Local Glucocorticoid Administration Impairs Embryonic Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of Dexamethasone-Beads
2.3. Chicken Embryo Treatment and In-Ovo Bead Implantation
2.4. Histological Analysis
2.5. Immunohistochemical Analysis
2.6. Whole Mount In-Situ Hybridization (ISH)
2.7. Statistical Analysis
3. Results
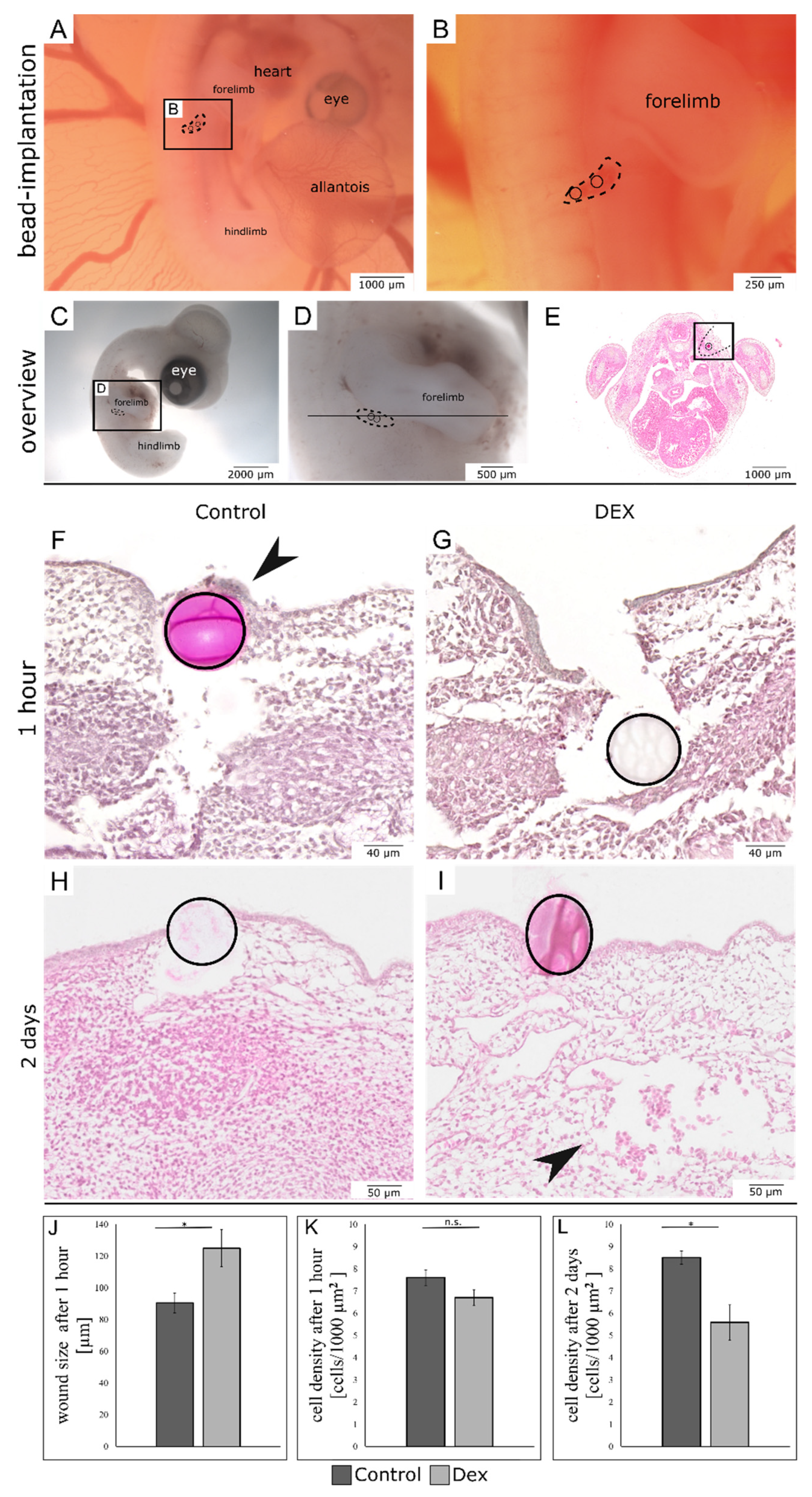
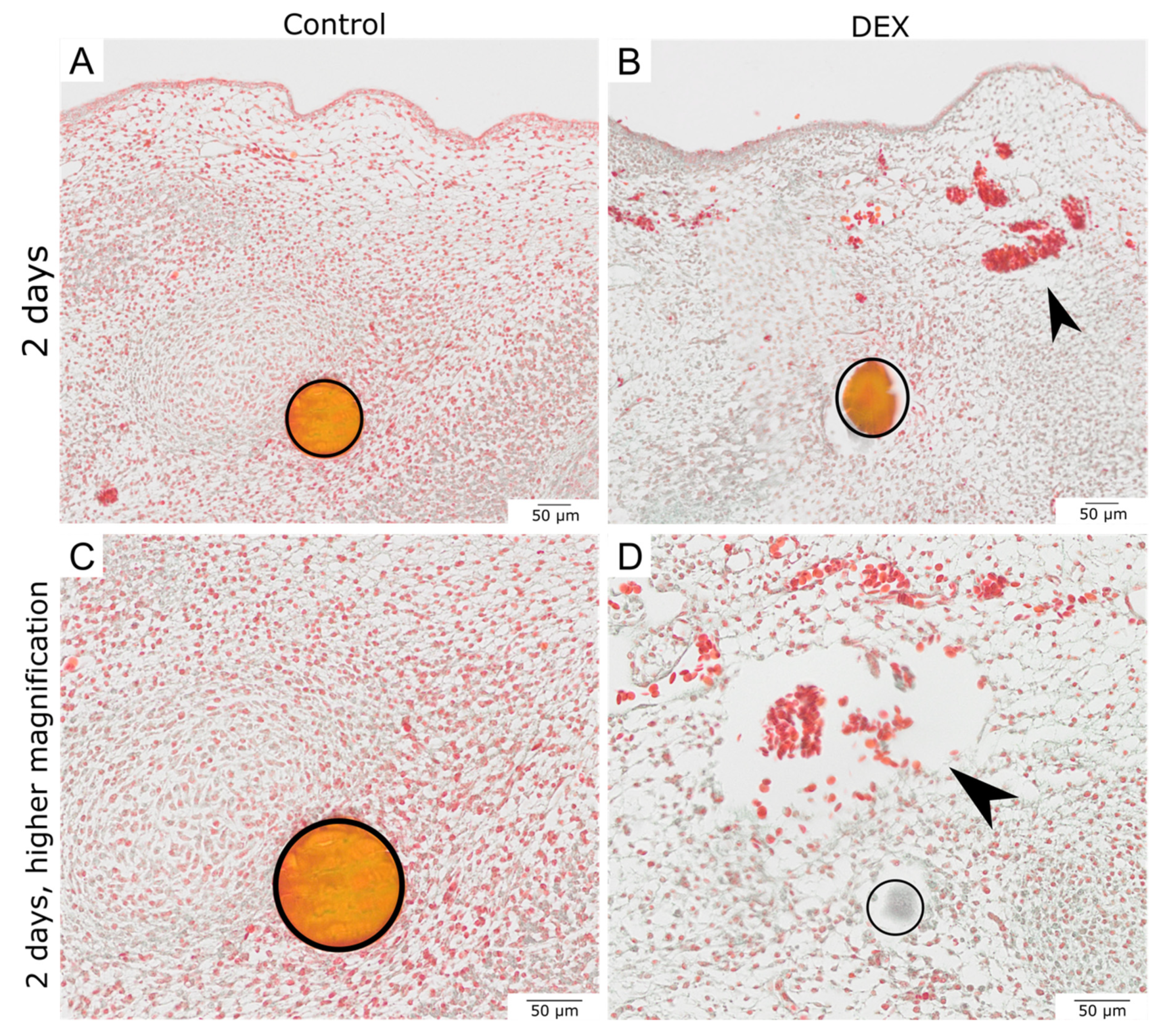
3.1. Local Dexamethasone Administration Impairs Embryonic Wound Healing
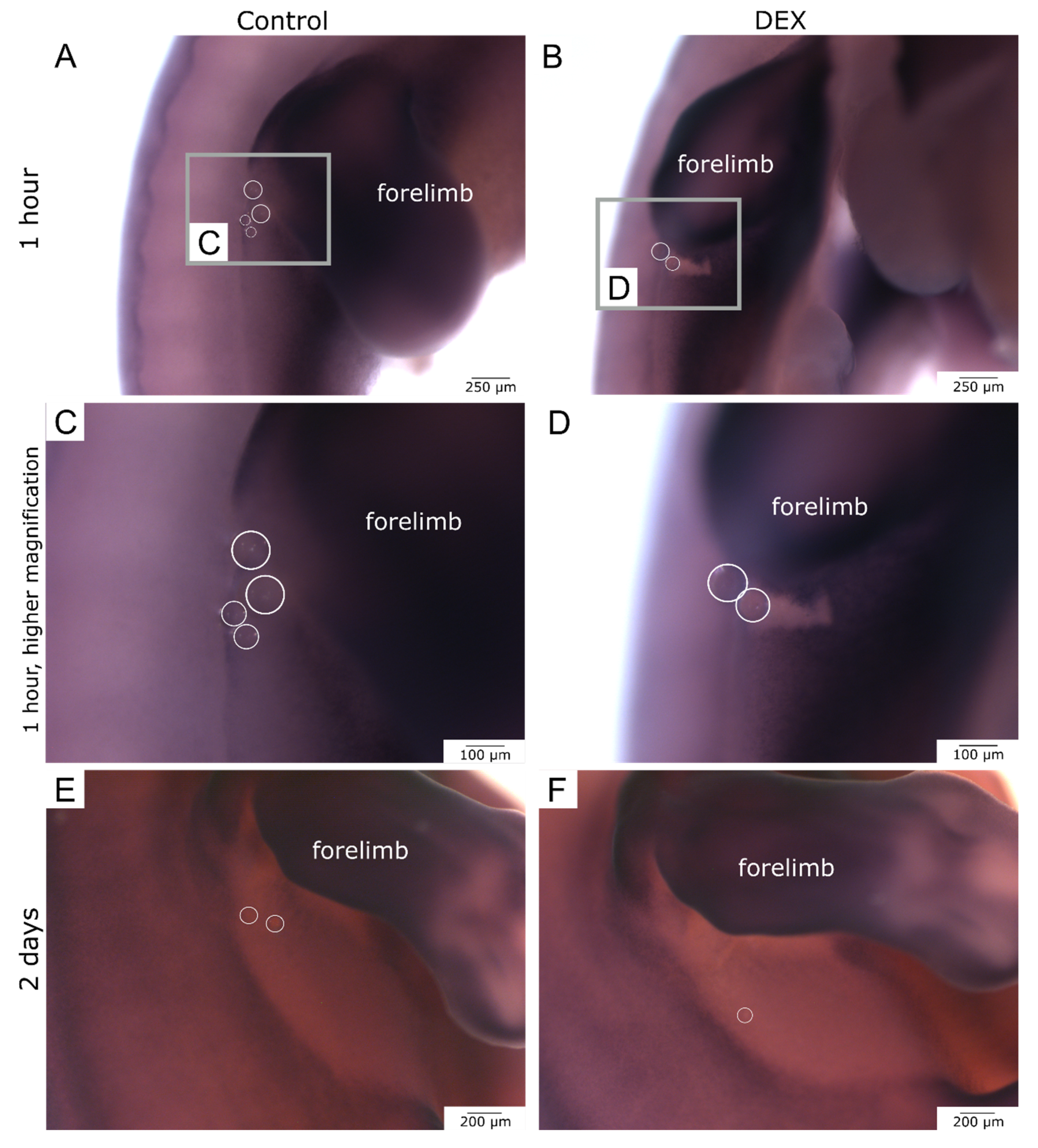
3.2. Local Dexamethasone Administration Reduces Vimentin Expression in Embryonic Wounds
3.3. Local Dexamethasone Administration Reduces Fibronectin Expression in Embryonic Wounds
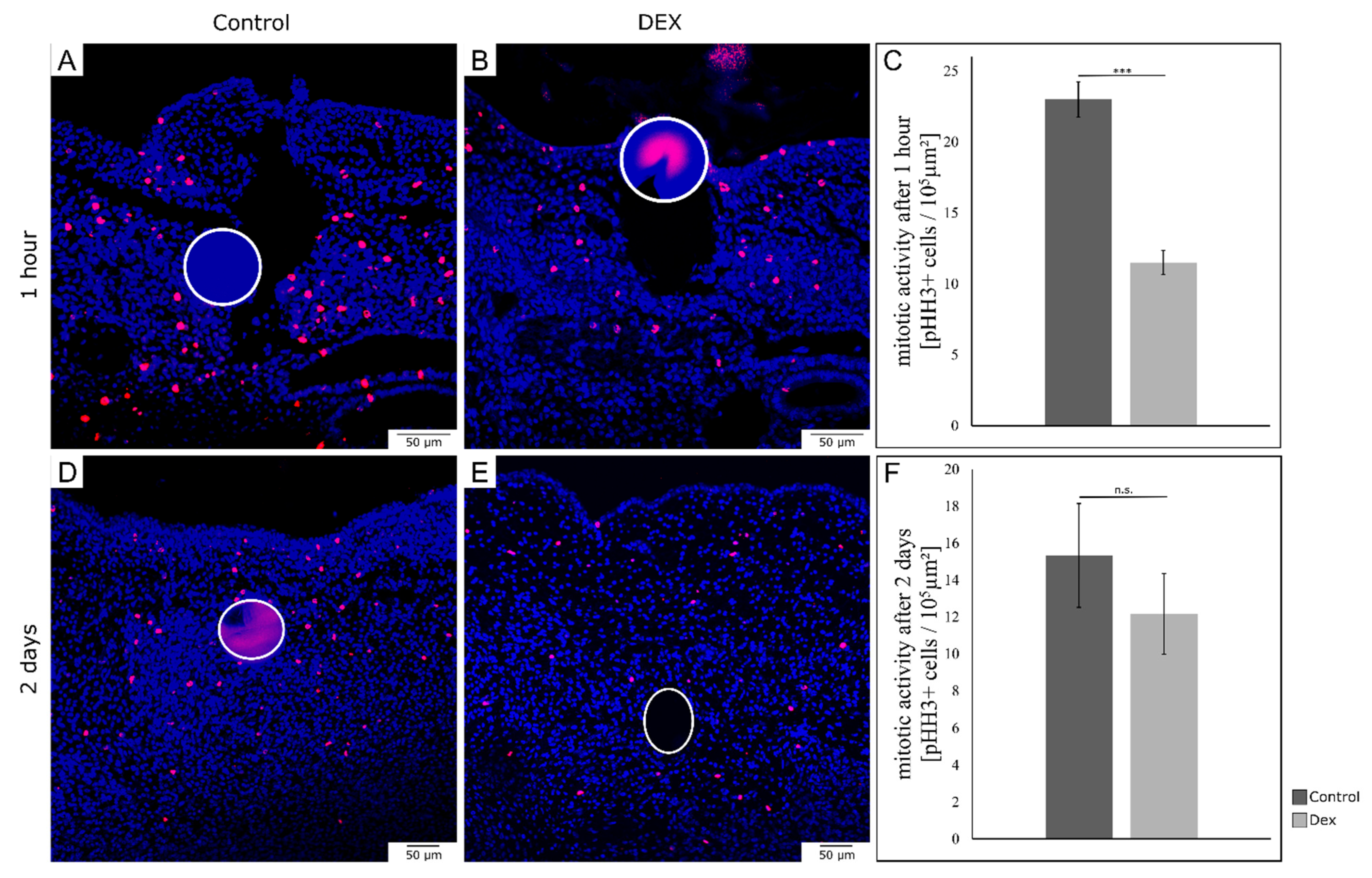
3.4. Local Dexamethasone Administration Temporarily Reduces Mitotic Activity of Embryonic Wound Tissue
3.5. Local Dexamethasone Administration Increases E-Cadherin Expression during Embryonic Wound Healing
3.6. Local Dexamethasone Administration Temporarily Disturbs Expression of Dermo-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sampat, K.; Losty, P.D. Fetal Surgery. Br. J. Surg. 2021, 108, 632–637. [Google Scholar] [CrossRef]
- Baumgarten, H.D.; Flake, A.W. Fetal Surgery. Pediatr. Clin. N. Am. 2019, 66, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S.; Harrison, M.R. Fetal Surgical Therapy. Lancet 1994, 343, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Adzick, N.S.; Harrison, M.R.; Glick, P.L.; Beckstead, J.H.; Villa, R.L.; Scheuenstuhl, H.; Goodson, W.H. Comparison of Fetal, Newborn, and Adult Wound Healing by Histologic, Enzyme-Histochemical, and Hydroxyproline Determinations. J. Pediatr. Surg. 1985, 20, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound Healing: An Overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Martin, P.; Lewis, J. Actin Cables and Epidermal Movement in Embryonic Wound Healing. Nature 1992, 360, 179–183. [Google Scholar] [CrossRef]
- Nodder, S.; Martin, P. Wound Healing in Embryos: A Review. Anat. Embryol. 1997, 195, 215–228. [Google Scholar] [CrossRef]
- Martin, P. Mechanisms of Wound Healing in the Embryo and Fetus. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 1996; pp. 175–203. [Google Scholar]
- McCluskey, J.; Martin, P. Analysis of the Tissue Movements of Embryonic Wound Healing—DiI Studies in the Limb Bud Stage Mouse Embryo. Dev. Biol. 1995, 170, 102–114. [Google Scholar] [CrossRef]
- Longaker, M.T.; Adzick, N.S. The Biology of Fetal Wound Healing: A Review. Plast. Reconstr. Surg. 1991, 87, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Whitby, D.J.; Longaker, M.T.; Harrison, M.R.; Adzick, N.S.; Ferguson, M.W. Rapid Epithelialisation of Fetal Wounds Is Associated with the Early Deposition of Tenascin. J. Cell Sci. 1991, 99 Pt 3, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired Wound Healing in Embryonic and Adult Mice Lacking Vimentin. J. Cell Sci. 2000, 113, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.V.; Lee, D.M.; Harris, T.J.C.; Fernandez-Gonzalez, R. Polarized E-Cadherin Endocytosis Directs Actomyosin Remodeling during Embryonic Wound Repair. J. Cell Biol. 2015, 210, 801–816. [Google Scholar] [CrossRef]
- Scaal, M.; Füchtbauer, E.-M.; Brand-Saberi, B. CDermo-1 Expression Indicates a Role in Avian Skin Development. Anat. Embryol. 2001, 203, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Cserjesi, P.; Olson, E.N. Dermo-1: A Novel Twist-Related BHLH Protein Expressed in the Developing Dermis. Dev. Biol. 1995, 172, 280–292. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal Corticosteroids for Accelerating Fetal Lung Maturation for Women at Risk of Preterm Birth. Cochrane Database Syst. Rev. 2020, 12, CD004454. [Google Scholar]
- Haq, S.U.; Bhat, U.A.; Kumar, A. Prenatal Stress Effects on Offspring Brain and Behavior: Mediators, Alterations and Dysregulated Epigenetic Mechanisms. J. Biosci. 2021, 46, 34. [Google Scholar] [CrossRef]
- Rog-Zielinska, E.A.; Richardson, R.V.; Denvir, M.A.; Chapman, K.E. Glucocorticoids and Foetal Heart Maturation; Implications for Prematurity and Foetal Programming. J. Mol. Endocrinol. 2014, 52, R125–R135. [Google Scholar] [CrossRef]
- Noorlander, C.W.; Visser, G.H.A.; Ramakers, G.M.J.; Nikkels, P.G.J.; de Graan, P.N.E. Prenatal Corticosteroid Exposure Affects Hippocampal Plasticity and Reduces Lifespan. Dev. Neurobiol. 2008, 68, 237–246. [Google Scholar] [CrossRef]
- Gitau, R.; Cameron, A.; Fisk, N.M.; Glover, V. Fetal Exposure to Maternal Cortisol. Lancet 1998, 352, 707–708. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Reul, J.M.H.M.; Sutanto, W. Corticosteroids and the Brain. J. Steroid Biochem. Mol. Biol. 1990, 37, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Niculet, E.; Bobeica, C.; Tatu, A.L. Glucocorticoid-Induced Skin Atrophy: The Old and the New. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Carolina, E.; Kato, T.; Khanh, V.C.; Moriguchi, K.; Yamashita, T.; Takeuchi, K.; Hamada, H.; Ohneda, O. Glucocorticoid Impaired the Wound Healing Ability of Endothelial Progenitor Cells by Reducing the Expression of CXCR4 in the PGE2 Pathway. Front. Med. 2018, 5, 276. [Google Scholar] [CrossRef]
- Wang, A.S.; Armstrong, E.J.; Armstrong, A.W. Corticosteroids and Wound Healing: Clinical Considerations in the Perioperative Period. Am. J. Surg. 2013, 206, 410–417. [Google Scholar] [CrossRef]
- Anstead, G.M. Steroids, Retinoids, and Wound Healing. Adv. Wound Care 1998, 11, 277–285. [Google Scholar]
- de Groef, B.; Grommen, S.V.H.; Darras, V.M. The Chicken Embryo as a Model for Developmental Endocrinology: Development of the Thyrotropic, Corticotropic, and Somatotropic Axes. Mol. Cell. Endocrinol. 2008, 293, 17–24. [Google Scholar] [CrossRef]
- Fonseca, B.B.; da Silva, M.V.; de Morais Ribeiro, L.N. The Chicken Embryo as an in Vivo Experimental Model for Drug Testing: Advantages and Limitations. Lab. Anim. 2021, 50, 138–139. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Essa, M.E.A. In Ovo Injection Corticosterone Method for Physiological and Behavioral Studies in Chickens. MethodsX 2020, 7, 100908. [Google Scholar] [CrossRef]
- Janczak, A.M.; Braastad, B.O.; Bakken, M. Behavioural Effects of Embryonic Exposure to Corticosterone in Chickens. Appl. Anim. Behav. Sci. 2006, 96, 69–82. [Google Scholar] [CrossRef]
- Yahya, I.; van Lin, D.J.M.; Böing, M.; Brand-Saberi, B.; Morosan-Puopolo, G. In Ovo Technique for Cell Injection in the CPM Followed by Bead Implantation in the BA2 of Chicken Embryos. MethodsX 2020, 7, 100792. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Liu, X.; Liu, Y.; Zhang, D.; Xue, L. The Relationship between Maternal Corticosteroid Use and Orofacial Clefts-a Meta-Analysis. Reprod. Toxicol. 2017, 69, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, H.; Yan, Y.; Wang, G.; Berman, Z.; Chuai, M.; Yang, X. Usage of Dexamethasone Increases the Risk of Cranial Neural Crest Dysplasia in the Chick Embryo. Toxicol. Sci. 2017, 158, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Patel, K.; Wilkinson, D.G. In Situ Hybridization Analysis of Chick Embryos in Whole Mount and Tissue Sections. Methods Cell Biol. 1996, 51, 219–235. [Google Scholar] [CrossRef]
- Yahya, I.; Böing, M.; Brand-Saberi, B.; Morosan-Puopolo, G. How to Distinguish between Different Cell Lineages Sharing Common Markers Using Combinations of Double In-Situ-Hybridization and Immunostaining in Avian Embryos: CXCR4-Positive Mesodermal and Neural Crest-Derived Cells. Histochem. Cell Biol. 2021, 155, 145–155. [Google Scholar] [CrossRef]
- Jozic, I.; Vukelic, S.; Stojadinovic, O.; Liang, L.; Ramirez, H.A.; Pastar, I.; Tomic Canic, M. Stress Signals, Mediated by Membranous Glucocorticoid Receptor, Activate PLC/PKC/GSK-3β/β-Catenin Pathway to Inhibit Wound Closure. J. Investig. Dermatol. 2017, 137, 1144–1154. [Google Scholar] [CrossRef]
- Lee, B.; Vouthounis, C.; Stojadinovic, O.; Brem, H.; Im, M.; Tomic-Canic, M. From an Enhanceosome to a Repressosome: Molecular Antagonism between Glucocorticoids and EGF Leads to Inhibition of Wound Healing. J. Mol. Biol. 2005, 345, 1083–1097. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin Coordinates Fibroblast Proliferation and Keratinocyte Differentiation in Wound Healing via TGF-β–Slug Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, W.; Wang, X.; Tang, Y.; Song, J. Glucocorticoid Induces Mesenchymal-to-Epithelial Transition and Inhibits TGF-Β1-Induced Epithelial-to-Mesenchymal Transition and Cell Migration. FEBS Lett. 2010, 584, 4646–4654. [Google Scholar] [CrossRef]
- Lee, H.M.; Yang, H.-W.; Lee, S.A.; Park, J.-H.; Heo, M.; Shin, J.-M. Inhibitory Effect of Glucocorticoids on TGF-Β1-Mediated Epithelial-to-Mesenchymal Transition of Airway Epithelium via MAPK and Snail/Slug Signaling Pathways. J. Allergy Clin. Immunol. 2018, 141, AB66. [Google Scholar] [CrossRef]
- Oliver, N.; Newby, R.F.; Furcht, L.T.; Bourgeois, S. Regulation of Fibronectin Biosynthesis by Glucocorticoids in Human Fibrosarcoma Cells and Normal Fibroblasts. Cell 1983, 33, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Guller, S.; Wozniak, R.; Krikun, G.; Burnham, J.M.; Kaplan, P.; Lockwood, C.J. Glucocorticoid Suppression of Human Placental Fibronectin Expression: Implications in Uterine-Placental Adherence. Endocrinology 1993, 133, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Patten, J.; Wang, K. Fibronectin in Development and Wound Healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef]
- Whitby, D.J.; Ferguson, M.W. The Extracellular Matrix of Lip Wounds in Fetal, Neonatal and Adult Mice. Development 1991, 112, 651–668. [Google Scholar] [CrossRef]
- Clark, R.A.; Lanigan, J.M.; DellaPelle, P.; Manseau, E.; Dvorak, H.F.; Colvin, R.B. Fibronectin and Fibrin Provide a Provisional Matrix for Epidermal Cell Migration during Wound Reepithelialization. J. Investig. Dermatol. 1982, 79, 264–269. [Google Scholar] [CrossRef]
- Clark, R.A.; Winn, H.J.; Dvorak, H.F.; Colvin, R.B. Fibronectin beneath Reepithelializing Epidermis in Vivo: Sources and Significance. J. Investig. Dermatol. 1983, 80, 26s–30s. [Google Scholar] [CrossRef]
- Sethi, K.K.; Yannas, I.V.; Mudera, V.; Eastwood, M.; McFarland, C.; Brown, R.A. Evidence for Sequential Utilization of Fibronectin, Vitronectin, and Collagen during Fibroblast-Mediated Collagen Contraction. Wound Repair Regen. 2002, 10, 397–408. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Ramalingam, A.; Hirai, A.; Thompson, E.A. Glucocorticoid Inhibition of Fibroblast Proliferation and Regulation of the Cyclin Kinase Inhibitor P21Cip1. Mol. Endocrinol. 1997, 11, 577–586. [Google Scholar] [CrossRef]
- King, K.L.; Cidlowski, J.A. Cell Cycle Regulation and Apoptosis. Annu. Rev. Physiol. 1998, 60, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Hornik, C.; Krishan, K.; Yusuf, F.; Scaal, M.; Brand-Saberi, B. CDermo-1 Misexpression Induces Dense Dermis, Feathers, and Scales. Dev. Biol. 2005, 277, 42–50. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bablok, M.; Gellisch, M.; Brand-Saberi, B.; Morosan-Puopolo, G. Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines 2022, 10, 3125. https://doi.org/10.3390/biomedicines10123125
Bablok M, Gellisch M, Brand-Saberi B, Morosan-Puopolo G. Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines. 2022; 10(12):3125. https://doi.org/10.3390/biomedicines10123125
Chicago/Turabian StyleBablok, Martin, Morris Gellisch, Beate Brand-Saberi, and Gabriela Morosan-Puopolo. 2022. "Local Glucocorticoid Administration Impairs Embryonic Wound Healing" Biomedicines 10, no. 12: 3125. https://doi.org/10.3390/biomedicines10123125
APA StyleBablok, M., Gellisch, M., Brand-Saberi, B., & Morosan-Puopolo, G. (2022). Local Glucocorticoid Administration Impairs Embryonic Wound Healing. Biomedicines, 10(12), 3125. https://doi.org/10.3390/biomedicines10123125




