Abstract
Patients with stage IV colorectal cancer (CRC) who have not undergone primary tumor resection (PTR) are at risk of sudden medical emergencies. Despite the ongoing controversy over the necessity and timing of PTR in patients with stage IV CRC, studies comparing the survival outcomes of elective and emergency surgery in this population are scarce. This is a retrospective study conducted at a single institute. The patients were divided into two groups: the elective surgery (ELS) group (n = 46) and the emergency surgery (EMS) group (n = 26). The primary outcome was 2-year overall survival (OS). During a median follow-up period of 27.0 months, the 2-year OS was significantly better in the ELS group (80% vs. 42.9%, p = 0.002). No significant differences were observed in the 2-year relapse-free survival and 30-day postoperative complication rates. Planning and performing elective surgery could help increase the survival rate of patients with synchronous stage IV CRC, especially those that undergo simultaneous or staged metastasectomy.
1. Introduction
Approximately 25% of patients with colorectal cancer (CRC) are initially diagnosed with stage IV disease [1]. Patients with stage IV CRC present with heterogeneous clinical features [2], meaning that the treatment strategies required are diverse, complex, and influenced by several factors. Regarding surgical treatment, only a limited number of patients undergo surgery with curative intent. The majority have unresectable stage IV disease [3,4]. While surgery may be necessary for symptomatic stage IV CRC [5], its role and optimal timing in patients with unresectable stage IV CRC are controversial [6,7]. Even in patients with resectable metastasis, the timing of surgery for the primary and metastatic lesions has been debated.
Patients with unresected stage IV CRC frequently experience disease-related emergencies, such as bowel obstruction, perforation, and bleeding [8,9,10]. In addition, chemotherapy and targeted treatments may cause emergency events, such as bowel perforation and bleeding [11]. According to the SEER database, despite advances in surgical techniques and chemotherapy, the 5-year relative survival rate is only 15.1% [12]. Within this limited lifespan, preemptive PTR to prevent bowel complications should be carefully performed after weighing the pros and cons. Therefore, several studies have evaluated the survival benefit of preemptive PTR. Some advocate preemptive PTR to prevent tumor-related symptoms and increase the effectiveness of chemotherapy by reducing the tumor burden [13,14]. However, other studies have found that primary tumor-related intestinal complications during chemotherapy occur in only 13–20% of patients and have failed to show a survival benefit with preventive surgery [15,16]. Comparing the survival outcomes of elective and emergency PTR would be an important reference in determining the usefulness of preemptive PTR. However, there are few studies comparing the survival outcomes after elective and emergency surgery for stage IV CRC. We hypothesized that there would be a difference in perioperative and survival outcomes between patients undergoing planned or unplanned surgery. Hence, we aimed to compare the immediate postoperative results and survival outcomes of elective and emergent PTR in patients with stage IV CRC and determine whether elective PTR, with or without metastasectomy, is associated with a better prognosis.
2. Materials and Methods
- (1)
- IRB
After obtaining approval from the Institutional Review Board of St. Vincent Hospital at the Catholic University of Korea (VC22RISI0200), we retrospectively reviewed the patients’ data and clinical information.
- (2)
- Study design and patients
Between January 2017 and December 2019, 86 patients were initially diagnosed with synchronous metastatic CRC at St. Vincent Hospital, Catholic University of Korea. We excluded 14 patients who did not undergo PTR for CRC. Finally, 72 patients were eligible for this study and were divided into two groups: elective surgery (ELS) and emergency surgery (EMS).
- (3)
- Definitions
Emergency surgery was defined as an operation performed because of cancer obstruction, bowel perforation, or bleeding symptoms. Elective surgery was defined as an operation performed based on a previously set treatment strategy.
- (4)
- Elective group
All patients who were pathologically diagnosed with colorectal adenocarcinoma underwent staging work-up with abdominopelvic and chest computed tomography (CT). Liver magnetic resonance imaging (MRI) was performed when a suspicious liver lesion was observed on abdominopelvic CT (APCT). Positron emission tomography-CT was performed in patients diagnosed with extrahepatic metastatic lesions on APCT. After cancer evaluation, the treatment plan was determined by a multidisciplinary team consisting of surgeons, oncologists, radiologists, and other related specialists.
The patients in the elective group were classified according to whether they underwent chemotherapy or surgery first. Patients with unresectable metastatic lesions or oligometastasis initially underwent chemotherapy. In addition, even in patients with resectable metastases, chemotherapy was administered to reduce their size, determine responsiveness to chemotherapy, and avoid local therapy for patients with early disease.
Patients underwent surgery first when they had almost complete intestinal obstruction, despite the absence of symptoms, or if an R0 (locally or totally) resection could be achieved. All patients were routinely treated with postoperative chemotherapy (FOLFOX or FOLFIRI regimen), with or without targeted agents, except those who refused chemotherapy or were unable to undergo treatment due to a poor general condition or underlying disease. Postoperative follow-up evaluations, including carcinoembryonic antigen (CEA) and imaging studies, were performed every 3 cycles of chemotherapy.
- (5)
- Emergency group
Patients in the emergency group were divided into two subgroups: those who presented to an emergency room with symptoms related to CRC before being formally diagnosed, and those who developed symptoms during chemotherapy.
Patients who visited the emergency room due to an initial presentation of CRC underwent emergency resection of the primary lesion without a histological diagnosis. The pathological diagnosis was confirmed using surgical specimens.
Staging workup was performed with APCT performed in the emergency room, chest CT, and other imaging studies after the pathological diagnosis. Patients with a sudden onset of symptoms during chemotherapy were referred to a surgeon for emergency intervention. After surgery and postoperative recuperation, chemotherapy was restarted using the same regimen or a 2nd line regimen, depending on the patient’s condition and tumor status. Follow-up evaluation was performed every 3 cycles of chemotherapy, in the same manner as in the elective group.
- (6)
- Surgery
The choice of surgical procedure depended on the site of the primary tumor. At our institution, we perform central vascular ligation (CVL) with complete mesocolic excision (CME) for colon cancer, and total mesorectal excision (TME) or tumor-specific mesorectal excision (TSME) for rectal cancer. We performed an extended radical resection if the primary tumor lesion had invaded the adjacent organs. After resection of the primary lesion, restoration of bowel continuity was performed, except in patients with septic shock, fecal peritonitis, or preoperative organ failure. If microscopic tumor infiltration of the distal or circumferential margin was observed on postoperative pathological examination, the operation was defined as an R1 resection in which R0 resection was intended. Metastasectomy was performed either simultaneously or in stages.
- (7)
- Outcome
The primary outcome of this study was the 2-year overall survival (OS) in the ELS and EMS groups. We calculated the overall survival period from the date of the diagnosis of synchronous stage IV CRC to the date of death or end of follow-up. Relapse-free survival (RFS) was defined as the period from the start of treatment (surgery or chemotherapy) to the detection of disease progression or recurrence. The immediate postoperative outcomes (30-day complication and mortality rates) were analyzed as secondary outcomes.
- (8)
- Statistical analyses
Statistical analysis was performed using the SPSS software, version 28.0 (IBM SPSS Statistics®, Armonk, NY, USA). Fisher’s exact test was used to compare categorical data and Student’s t-test was used for continuous data. The results are presented as mean values and standard deviations (SD) for continuous normally distributed variables, and as counts and percentages for categorical data. Survival analysis was performed using the Kaplan–Meier (log-rank test) method. Univariate and multivariate analyses of factors associated with 2-year OS were performed using the following variables: age, sex, ASA score, elective or emergency surgery, metastasectomy, and postoperative chemotherapy. Statistical significance was set at p < 0.05.
3. Results
A total of 72 patients who underwent PTR were included in this study (flow diagram shown in Figure 1). Among them, 26 patients underwent emergent CRC resection because of obstruction (23 patients) and perforation (3 patients). We compared the patients’ demographic and pathological data between the elective and emergency groups. The ASA scores (p = 0.015) and initial CEA levels (p = 0.006) were significantly higher in the EMS group. The proportion of patients who underwent preoperative chemotherapy did not differ between the ELS and EMS groups; however, the number of chemotherapy cycles administered before surgery was greater in the EMS group (p = 0.006) (Table 1).
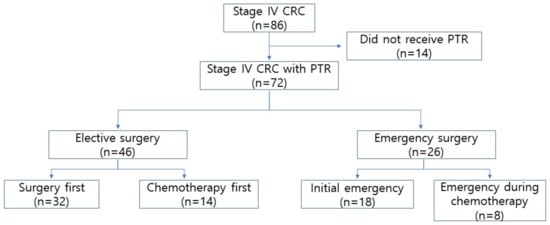
Figure 1.
Flow diagram CRC, colorectal cancer; PTR, primary tumor resection.

Table 1.
Baseline characteristics.
There were significant differences in terms of primary tumor pathological (p) T classification, where pT4 tumors (p = 0.015) and multiorgan metastases (p = 0.005) were significantly more common in the EMS group than in the ELS group. Resectable metastatic lesions were more common (p = 0.003) and metastasectomy was performed more frequently in the ELS group (p < 0.001). Although there was no difference in the radicality of the primary tumor lesion, R0 status, including for the metastatic lesions, was significantly more common in the ELS group (Table 2). There was no difference in 30-day postoperative complication rates between the two groups (Table 3). At a median follow-up of 27.0 months, the 2-year OS rate was higher in the ELS group (80% vs. 42.9%, p = 0.002), but there was no difference in the 2-year RFS rate (Figure 2).

Table 2.
Tumor characteristics.

Table 3.
Perioperative outcomes.
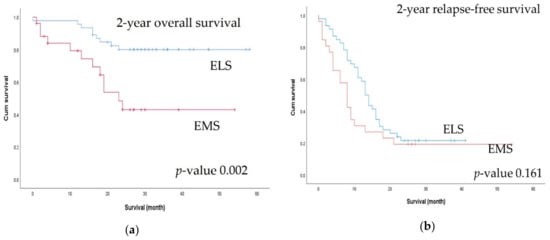
Figure 2.
Comparison of 2-year survival outcomes between the elective surgery (ELS) and emergency surgery (EMS) groups: (a) 2-year overall survival; (b) 2-year relapse-free survival.
Since the two groups differed with respect to initial resectability, we performed a subgroup analysis of 2-year OS according to initial resectability. Among patients with initially resectable metastatic lesions, the 2-year OS rate was significantly better in the ELS group (86.21% vs. 34.29%, p = 0.01). Among patients with unresectable lesions, the ELS group showed better 2-year OS, but the difference was not statistically significant (75.49% vs. 46.44%, p = 0.098) (Figure 3).
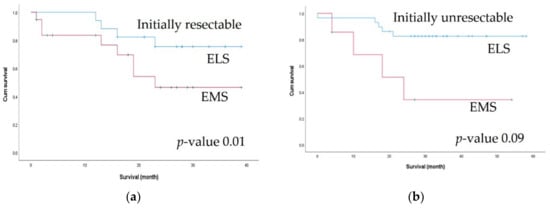
Figure 3.
Comparison of 2-year survival outcomes between the elective surgery (ELS) and emergency surgery (EMS) groups according to initial resectability: (a) 2-year overall survival in initially resectable patients; (b) 2-year overall survival in initially unresectable patients.
Table 4 shows the results of the univariate and multivariate Cox hazard analyses for 2-year OS. Emergent surgery (p = 0.002), higher ASA score (p < 0.001), no metastasectomy (p = 0.006), and postoperative chemotherapy (p = 0.014) were associated with worse 2-year OS. In the multivariate analysis, only a higher ASA score (p = 0.002) and no metastasectomy (p = 0.018) remained significant risk factors. In the univariate analysis of 2-year RFS, only the administration of postoperative chemotherapy (p = 0.043) was a statistically significant factor (Table A1).

Table 4.
Analysis of factors associated with 2-year overall survival.
We performed an additional subgroup analysis for survival outcomes between patients who underwent elective surgery as the first treatment (n = 32) and those who underwent emergency surgery while receiving chemotherapy (n = 8). There was a trend towards better 2-year OS in the elective group (74.3% and 37.5%), but the difference was not statistically significant (p = 0.052) (Figure 4). We also compared the effect of postoperative chemotherapy on patient survival. Of the 72 patients, 50 had received postoperative chemotherapy, with no significant difference in the rate of postoperative chemotherapy between the ELS and EMS groups (76.1% vs. 57.7%, p = 0.104). The patients were accordingly divided into four subgroups according to the treatment received: ELS with/without postoperative chemotherapy and EMS with/without postoperative chemotherapy. The subgroup of patients who underwent elective surgery followed by chemotherapy exhibited the best 2-year OS (p < 0.001) (Figure 5).
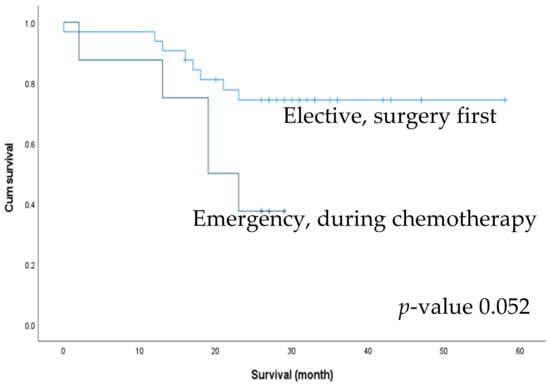
Figure 4.
Comparison of 2-year survival outcomes between surgery first patients in the elective group and patients with an emergent event during chemotherapy.
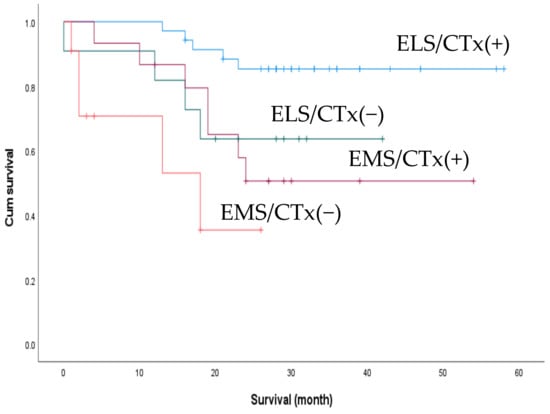
Figure 5.
C Comparison of 2-year overall survival between patients who received postoperative adjuvant chemotherapy (post-op CTx) and those who did not. ELS/CTx(+), elective surgery + post-op CTx; ELS/CTx(−), elective surgery + no post-op CTx; EMS/CTx(+), emergency surgery + post-op CTx; EMS/CTx(−), emergency surgery + no post-op CTx.
We also analyzed stoma-free survival between the ELS and EMS groups. Stoma formation was performed in 19 patients in the ELS group and 4 in the EMS group. Among these, stoma-takedown surgery was performed in 63.2% of patients in the ELS group and 50% of those in the EMS group (Table 5). The ELS group showed better stoma-free survival (26.16 vs. 13.25 months), but the difference was not statistically significant (p = 0.701) (Figure 6).

Table 5.
Stoma creation in patients with left-sided cancer.
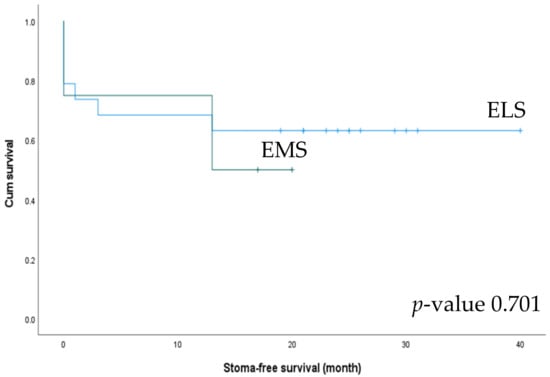
Figure 6.
Stoma-free survival of patients undergoing left-side colectomy in the elective surgery (ELS) and emergency surgery (EMS) groups.
4. Discussion
In patients with stage IV CRC, the primary tumor is usually at an advanced stage of T3 or higher, putting them at risk of emergencies, such as obstruction, perforation, and bleeding. Approximately 7–29% of CRC patients present with bowel obstruction [17], and the morbidity, mortality, and stoma formation rates are higher for patients requiring emergent intervention than for those managed electively [8]. However, few studies have investigated the outcomes of emergent PTR in stage IV CRC patients. Evaluating surgical outcome of stage IV CRC patients who underwent emergency PTR, this study demonstrates that a more advanced pathological T stage (T4) was more common in the EMS group, and 23 of the 26 patients underwent emergency PTR for bowel obstruction.
Surgical treatment of obstructive CRC is different depending on the location of the cancer [18]. Emergency PTR with primary anastomosis is mainly performed for right (Rt.) colon cancer obstruction. However, with the development of colonoscopy, staged operation after self-expandable metal stent (SEMS) can be considered in Rt. colon cancer obstruction [19], if the patient is stable and the cancer is not located at the IC valve or cecum. More diverse treatment can be considered in left (Lt.) colon cancer obstruction than in Rt. Colon cancer. Hartmann’s procedure is the most preferred surgical treatment in high-risk patients with Lt. colon cancer obstruction [20]. Fecal diversion without PTR could be another option. If the patient’s vital sign is stable and there is no sign of impending perforation or ischemic change, staged operation after SEMS is recommended [21,22,23].
In this study, patients with Rt. Colon and proximal Lt. sided colon cancer obstruction underwent PTR without a trial of SEMS. In patients with distal Lt. sided colon cancer obstruction, PTR and primary anastomosis was performed when the patient’s vital sign and bowel condition were acceptable. Moreover, PTR with or without anastomosis was performed inevitably due to the failure of the SEMS or ischemic change of the bowel.
Although the difference was not statistically significant, it is notable that there were two local R2 resections in the EMS group compared to none in the ELS group. Furthermore, the colostomy rate was high, and the stoma closure rate was low in the EMS group, even though the ELS group included 4 APR cases. Based on these results, it is possible to prevent local R2 resection and perform simultaneous metastasectomy by staged operation after SEMS insertion or planned surgery if the primary tumor is resectable.
Patients who underwent elective surgery showed a significantly better 2-year OS than those who underwent emergency surgery. As the initial resectability rates differed between the ELS and EMS groups, we compared the 2-year OS between the two groups according to resectability. Although statistical significance was confirmed only in patients with initially resectable tumors, the ELS group showed improved 2-year OS for both initially resectable and unresectable metastatic lesions. This result supports the possibility that elective surgery contributes to a better survival outcome, regardless of the initial resectability of the metastatic lesion. In the multivariate analysis, ASA score and whether metastasectomy was performed were the most significant factors associated with survival. Patients in the ELS group showed better ASA scores, more resectable metastatic lesions, and underwent more metastasectomies than those in the EMS group. Several retrospective and prospective studies on stage IV CRC have demonstrated that curative metastasectomy improves survival and may even be curative [24,25]. The results of this study are consistent with these previous findings.
In asymptomatic patients with stage IV CRC, the benefit of PTR as the initial treatment is controversial. While it may reduce the tumor burden, previous studies have shown that PTR increases postoperative morbidity and mortality rates without improving survival [12,26]. However, unresected primary tumor can also cause emergent events that start chemotherapy first. Therefore, we performed a subgroup analysis to compare asymptomatic patients who underwent elective surgery first with those who underwent emergency surgery while receiving chemotherapy. Although the difference was not statistically significant, asymptomatic patients who underwent elective surgery as the initial treatment tended to have improved survival rates.
Regarding postoperative chemotherapy, there was no significant difference in its frequency, its timing after surgery, and the number of cycles between the ELS and EMS groups. However, the univariate analysis showed that the administration of postoperative chemotherapy was significantly associated with OS, and was the only factor associated with RFS. Furthermore, in the subgroup analysis, patients who received chemotherapy after elective surgery showed significantly better survival outcomes than other cohorts. Hirotoshi et al. demonstrated that adjuvant chemotherapy improved OS after curative resection for stage IV CRC [27]. In the ELS group, R0 metastasectomy was more common than in the EMS group. Thus, the results of the subgroup analysis with respect to postoperative chemotherapy are consistent with previous findings.
As the clinical outcome for patients with stage IV CRC has improved [28], their quality of life (QOL) has become an increasingly important consideration. In a UK survey [29] of CRC survivors, colostomy was clearly associated with reduced QOL. Therefore, we analyzed stoma-related outcomes, finding that while there was no difference in stoma closure rate between the groups, the ELS group showed a trend towards better stoma-free survival. While the difference in survival was not significant, given the small number of patients who underwent stoma takedown surgery in the EMS group, it is still notable considering that four patients in the ELS group had undergone abdominoperineal resections.
This study has some limitations. First, this was a retrospective, non-randomized, study conducted at a single center with a small number of patients. Since there is no consensus on the superiority of simultaneous or staged resection of metastatic lesions [3,30,31], we determined the timing of metastasectomy individually for each patient after multidisciplinary consultation. Finally, the fact that the clinicopathological characteristics of patients were worse in the EMS group may have affected their poor survival outcomes.
The strength of this study is that it is one of very few that have investigated the issue of survival after emergency surgery for stage 4 CRC. We have also analyzed the factors associated with stoma-free survival and the influence of chemotherapy. Additionally, emergency colorectal surgery is often performed by unspecialized general surgeons, which can reportedly influence the outcome of surgery [32]. However, in this study, surgical quality was guaranteed because all PTRs were performed by a specialized colorectal surgeon.
5. Conclusions
In conclusion, in patients who are fit for surgery, elective PTR showed better survival outcomes, especially when performed with simultaneous or staged metastasectomies. However, the benefit of elective PTR alone and surgery in asymptomatic patients remains unclear. Future prospective, observational, and large-scale studies are required to confirm these encouraging results. In addition, careful patient selection is needed to identify those who stand to benefit the most from PTR.
Author Contributions
Conceptualization, J.-Y.M., J.-E.K., N.Y., H.-M.C., H.K., H.-J.A. and B.-H.K.; methodology, J.-Y.M., J.-E.K., N.Y. and B.-H.K.; software, J.-Y.M. and B.-H.K.; validation, J.-Y.M. and B.-H.K.; formal analysis, J.-Y.M. and B.-H.K.; investigation, J.-Y.M. and J.-E.K.; resources, J.-Y.M. and B.-H.K.; data curation, J.-Y.M. and J.-E.K.; writing—original draft preparation, J.-Y.M., J.-E.K., N.Y. and B.-H.K.; writing—review and editing, J.-Y.M., N.Y., H.-M.C., H.K., H.-J.A. and B.-H.K.; visualization, J.-Y.M.; supervision, N.Y., H.-M.C., H.K., H.-J.A. and B.-H.K.; project administration, J.-Y.M. and B.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of St. Vincent’s Hospital (VC22RISI0200) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to retrospective study.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank all participating patients and people who set up the cancer databases.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Analysis of factors associated with 2-year relapse-free survival.
Table A1.
Analysis of factors associated with 2-year relapse-free survival.
| Factor | N | 2-Year OS (%) | Univariate Analysis |
|---|---|---|---|
| p-Value | |||
| Surgery | |||
| Elective | 46 | 21.51 | 0.173 |
| Emergency | 26 | 19.23 | |
| Age (years) | |||
| <65 | 35 | 28.57 | 0.123 |
| >65 | 37 | 16.22 | |
| Sex | |||
| Male | 45 | 17.5 | 0.499 |
| Female | 27 | 25.93 | |
| BMI (kg/m2) | |||
| <23 | 36 | 25 | 0.496 |
| >23 | 36 | 16.67 | |
| ASA score | |||
| I-II | 64 | 21.76 | 0.078 |
| III-IV | 8 | 12.5 | |
| Tumor sidedness | |||
| Right | 20 | 25 | 0.778 |
| Left | 52 | 19.04 | |
| Tumor location | |||
| Colon | 54 | 20.20 | 0.767 |
| Rectum | 18 | 22.22 | |
| Pathological T stage | |||
| T2-3 | 30 | 16 | 0.965 |
| T4 | 42 | 23.81 | |
| Pathological N stage | |||
| N0 | 6 | 16.67 | 0.758 |
| N+ | 66 | 21.10 | |
| Number of metastases | |||
| 1 | 55 | 23.50 | 0.145 |
| ≥2 | 17 | 11.76 | |
| Initial resectability | |||
| Yes | 36 | 16.67 | 0.488 |
| No | 36 | 24.69 | |
| Metastasectomy | |||
| Yes | 42 | 21.43 | 0.321 |
| No | 30 | 20 | |
| Postoperative chemotherapy | |||
| Yes | 50 | 23.83 | 0.043 |
| No | 22 | 13.64 | |
ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; HR, hazard ratio; RFS, relapse-free survival.
References
- Steinberg, S.M.; Barkin, J.S.; Kaplan, R.S.; Stablein, D.M. Prognostic indicators of colon tumors. The Gastrointestinal Tumor Study Group experience. Cancer 1986, 57, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.A.; Buell, J.F.; Yoshida, A.; Kazsuba, S.; Hurst, R.; Michelassi, F.; Millis, J.M.; Posner, M.C. Initial Presentation with Stage IV Colorectal Cancer: How aggressive should we be? Arch. Surg. 2000, 135, 530–534, discussion 534–535. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Pawlik, T.M.; Rodriguez-Bigas, M.A.; Monson, J.R.; Chang, G.J.; Larson, D.W. Timing of Surgical Resection for Curative Colorectal Cancer with Liver Metastasis. Ann. Surg. Oncol. 2018, 25, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated with Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Colon Cancer (Version 1.2022). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428 (accessed on 11 June 2022).
- Ahmed, S.; Leis, A.; Fields, A.; Chandra-Kanthan, S.; Haider, K.; Alvi, R.; Reeder, B.; Pahwa, P. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer 2014, 120, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients with Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef]
- Baer, C.; Menon, R.; Bastawrous, S.; Bastawrous, A. Emergency Presentations of Colorectal Cancer. Surg. Clin. North Am. 2017, 97, 529–545. [Google Scholar] [CrossRef]
- Gunnarsson, H.; Jennische, K.; Forssell, S.; Granström, J.; Jestin, P.; Ekholm, A.; Olsson, L.I. Heterogeneity of Colon Cancer Patients Reported as Emergencies. World J. Surg. 2014, 38, 1819–1826. [Google Scholar] [CrossRef]
- Golder, A.M.; McMillan, D.C.; Horgan, P.G.; Roxburgh, C.S.D. Determinants of emergency presentation in patients with colorectal cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 4366. [Google Scholar] [CrossRef]
- Ranpura, V.; Hapani, S.; Wu, S. Treatment-Related Mortality with Bevacizumab in Cancer Patients. JAMA 2011, 305, 487–494. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Bailey, C.E.; You, Y.N.; Skibber, J.M.; Rodriguez-Bigas, M.A.; Feig, B.W.; Chang, G.J. Time trend analysis of primary tumor resection for stage IV colorectal cancer: Less surgery, improved survival. JAMA Surg. 2015, 150, 245–251. [Google Scholar] [CrossRef]
- Ruo, L.; Gougoutas, C.; Paty, P.B.; Guillem, J.G.; Cohen, A.M.; Wong, D.W. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J. Am. Coll. Surg. 2003, 196, 722–728. [Google Scholar] [CrossRef]
- Kim, M.S.; Chung, M.; Ahn, J.B.; Kim, C.W.; Cho, M.S.; Shin, S.J.; Baek, S.J.; Hur, H.; Min, B.S.; Baik, S.H.; et al. Clinical significance of primary tumor resection in colorectal cancer patients with synchronous unresectable metastasis. J. Surg. Oncol. 2014, 110, 214–221. [Google Scholar] [CrossRef]
- Scheer, M.G.W.; Sloots, C.E.J.; van der Wilt, G.J.; Ruers, T.J.M. Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases. Ann. Oncol. 2008, 19, 1829–1835. [Google Scholar] [CrossRef]
- Seo, G.J.; Park, J.W.; Yoo, S.B.; Kim, S.Y.; Choi, H.S.; Chang, H.J.; Shin, A.; Jeong, S.-Y.; Kim, D.Y.; Oh, J.H. Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage IV colorectal cancer. J. Surg. Oncol. 2010, 102, 94–99. [Google Scholar] [CrossRef]
- Rodrigues-Pinto, E.; Morais, R.; Coelho, C.; Pereira, P.; Repici, A.; Macedo, G. Bridge-to-surgery versus emergency surgery in the management of left-sided acute malignant colorectal obstruction—Efficacy, safety and long-term outcomes. Dig. Liver Dis. 2019, 51, 364–372. [Google Scholar] [CrossRef]
- Decker, K.M.; Lambert, P.; Nugent, Z.; Biswanger, N.; Samadder, J.; Singh, H. Time Trends in the Diagnosis of Colorectal Cancer with Obstruction, Perforation, and Emergency Admission After the Introduction of Population-Based Organized Screening. JAMA Netw. Open 2020, 3, e205741. [Google Scholar] [CrossRef]
- Boeding, J.R.E.; Ramphal, W.; Rijken, A.M.; Crolla, R.M.P.H.; Verhoef, C.; Gobardhan, P.D.; Schreinemakers, J.M.J. A Systematic Review Comparing Emergency Resection and Staged Treatment for Curable Obstructing Right-Sided Colon Cancer. Ann. Surg. Oncol. 2021, 28, 3545–3555. [Google Scholar] [CrossRef]
- Kube, R.; Granowski, D.; Stübs, P.; Mroczkowski, P.; Ptok, H.; Schmidt, U.; Gastinger, I.; Lippert, H. Surgical practices for malignant left colonic obstruction in Germany. Eur. J. Surg. Oncol. 2010, 36, 65–71. [Google Scholar] [CrossRef]
- Huang, X.; Lv, B.; Zhang, S.; Meng, L. Preoperative Colonic Stents Versus Emergency Surgery for Acute Left-Sided Malignant Colonic Obstruction: A Meta-analysis. J. Gastrointest. Surg. 2014, 18, 584–591. [Google Scholar] [CrossRef]
- Arezzo, A.; Passera, R.; Secco, G.L.; Verra, M.; Bonino, M.A.; Targarona, E.; Morino, M. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: Results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest. Endosc. 2017, 86, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Van Hooft, J.E.; Veld, J.V.; Arnold, D.; Beets-Tan, R.G.; Everett, S.; Götz, M.; van Halsema, E.E.; Hill, J.; Manes, G.; Meisner, S.; et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 389–407. [Google Scholar] [CrossRef] [PubMed]
- De Haas, R.J.; Wicherts, D.A.; Andreani, P.; Pascal, G.; Saliba, F.; Ichai, P.; Adam, R.; Castaing, D.; Azoulay, D. Impact of Expanding Criteria for Resectability of Colorectal Metastases on Short- and Long-Term Outcomes After Hepatic Resection. Ann. Surg. 2011, 253, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.M.; Kneuertz, P.J.; Pawlik, T.M. Resection of non-hepatic colorectal cancer metastasis. J. Gastrointest. Oncol. 2012, 3, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, H.; Hinoi, T.; Shimomura, M.; Egi, H.; Hattori, M.; Ishizaki, Y.; Adachi, T.; Saito, Y.; Miguchi, M.; Sawada, H.; et al. Up-front systemic chemotherapy is a feasible option compared to primary tumor resection followed by chemotherapy for colorectal cancer with unresectable synchronous metastases. World J. Surg. Oncol. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kotake, K.; Sugihara, K.; Study Group for Peritoneal Metastasis from Colorectal Cancer by the Japanese Society for Cancer of the Colon and Rectum. Impact of Adjuvant Chemotherapy in Patients with Curatively Resected Stage IV Colorectal Cancer. Medicine 2015, 94, e696. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Downing, A.; Morris, E.J.; Richards, M.; Corner, J.; Wright, P.; Sebag-Montefiore, D.; Finan, P.; Kind, P.; Wood, C.C.; Lawton, S.; et al. Health-Related Quality of Life After Colorectal Cancer in England: A Patient-Reported Outcomes Study of Individuals 12 to 36 Months After Diagnosis. J. Clin. Oncol. 2015, 33, 616–624. [Google Scholar] [CrossRef]
- Feldman, H.A.; Zhou, N.; Antonoff, M.B.; Hofstetter, W.L.; Rajaram, R.; Rice, D.C.; Sepesi, B.; Vaporciyan, A.A.; Zorrilla-Vaca, A.; Mehran, R.J. Simultaneous versus staged resections for bilateral pulmonary metastases. J. Surg. Oncol. 2021, 123, 1633–1639. [Google Scholar] [CrossRef]
- Ejaz, A.; Semenov, E.; Spolverato, G.; Kim, Y.; Tanner, D.; Hundt, J.; Pawlik, T.M. Synchronous primary colorectal and liver metastasis: Impact of operative approach on clinical outcomes and hospital charges. HPB 2014, 16, 1117–1126. [Google Scholar] [CrossRef]
- Hall, G.M.; Shanmugan, S.; Bleier, J.I.S.; Jeganathan, A.N.; Epstein, A.J.; Paulson, E.C. Colorectal specialization and survival in colorectal cancer. Color. Dis. 2016, 18, O51–O60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).