Long-Term Synaptic Plasticity Tunes the Gain of Information Channels through the Cerebellum Granular Layer
Abstract
1. Introduction
2. Materials and Methods
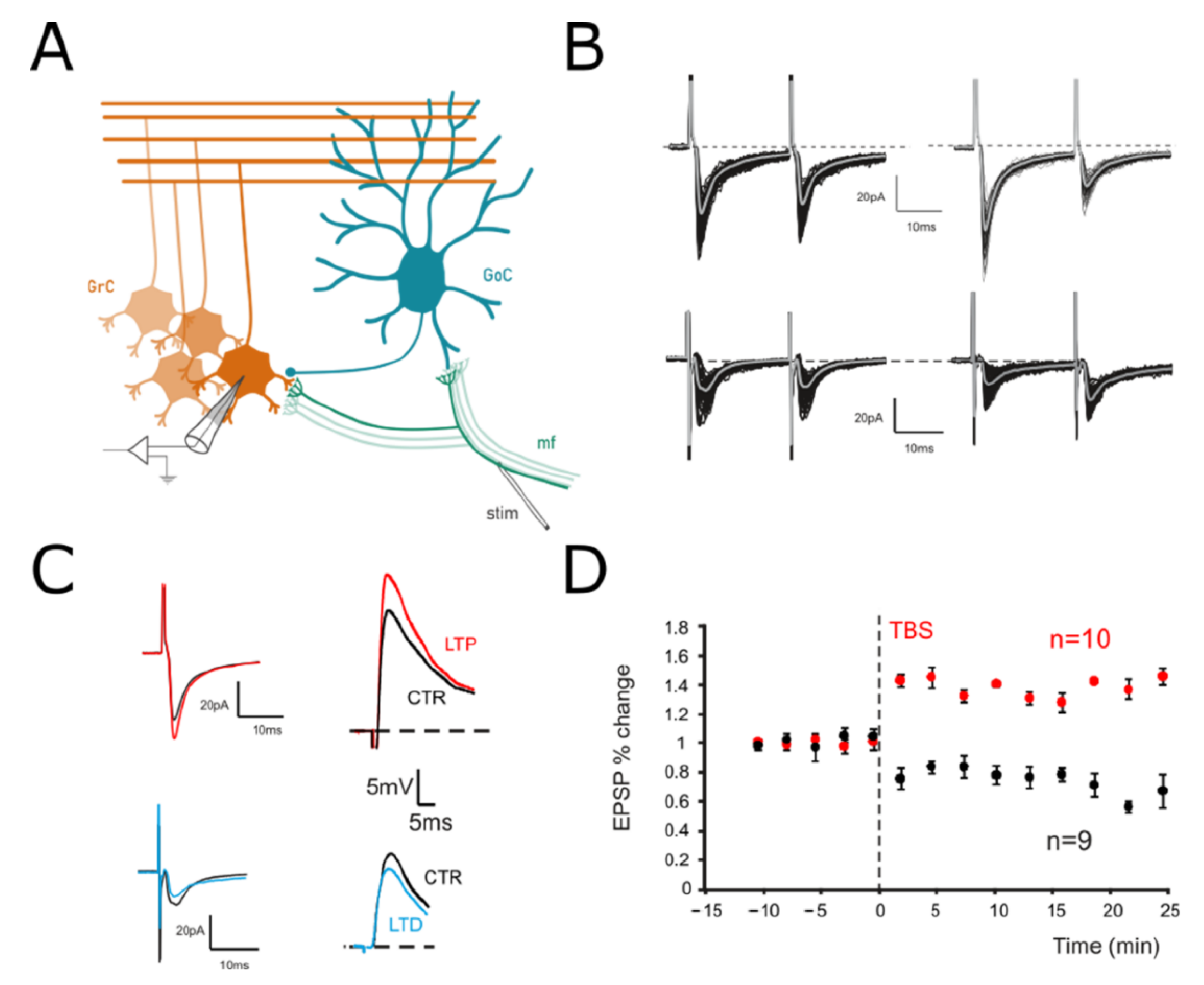
2.1. Recordings in Acute Cerebellar Slices
2.2. Long-Term Plasticity Induction and Analysis
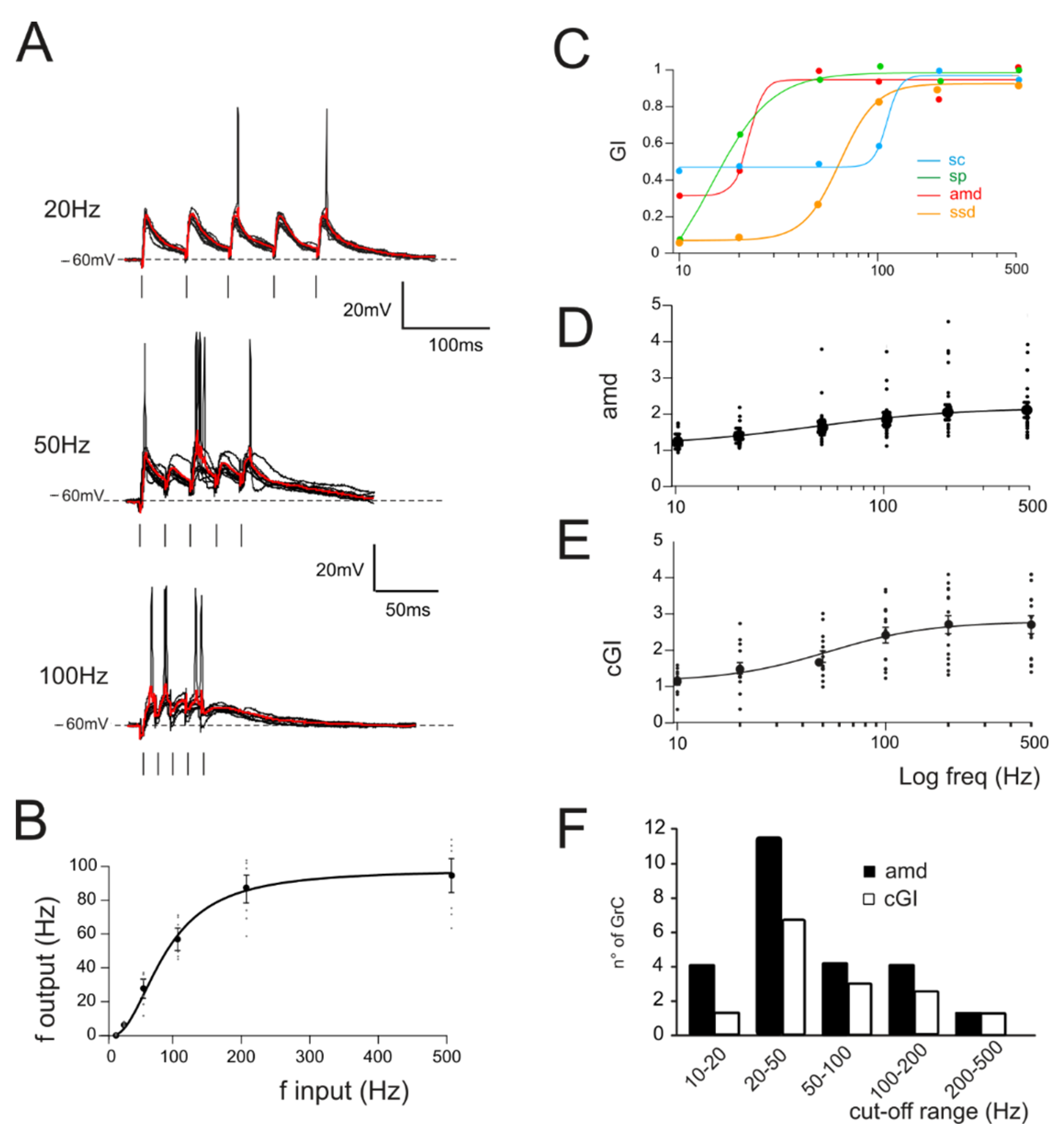
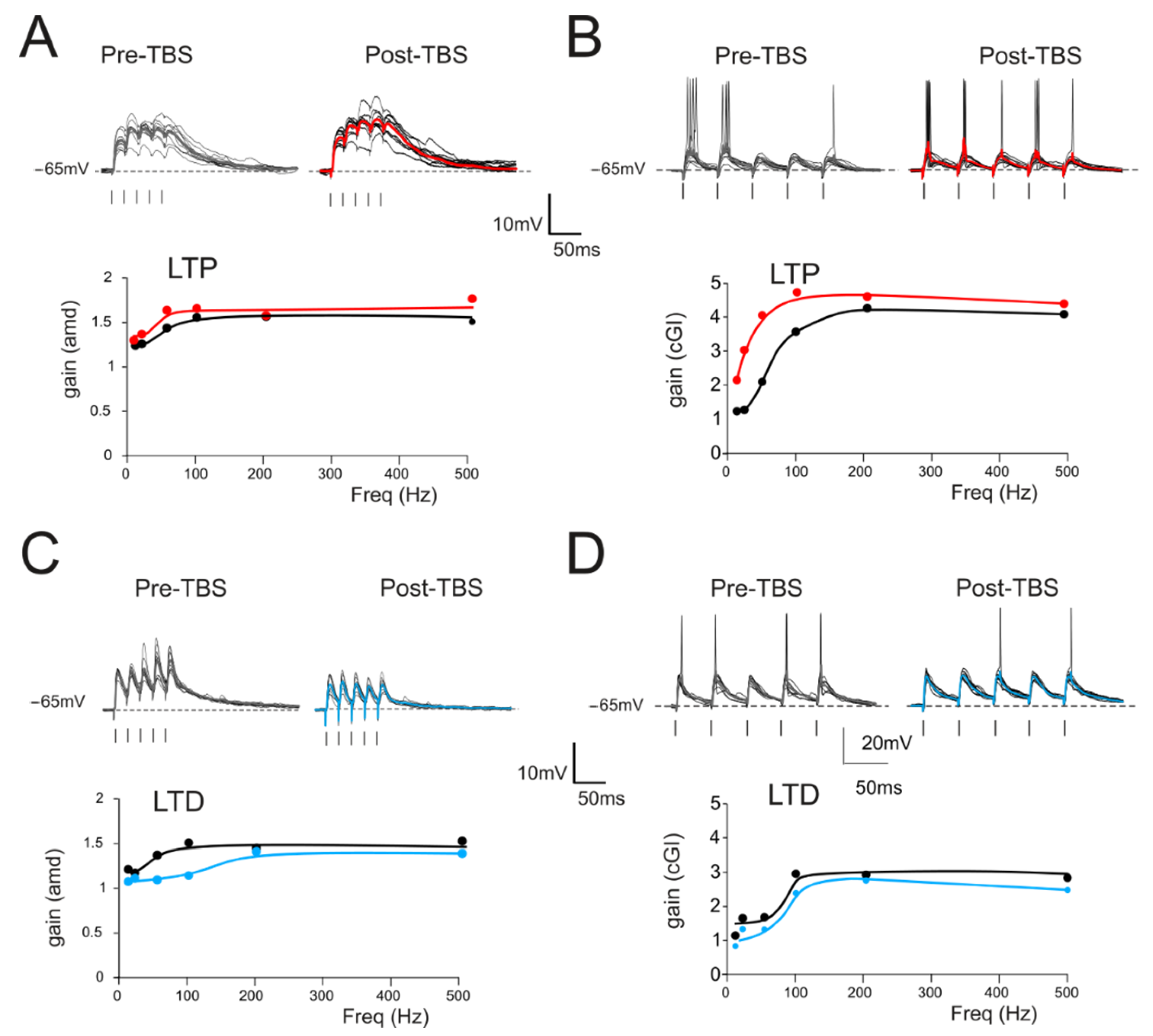
2.3. Gain Function Analysis
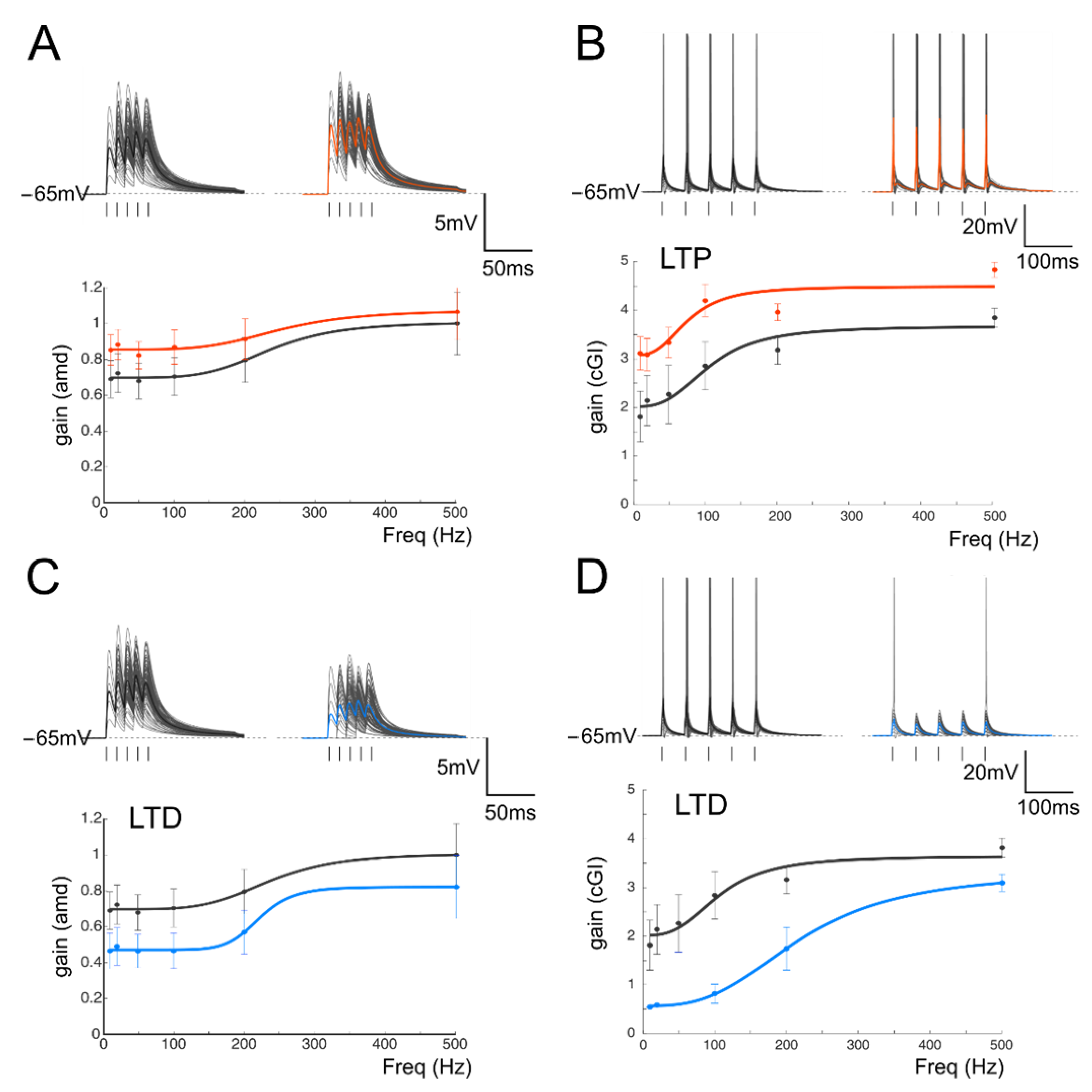
2.4. Mathematical Modeling
Synaptic Dynamics
3. Results
3.1. The Effect of Long-Term Synaptic Plasticity on a Single Granule Cell
3.2. The Impact of LTP and LTD on Filtering Properties
3.3. Mathematical Modeling of the Impact of Plasticity on Filtering Properties
4. Discussion
4.1. Gain Control by Synaptic Plasticity
4.2. Functional Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.S.; Lampl, I.; Gillespie, D.C.; Ferster, D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 2000, 290, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Chance, F.S.; Abbott, L.; Reyes, A.D. Gain Modulation from Background Synaptic Input. Neuron 2002, 35, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.S.; Cathala, L.; Steuber, V.; Silver, R.A. Synaptic depression enables neuronal gain control. Nature 2009, 457, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.R.; Koch, C. Shunting inhibition does not have a divisive effect on firing rates. Neural Comput. 1997, 9, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, D.; Mapelli, J.; D’Angelo, E. Long-Term Spatiotemporal Reconfiguration of Neuronal Activity Revealed by Voltage-Sensitive Dye Imaging in the Cerebellar Granular Layer. Neural Plast. 2015, 2015, 284986. [Google Scholar] [CrossRef]
- Bliss, T.V.; Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaes-thetized rabbit following stimulation of the perforant path. J. Physiol. 1973, 232, 331–356. [Google Scholar] [CrossRef]
- Mapelli, J.; Gandolfi, D.; Vilella, A.; Zoli, M.; Bigiani, A. Heterosynaptic GABAergic plasticity bidirec-tionally driven by the activity of pre- and postsynaptic NMDA receptors. Proc. Natl. Acad. Sci. USA 2016, 113, 9898–9903. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993, 16, 521–527. [Google Scholar] [CrossRef]
- Romano, V.; de Propris, L.; Bosman, L.W.; Warnaar, P.; ten Brinke, M.M.; Lindeman, S.; Ju, C.; Velau-thapillai, A.; Spanke, J.K.; Middendorp Guerra, E.; et al. Potentiation of cerebellar Purkinje cells facilitates whisker reflex adaptation through increased simple spike activity. eLife 2018, 7, e38852. [Google Scholar] [CrossRef]
- Casali, S.; Tognolina, M.; Gandolfi, D.; Mapelli, J.; D’Angelo, E. Cellular-resolution mapping uncovers spatial adaptive filtering at the rat cerebellum input stage. Commun. Biol. 2020, 3, 635. [Google Scholar] [CrossRef]
- Gandolfi, D.; Bigiani, A.; Porro, C.A.; Mapelli, J. Inhibitory Plasticity: From Molecules to Computation and Beyond. IJMS 2020, 21, 1805. [Google Scholar] [CrossRef]
- Bear, M.F.; Malenka, R.C. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol. 1994, 4, 389–399. [Google Scholar] [CrossRef]
- Steuber, V.; Mittmann, W.; Hoebeek, F.E.; Silver, R.A.; de Zeeuw, C.I.; Häusser, M.; Schutter, E. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron 2007, 54, 121–136. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Malenka, R.C. Striatal plasticity and basal ganglia circuit function. Neuron 2008, 60, 543–554. [Google Scholar] [CrossRef]
- Kohonen, T.; Oja, E. Computing with neural networks. Science 1987, 235, 1227a. [Google Scholar] [CrossRef][Green Version]
- Lehky, S.R.; Sejnowski, T.J.; Desimone, R. Predicting responses of nonlinear neurons in monkey striate cortex to complex patterns. J. Neurosci. 1992, 12, 3568–3581. [Google Scholar] [CrossRef]
- Hopfield, J.J. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl. Acad. Sci. USA 1982, 79, 2554–2558. [Google Scholar] [CrossRef]
- Mittmann, W.; Häusser, M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J. Neurosci. 2007, 27, 5559–5570. [Google Scholar] [CrossRef]
- Mapelli, J.; Gandolfi, D.; D’Angelo, E. Combinatorial responses controlled by synaptic inhibition in the cerebellum granular layer. J. Neurophysiol. 2010, 103, 250–261. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Silver, R. Shunting Inhibition Modulates Neuronal Gain during Synaptic Excitation. Neuron 2003, 38, 433–445. [Google Scholar] [CrossRef]
- D’Angelo, E.; de Filippi, G.; Rossi, P.; Taglietti, V. Synaptic excitation of individual rat cerebellar granule cells in situ: Evidence for the role of NMDA receptors. J. Physiol. 1995, 484 Pt 2, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.J.; Rothman, J.S.; Dugué, G.P.; Diana, M.; Rousseau, C.; Silver, R.A.; Dieudonné, S. NMDA receptors with incomplete Mg2⁺ block enable low-frequency transmission through the cerebellar cortex. J. Neurosci. 2012, 32, 6878–6893. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, J.; Gandolfi, D.; D’Angelo, E. High-Pass Filtering and Dynamic Gain Regulation Enhance Vertical Bursts Transmission along the Mossy Fiber Pathway of Cerebellum. Front. Cell. Neurosci. 2010, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Rossi, P.; Armano, S.; Taglietti, V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J. Neurophysiol. 1999, 81, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Gall, D.; Prestori, F.; Sola, E.; D’Errico, A.; Roussel, C.; Forti, L.; Rossi, P.; D’Angelo, E. Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J. Neurosci. 2005, 25, 4813–4822. [Google Scholar] [CrossRef]
- Gandolfi, D.; Cerri, S.; Mapelli, J.; Polimeni, M.; Tritto, S.; Fuzzati-Armentero, M.-T.; Bigiani, A.; Blandini, F.; Mapelli, L.; D’Angelo, E. Activation of the CREB/c-Fos Pathway during Long-Term Synaptic Plasticity in the Cerebellum Granular Layer. Front. Cell. Neurosci. 2017, 11, 184. [Google Scholar] [CrossRef]
- Locatelli, F.; Soda, T.; Montagna, I.; Tritto, S.; Botta, L.; Prestori, F.; D’Angelo, E. Calcium Channel-Dependent Induction of Long-Term Synaptic Plasticity at Excitatory Golgi Cell Synapses of Cerebellum. J. Neurosci. 2021, 41, 3307–3319. [Google Scholar] [CrossRef]
- Nieus, T.; Sola, E.; Mapelli, J.; Saftenku, E.; Rossi, P.; D’Angelo, E. LTP regulates burst initiation and frequency at mossy fiber-granule cell synapses of rat cerebellum: Experimental observations and theoretical predictions. J. Neurophysiol. 2006, 95, 686–699. [Google Scholar] [CrossRef]
- Arleo, A.; Nieus, T.; Bezzi, M.; D’Errico, A.; D’Angelo, E.; Coenen, O.J.-M.D. How synaptic release probability shapes neuronal transmission: Information-theoretic analysis in a cerebellar granule cell. Neural Comput. 2010, 22, 2031–2058. [Google Scholar] [CrossRef]
- Garthwaite, J.; Brodbelt, A.R. Synaptic activation of N-methyl-d-aspartate and non-N-methyl-d-aspartate receptors in the mossy fibre pathway in adult and immature rat cerebellar slices. Neuroscience 1989, 29, 401–412. [Google Scholar] [CrossRef]
- Solinas, S.; Nieus, T.; D’Angelo, E. A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal filtering properties. Front. Cell. Neurosci. 2010, 4, 12. [Google Scholar] [CrossRef]
- Cesana, E.; Pietrajtis, K.; Bidoret, C.; Isope, P.; D’Angelo, E.; Dieudonné, S.; Forti, L. Granule cell ascending axon excitatory synapses onto Golgi cells implement a potent feedback circuit in the cerebellar granular layer. J. Neurosci. 2013, 33, 12430–12446. [Google Scholar] [CrossRef]
- Mapelli, J.; D’Angelo, E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J. Neurosci. 2007, 27, 1285–1296. [Google Scholar] [CrossRef]
- Heck, D.H.; de Zeeuw, C.I.; Jaeger, D.; Khodakhah, K.; Person, A.L. The neuronal code(s) of the cerebellum. J. Neurosci. 2013, 33, 17603–17609. [Google Scholar] [CrossRef]
- Dean, P.; Porrill, J. Evaluating the adaptive-filter model of the cerebellum. J. Physiol. 2011, 589, 3459–3470. [Google Scholar] [CrossRef]
- Mapelli, J.; Gandolfi, D.; Giuliani, E.; Casali, S.; Congi, L.; Barbieri, A.; D’Angelo, E.; Bigiani, A. The effects of the general anesthetic sevoflurane on neurotransmission: An experimental and computational study. Sci. Rep. 2021, 11, 4335. [Google Scholar] [CrossRef]
- Mapelli, J.; Gandolfi, D.; Giuliani, E.; Prencipe, F.P.; Pellati, F.; Barbieri, A.; D’Angelo, E.; Bigiani, A. The effect of desflurane on neuronal communication at a central synapse. PLoS ONE 2015, 10, e0123534. [Google Scholar] [CrossRef]
- Fassio, A.; Merlo, D.; Mapelli, J.; Menegon, A.; Corradi, A.; Mete, M.; Zappettini, S.; Bonanno, G.; Valtorta, F.; D’Angelo, E.; et al. The synapsin domain E accelerates the exoendocytotic cycle of synaptic vesicles in cerebellar Purkinje cells. J. Cell Sci. 2006, 119, 4257–4268. [Google Scholar] [CrossRef][Green Version]
- Prestori, F.; Bonardi, C.; Mapelli, L.; Lombardo, P.; Goselink, R.; de Stefano, M.E.; Gandolfi, D.; Mapelli, J.; Bertrand, D.; Schonewille, M.; et al. Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS ONE 2013, 8, e64828. [Google Scholar] [CrossRef]
- D’Angelo, E.; Rossi, P.; Taglietti, V. Different proportions of N-methyl-d-aspartate and non-N-methyl-d-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience 1993, 53, 121–130. [Google Scholar] [CrossRef]
- Silver, R.A.; Colquhoun, D.; Cull-Candy, S.G.; Edmonds, B. Deactivation and desensitization of non-NMDA receptors in patches and the time course of EPSCs in rat cerebellar granule cells. J. Physiol. 1996, 493 Pt 1, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sola, E.; Prestori, F.; Rossi, P.; Taglietti, V.; D’Angelo, E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J. Physiol. 2004, 557, 843–861. [Google Scholar] [CrossRef] [PubMed]
- Armano, S.; Rossi, P.; Taglietti, V.; D’Angelo, E. Long-Term Potentiation of Intrinsic Excitability at the Mossy Fiber–Granule Cell Synapse of Rat Cerebellum. J. Neurosci. 2000, 20, 5208–5216. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, S.; Magistretti, J.; Goldfarb, M.; Naldi, G.; D’Angelo, E. Axonal Na+ channels ensure fast spike activation and back-propagation in cerebellar granule cells. J. Neurophysiol. 2009, 101, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Chadderton, P.; Margrie, T.W.; Häusser, M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 2004, 428, 856–860. [Google Scholar] [CrossRef]
- D’Angelo, E.; Nieus, T.; Maffei, A.; Armano, S.; Rossi, P.; Taglietti, V.; Fontana, A.; Naldi, G. Theta-frequency bursting and resonance in cerebellar granule cells: Experimental evidence and modeling of a slow k+-dependent mechanism. J. Neurosci. 2001, 21, 759–770. [Google Scholar] [CrossRef]
- Tsodyks, M.; Uziel, A.; Markram, H. Synchrony generation in recurrent networks with frequency-dependent synapses. J. Neurosci. 2000, 20, RC50. [Google Scholar] [CrossRef]
- Solinas, S.; Forti, L.; Cesana, E.; Mapelli, J.; de Schutter, E.; D’Angelo, E. Fast-reset of pacemaking and theta-frequency resonance patterns in cerebellar golgi cells: Simulations of their impact in vivo. Front. Cell. Neurosci. 2007, 1, 4. [Google Scholar] [CrossRef]
- Solinas, S.; Forti, L.; Cesana, E.; Mapelli, J.; de Schutter, E.; D’Angelo, E. Computational reconstruction of pacemaking and intrinsic electroresponsiveness in cerebellar golgi cells. Front. Cell. Neurosci. 2007, 1, 2. [Google Scholar] [CrossRef]
- Vos, B.P.; Volny-Luraghi, A.; Maex, R.; De Schutter, E. Precise spike timing of tactile-evoked cerebellar Golgi cell responses: A reflection of combined mossy fiber and parallel fiber activation? Prog. Brain Res. 2000, 124, 95–106. [Google Scholar] [CrossRef]
- Jörntell, H.; Ekerot, C.F. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J. Neurosci. 2006, 26, 11786–11797. [Google Scholar] [CrossRef]
- Nieus, T.R.; Mapelli, L.; D’Angelo, E. Regulation of output spike patterns by phasic inhibition in cerebellar granule cells. Front. Cell. Neurosci. 2014, 8, 246. [Google Scholar] [CrossRef]
- Mapelli, L.; Rossi, P.; Nieus, T.; D’Angelo, E. Tonic Activation of GABA(B) Receptors Reduces Release Probability at Inhibitory Connections in the Cerebellar Glomerulus. J. Neurophysiol. 2009, 101, 3089–3099. [Google Scholar] [CrossRef]
- Gandolfi, D.; Boiani, G.M.; Bigiani, A.; Mapelli, J. Modeling Neurotransmission: Computational Tools to Investigate Neurological Disorders. IJMS 2021, 22, 4565. [Google Scholar] [CrossRef]
- Gandolfi, D.; Pozzi, P.; Tognolina, M.; Chirico, G.; Mapelli, J.; D’Angelo, E. The spatiotemporal organization of cerebellar network activity resolved by two-photon imaging of multiple single neurons. Front. Cell. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef]
- Pozzi, P.; Gandolfi, D.; Tognolina, M.; Chirico, G.; Mapelli, J.; D’Angelo, E. High-throughput spatial light modulation two-photon microscopy for fast functional imaging. Neurophotonics 2015, 2, 15005. [Google Scholar] [CrossRef]
- Kuhn, B.; Denk, W.; Bruno, R.M. In vivo two-photon voltage-sensitive dye imaging reveals top-down control of cortical layers 1 and 2 during wakefulness. Proc. Natl. Acad. Sci. USA 2008, 105, 7588–7593. [Google Scholar] [CrossRef]
- Konnerth, A.; Orkand, R.K. Voltage-sensitive dyes measure potential changes in axons and glia of the frog optic nerve. Neurosci. Lett. 1986, 66, 49–54. [Google Scholar] [CrossRef]
- Markram, H.; Muller, E.; Ramaswamy, S.; Reimann, M.W.; Abdellah, M.; Sanchez, C.A.; Ailamaki, A.; Alonso-Nanclares, L.; Antille, N.; Arsever, S.; et al. Reconstruction and Simulation of Neocortical Mi-crocircuitry. Cell 2015, 163, 456–492. [Google Scholar] [CrossRef]
- D’Errico, A.; Prestori, F.; D’Angelo, E. Differential induction of bidirectional long-term changes in neurotransmitter release by frequency-coded patterns at the cerebellar input. J. Physiol. 2009, 587, 5843–5857. [Google Scholar] [CrossRef]
- Forti, L.; Cesana, E.; Mapelli, J.; D’Angelo, E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J. Physiol. 2006, 574, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Kanichay, R.T.; Silver, R.A. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J. Neurosci. 2008, 28, 8955–8967. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, D.; Lombardo, P.; Mapelli, J.; Solinas, S.; D’Angelo, E. θ-Frequency resonance at the cerebellum input stage improves spike timing on the millisecond time-scale. Front. Neural Circuits 2013, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Marr, D. A theory of cerebellar cortex. J. Physiol. 1969, 202, 437–470. [Google Scholar] [CrossRef] [PubMed]
- Albus, J.S. A theory of cerebellar function. Math. Biosci. 1971, 10, 25–61. [Google Scholar] [CrossRef]
- D’Angelo, E.; Mazzarello, P.; Prestori, F.; Mapelli, J.; Solinas, S.; Lombardo, P.; Cesana, E.; Gandolfi, D.; Congi, L. The cerebellar network: From structure to function and dynamics. Brain Res. Rev. 2011, 66, 5–15. [Google Scholar] [CrossRef]
- Kase, M.; Miller, D.C.; Noda, H. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J. Physiol. 1980, 300, 539–555. [Google Scholar] [CrossRef]
- Lisberger, S.G.; Fuchs, A.F. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J. Neurophysiol. 1978, 41, 764–777. [Google Scholar] [CrossRef]
- Rancz, E.A.; Ishikawa, T.; Duguid, I.; Chadderton, P.; Mahon, S.; Häusser, M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 2007, 450, 1245–1248. [Google Scholar] [CrossRef]
- D’Angelo, E.; Solinas, S.; Mapelli, J.; Gandolfi, D.; Mapelli, L.; Prestori, F. The cerebellar Golgi cell and spatiotemporal organization of granular layer activity. Front. Neural Circuits 2013, 7, 93. [Google Scholar] [CrossRef]
- D’Angelo, E.; Solinas, S.; Garrido, J.; Casellato, C.; Pedrocchi, A.; Mapelli, J.; Gandolfi, D.; Prestori, F. Realistic modeling of neurons and networks: Towards brain simulation. Funct. Neurol. 2013, 28, 153–166. [Google Scholar] [CrossRef]
- Gandolfi, D.; Mapelli, J.; Solinas, S.; de Schepper, R.; Geminiani, A.; Casellato, C.; D’Angelo, E.; Migliore, M. A realistic morpho-anatomical connection strategy for modelling full-scale point-neuron microcircuits. Sci. Rep. 2022, 12, 13864. [Google Scholar] [CrossRef]
- Gandolfi, D.; Puglisi, F.M.; Boiani, G.M.; Pagnoni, G.; Friston, K.J.; D’Angelo, E.; Mapelli, J. Emergence of associative learning in a neuromorphic inference network. J. Neural Eng. 2022, 19, 036022. [Google Scholar] [CrossRef]
- Florini, D.; Gandolfi, D.; Mapelli, J.; Benatti, L.; Pavan, P.; Puglisi, F.M. A Hybrid CMOS-Memristor Spiking Neural Network Supporting Multiple Learning Rules. IEEE Trans. Neural Netw. Learn. Syst. 2022. [Google Scholar] [CrossRef]



| Ionic Channel | Conductance, nS | Localization |
|---|---|---|
| Na | 8.589 | Axon |
| KDR K-A K-IR K-Ca Ca K-slow Lkg1 Lkg2 | 2.338 2.336 3.382 0.951 0.707 0.108 0.264 0.17 0.042 | Hillock Axon, Hillock Soma Soma Dend(4) Dend(4) Soma All compartments Dend(1,2,3,4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapelli, J.; Boiani, G.M.; D’Angelo, E.; Bigiani, A.; Gandolfi, D. Long-Term Synaptic Plasticity Tunes the Gain of Information Channels through the Cerebellum Granular Layer. Biomedicines 2022, 10, 3185. https://doi.org/10.3390/biomedicines10123185
Mapelli J, Boiani GM, D’Angelo E, Bigiani A, Gandolfi D. Long-Term Synaptic Plasticity Tunes the Gain of Information Channels through the Cerebellum Granular Layer. Biomedicines. 2022; 10(12):3185. https://doi.org/10.3390/biomedicines10123185
Chicago/Turabian StyleMapelli, Jonathan, Giulia Maria Boiani, Egidio D’Angelo, Albertino Bigiani, and Daniela Gandolfi. 2022. "Long-Term Synaptic Plasticity Tunes the Gain of Information Channels through the Cerebellum Granular Layer" Biomedicines 10, no. 12: 3185. https://doi.org/10.3390/biomedicines10123185
APA StyleMapelli, J., Boiani, G. M., D’Angelo, E., Bigiani, A., & Gandolfi, D. (2022). Long-Term Synaptic Plasticity Tunes the Gain of Information Channels through the Cerebellum Granular Layer. Biomedicines, 10(12), 3185. https://doi.org/10.3390/biomedicines10123185







