI) | Equation | |
|---|---|---|---|---|---|---|---|---|---|
| Healthy individuals | 85 | <0.001 | 0.61 | 0.60 | 11.42 | 9.40 | −4.85 (−5.70 to −4.01) | Y = −14.89 − 4.85X | |
| Autoimmune patients | Active | 66 | <0.001 | 0.25 | 0.24 | 12.99 | 10.37 | −1.75 (−2.51 to −0.99) | Y = 13.54 − 1.75X |
| Inactive | 24 | 0.004 | 0.32 | 0.29 | 14.89 | 12.78 | −2.62 (−4.29 to −0.95) | Y = 6.61 − 2.62X | |
| Total | 90 | <0.001 | 0.26 | 0.25 | 13.47 | 11.04 | −1.91 (−2.60 to −1.23) | Y = 12.25 − 1.91X | |
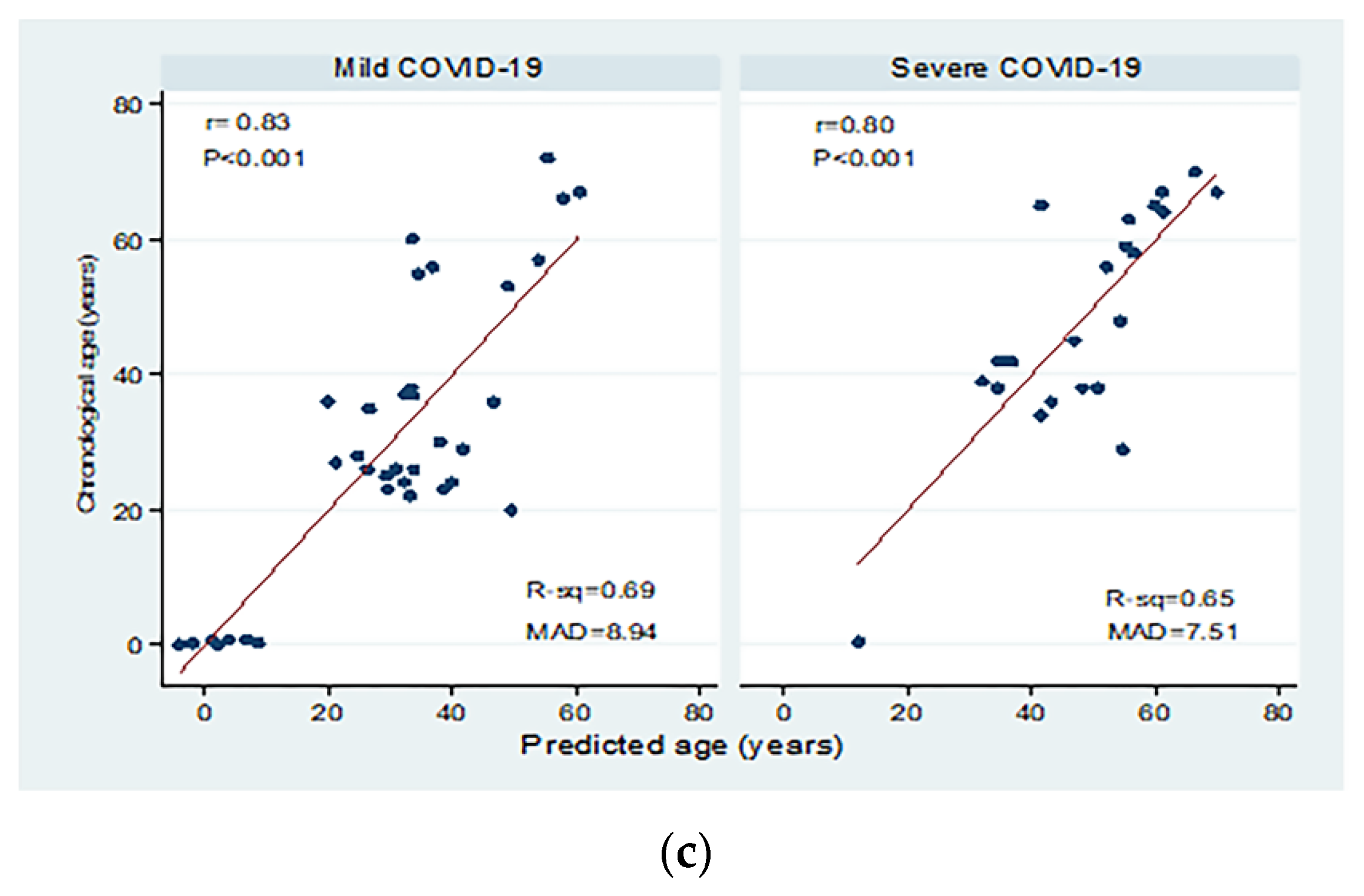
| COVID-19 patients | Mild | 35 | <0.001 | 0.69 | 0.68 | 11.69 | 8.94 | −6.48 (−8.01 to −4.95) | Y = −44.24 − 6.48X |
| Severe | 23 | <0.001 | 0.65 | 0.63 | 10.05 | 7.51 | −5.91 (−7.89 to −3.93) | Y = −24.56 − 5.91X | |
| Total | 58 | <0.001 | 0.64 | 0.64 | 12.60 | 9.71 | −6.68 (−8.01 to −5.36) | Y = −41.63 − 6.68X | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, A.A.; Kharboush, T.G.; Ibrahim, N.H.; Darwish, M.; Fawzy, I.M.; Bayomy, H.E.-S.; Abdelmotaleb, D.S.; Abdul Basset, S.A.B.; Abdel-Kareim, A.M.; Al mohaini, M.; et al. Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence. Biomedicines 2022, 10, 3193. https://doi.org/10.3390/biomedicines10123193
Farag AA, Kharboush TG, Ibrahim NH, Darwish M, Fawzy IM, Bayomy HE-S, Abdelmotaleb DS, Abdul Basset SAB, Abdel-Kareim AM, Al mohaini M, et al. Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence. Biomedicines. 2022; 10(12):3193. https://doi.org/10.3390/biomedicines10123193
Chicago/Turabian StyleFarag, Amina A., Taghrid G. Kharboush, Noha H. Ibrahim, Mohamed Darwish, Iman M. Fawzy, Hanaa El-Sayed Bayomy, Dina Saad Abdelmotaleb, Shaza Abdul Basset Abdul Basset, Amal M. Abdel-Kareim, Mohammed Al mohaini, and et al. 2022. "Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence" Biomedicines 10, no. 12: 3193. https://doi.org/10.3390/biomedicines10123193
APA StyleFarag, A. A., Kharboush, T. G., Ibrahim, N. H., Darwish, M., Fawzy, I. M., Bayomy, H. E.-S., Abdelmotaleb, D. S., Abdul Basset, S. A. B., Abdel-Kareim, A. M., Al mohaini, M., Ahmed, I. A., & Fakher, H. M. (2022). Exploiting Signal Joint T Cell Receptor Excision Circle to Investigate the Impact of COVID-19 and Autoimmune Diseases on Age Prediction and Immunosenescence. Biomedicines, 10(12), 3193. https://doi.org/10.3390/biomedicines10123193






