Tissue Characterization in Cardiac Amyloidosis
Abstract
1. Introduction
2. Tissue Biopsy for the Diagnosis of Amyloidosis
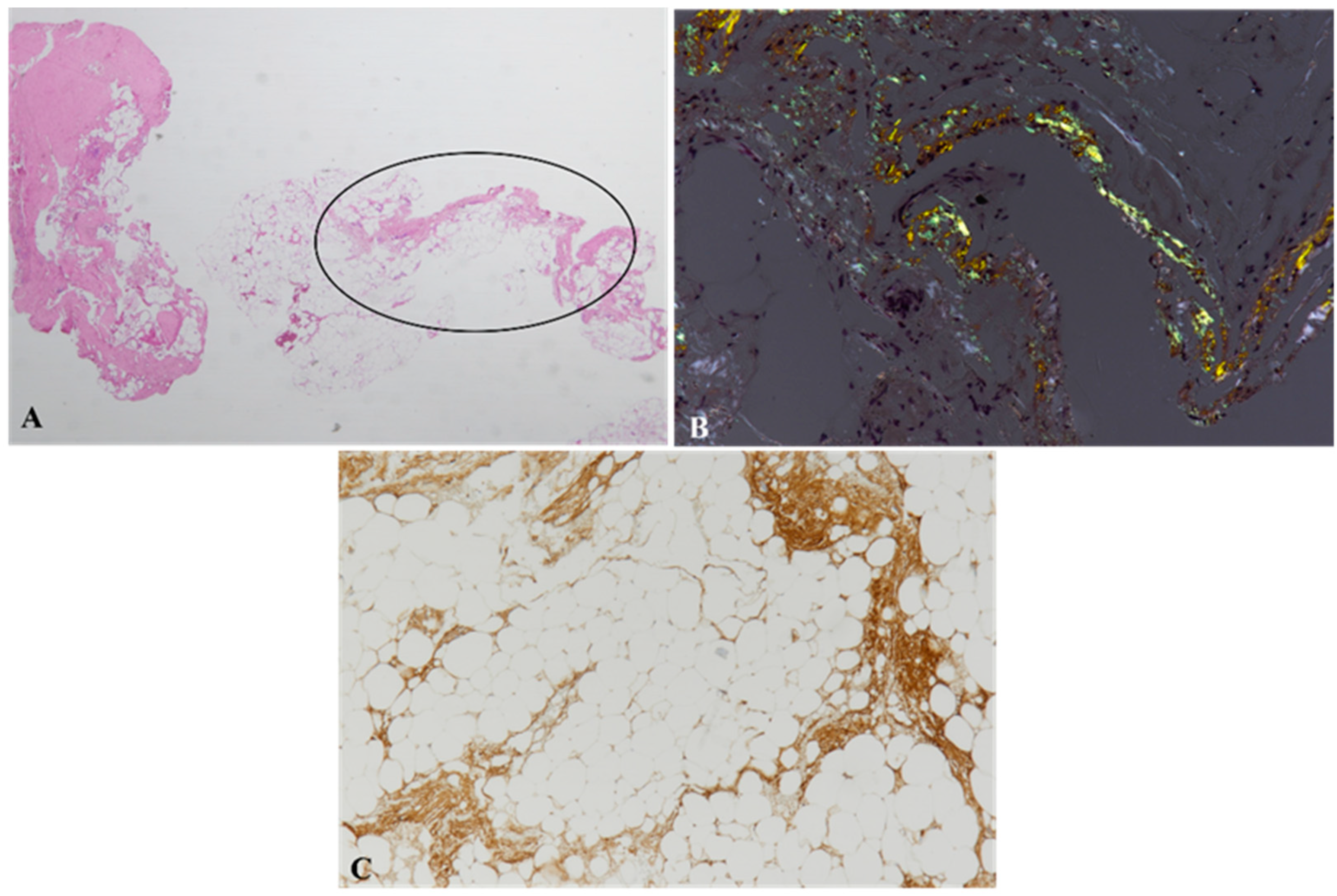
2.1. Abdominal Fat Biopsy
2.2. Endomyocardial Biopsy
2.3. Other Biopsy Sites
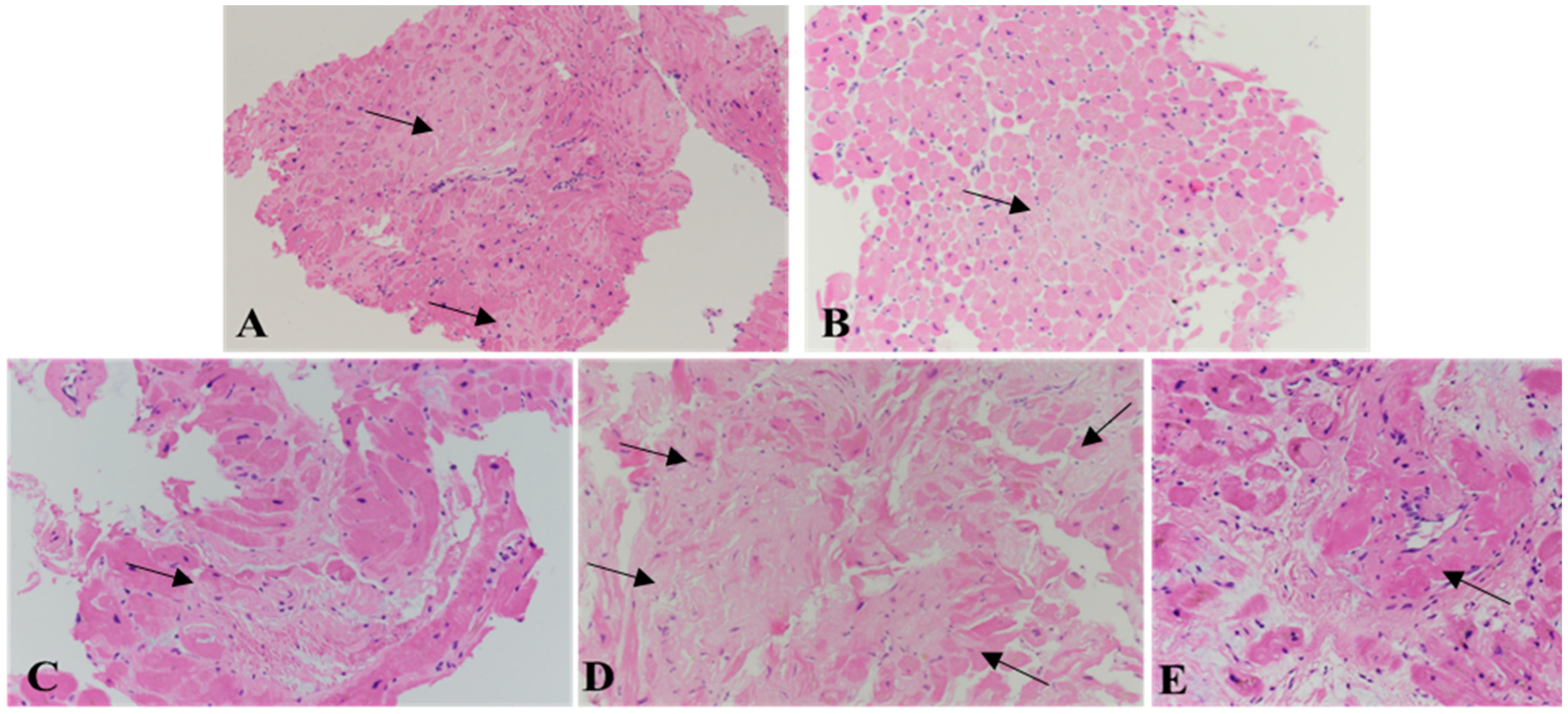
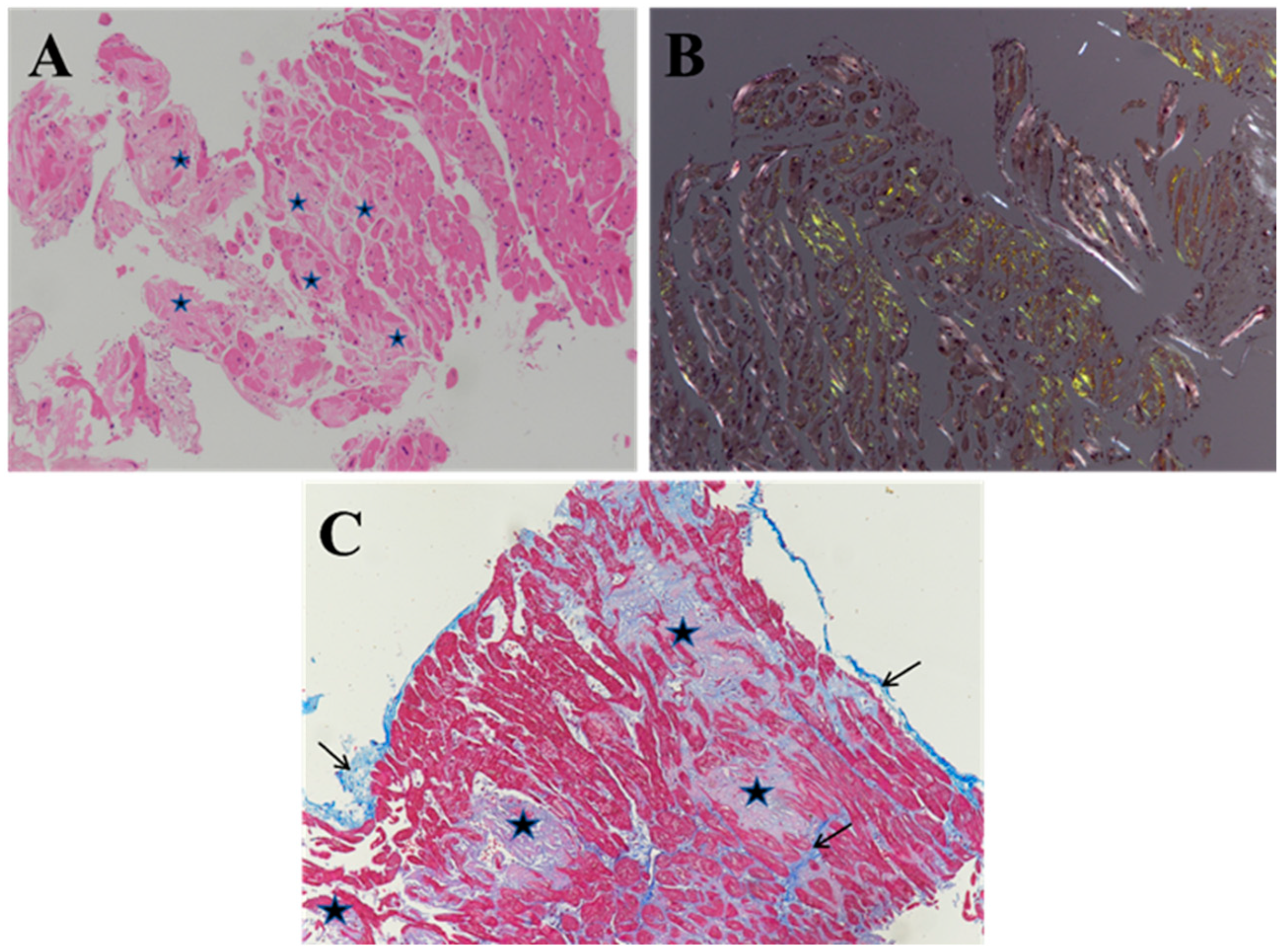
3. Histology of Cardiac Amyloidosis
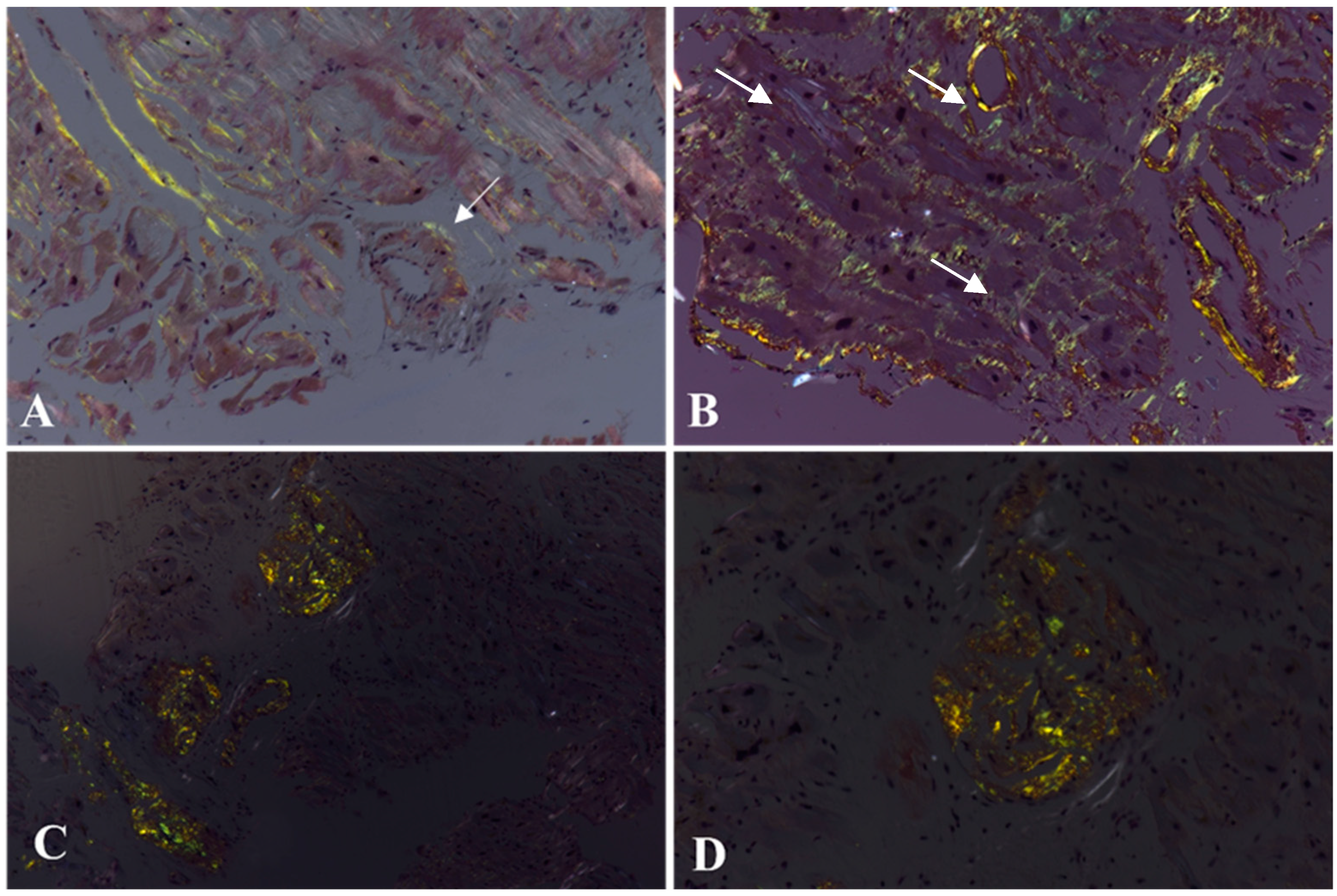
4. Histochemistry of Cardiac Amyloidosis
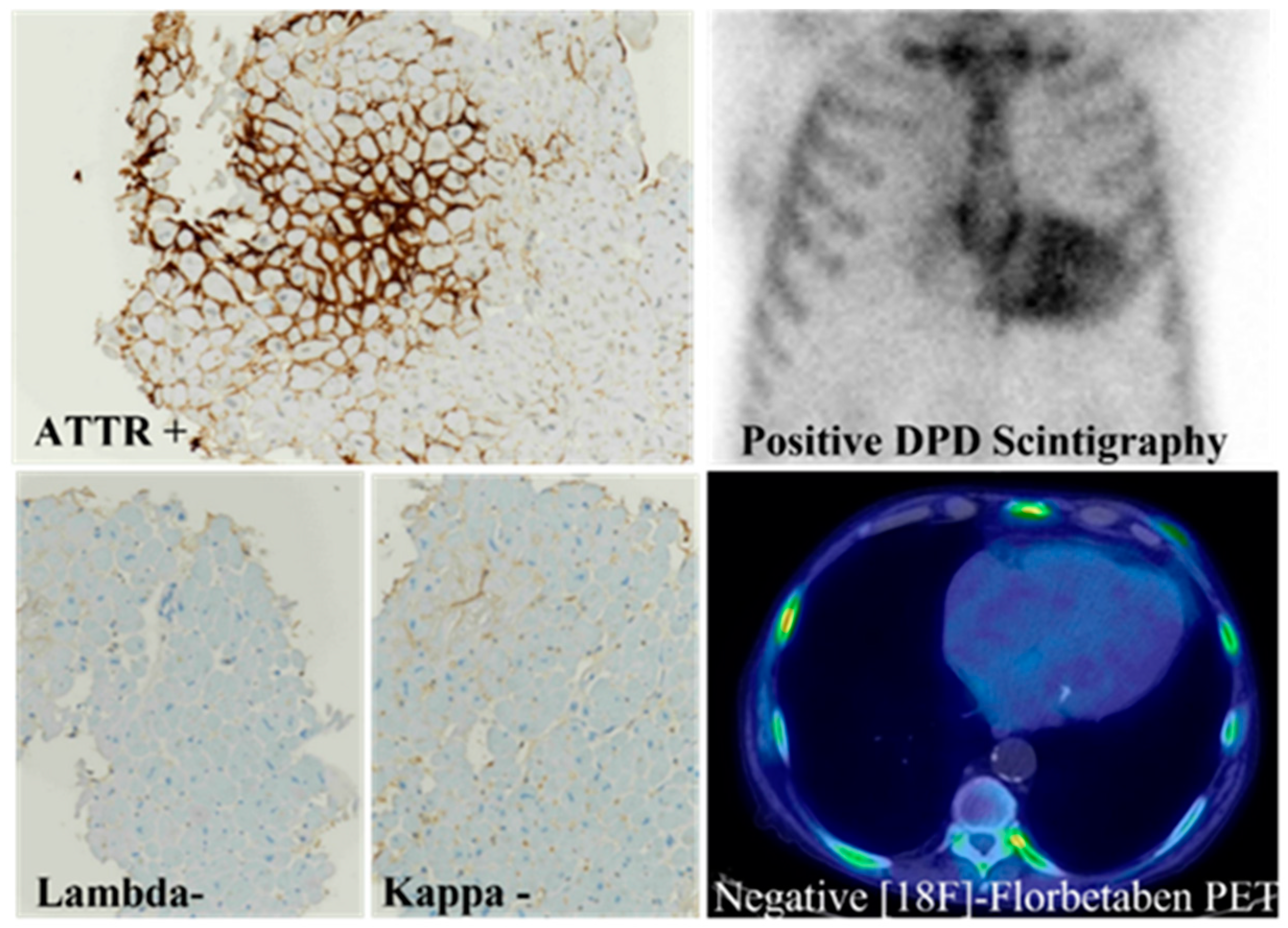
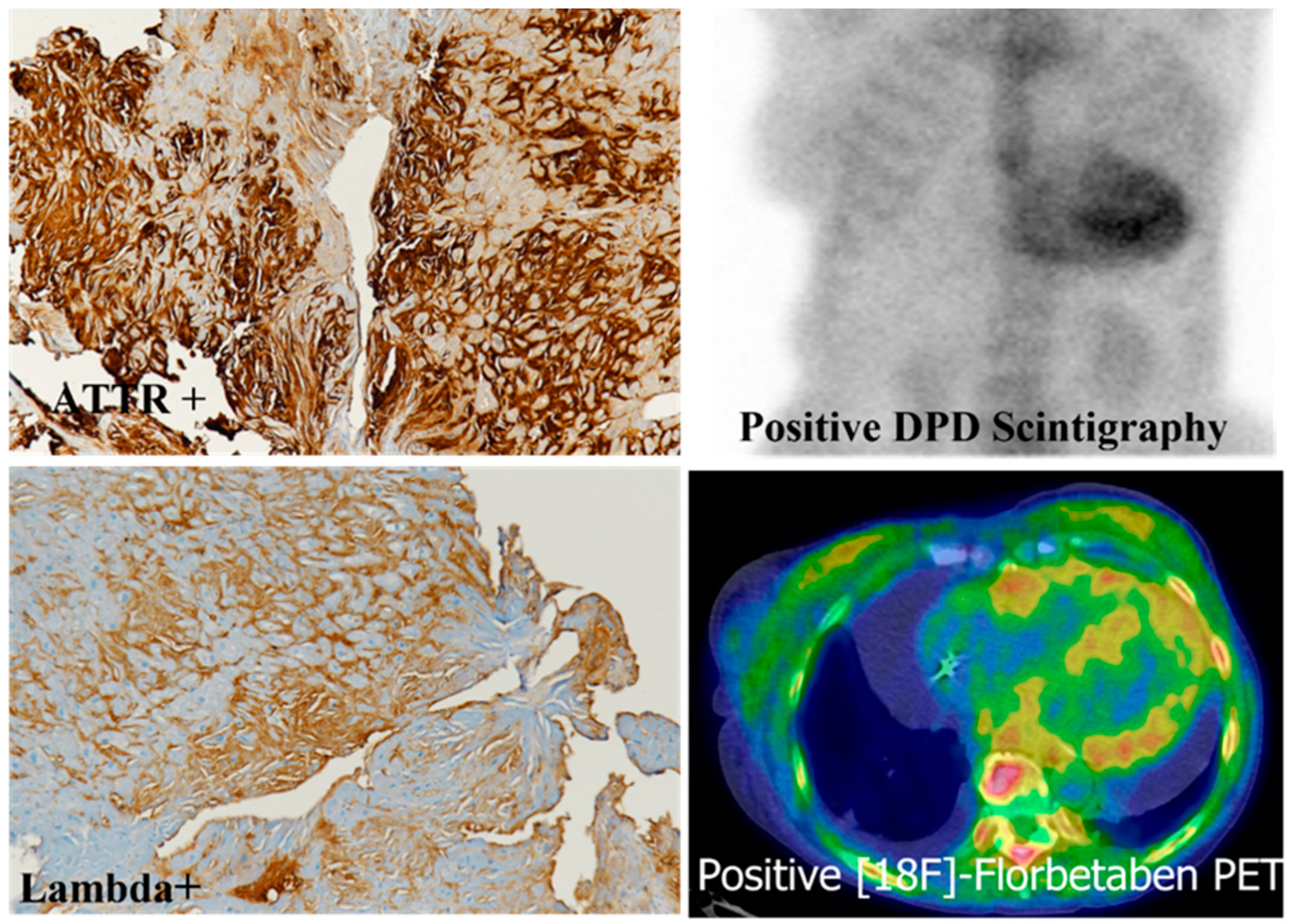
5. Immunohistochemistry of Cardiac Amyloidosis
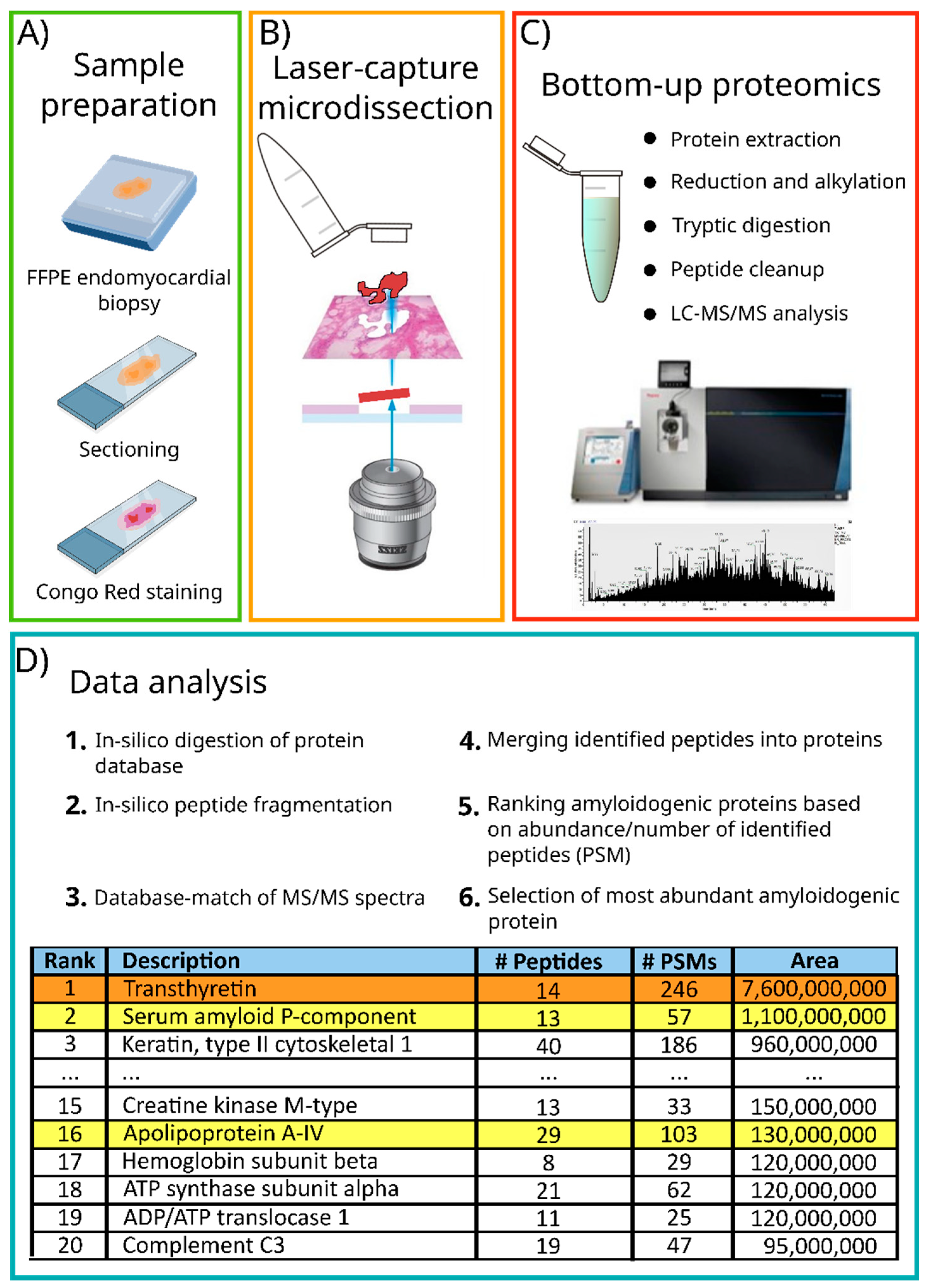
6. Mass Spectrometry-Based Proteomic Analysis of Cardiac Amyloidosis
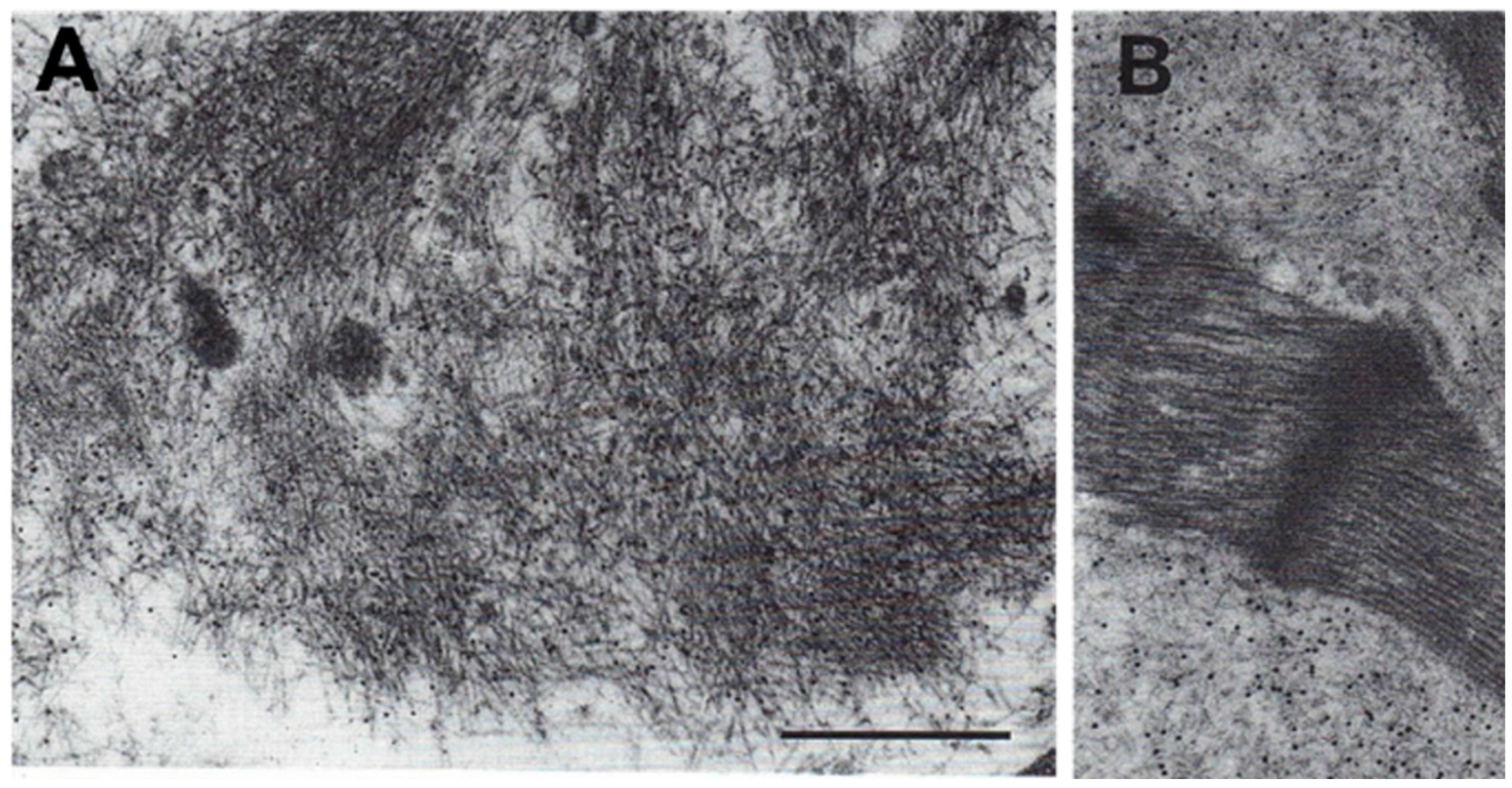
7. Immuno-Electron Microscopy of Cardiac Amyloidosis
8. Immunofluorescence and Confocal Laser Scanning Microscopy
9. Future Directions
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merlini, G.; Bellotti, V. Molecular Mechanisms of Amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Flodrova, P.; Flodr, P.; Pika, T.; Vymetal, J.; Holub, D.; Dzubak, P.; Hajduch, M.; Scudla, V. Cardiac amyloidosis: From clinical suspicion to morphological diagnosis. Pathology 2018, 50, 261–268. [Google Scholar] [CrossRef]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.-I.; Merlini, G.; Saraiva, M.J.; Westermark, P. Nomenclature 2014: Amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 2014, 21, 221–224. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Rapezzi, C.; Franzini, M.; Panichella, G.; Vergaro, G.; Gillmore, J.; Fontana, M.; Passino, C.; Emdin, M. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat. Rev. Cardiol. 2022, 19, 655–667. [Google Scholar] [CrossRef]
- Merlo, M.; Pagura, L.; Porcari, A.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: Results from Phase 2 of the AC-TIVE study, an Italian nationwide survey. Eur. J. Heart Fail. 2022, 24, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, G.; Aimo, A.; Barison, A.; Genovesi, D.; Buda, G.; Passino, C.; Emdin, M. Keys to early diagnosis of cardiac amyloidosis: Red flags from clinical, laboratory and imaging findings. Eur. J. Prev. Cardiol. 2019, 27, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Wisniowski, B.; Wechalekar, A. Confirming the Diagnosis of Amyloidosis. Acta Haematol. 2020, 143, 312–321. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Suhr, O.B.; Lundgren, E.; Westermark, P. One mutation, two distinct disease variants: Unravelling the impact of transthyretin amyloid fibril composition. J. Intern. Med. 2017, 281, 337–347. [Google Scholar] [CrossRef]
- Maleszewski, J.J. Cardiac amyloidosis: Pathology, nomenclature, and typing. Cardiovasc. Pathol. 2015, 24, 343–350. [Google Scholar] [CrossRef]
- Kristen, A.V.; Brokbals, E.; Siepen, F.A.D.; Bauer, R.; Hein, S.; Aurich, M.; Riffel, J.; Behrens, H.-M.; Krüger, S.; Schirmacher, P.; et al. Cardiac Amyloid Load. J. Am. Coll. Cardiol. 2016, 68, 13–24. [Google Scholar] [CrossRef]
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2020: Update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020, 27, 217–222. [Google Scholar] [CrossRef]
- Ruberg, F.L.; Grogan, M.; Hanna, M.; Kelly, J.W.; Maurer, M.S. Transthyretin Amyloid Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 73, 2872–2891. [Google Scholar] [CrossRef]
- Siegismund, C.S.; Escher, F.; Lassner, D.; Kühl, U.; Gross, U.; Fruhwald, F.; Wenzel, P.; Münzel, T.; Frey, N.; Linke, R.P.; et al. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur. J. Heart Fail. 2017, 20, 751–757. [Google Scholar] [CrossRef]
- Pucci, A.; Aimo, A.; Musetti, V.; Barison, A.; Vergaro, G.; Genovesi, D.; Giorgetti, A.; Masotti, S.; Arzilli, C.; Prontera, C.; et al. Amyloid Deposits and Fibrosis on Left Ventricular Endomyocardial Biopsy Correlate With Extracellular Volume in Cardiac Amyloidosis. J. Am. Heart Assoc. 2021, 10, e020358. [Google Scholar] [CrossRef]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef]
- Maurer, M.S.; Bokhari, S.; Damy, T.; Dorbala, S.; Drachman, B.M.; Fontana, M.; Grogan, M.; Kristen, A.V.; Lousada, I.; Nativi-Nicolau, J.; et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ. Heart Fail. 2019, 12, e006075. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schönland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Prim. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2011, 21, 245–274. [Google Scholar] [CrossRef]
- Brownrigg, J.; Lorenzini, M.; Lumley, M.; Elliott, P. Diagnostic performance of imaging investigations in detecting and differentiating cardiac amyloidosis: A systematic review and meta-analysis. ESC Heart Fail. 2019, 6, 1041–1051. [Google Scholar] [CrossRef]
- Chatzantonis, G.; Bietenbeck, M.; Elsanhoury, A.; Tschöpe, C.; Pieske, B.; Tauscher, G.; Vietheer, J.; Shomanova, Z.; Mahrholdt, H.; Rolf, A.; et al. Diagnostic value of cardiovascular magnetic resonance in comparison to endomyocardial biopsy in cardiac amyloidosis: A multi-centre study. Clin. Res. Cardiol. 2020, 110, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Baksi, A.J.; Hawkins, P.N.; Fontana, M. Diagnostic imaging of cardiac amyloidosis. Nat. Rev. Cardiol. 2020, 17, 413–426. [Google Scholar] [CrossRef]
- Mollee, P.; Renaut, P.; Gottlieb, D.; Goodman, H. How to diagnose amyloidosis. Intern. Med. J. 2014, 44, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Wechalekar, A.; Bird, J.; Cavenagh, J.; Hawkins, S.; Kazmi, M.; Lachmann, H.; Hawkins, P.N.; Pratt, G. The BCSH Committee Guidelines on the diagnosis and investigation of AL amyloidosis. Br. J. Haematol. 2014, 168, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Emdin, M.; Musetti, V.; Pucci, A.; Vergaro, G. Abdominal Fat Biopsy for the Diagnosis of Cardiac Amyloidosis. JACC Case Rep. 2020, 2, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.C.; Gonzalez-Lopez, E.; Gilbertson, J.A.; Botcher, N.; Rowczenio, D.; Petrie, A.; Rezk, T.; Youngstein, T.; Mahmood, S.; Sachchithanantham, S.; et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur. Heart J. 2017, 38, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- de Larrea, C.F.; Verga, L.; Morbini, P.; Klersy, C.; Lavatelli, F.; Foli, A.; Obici, L.; Milani, P.; Capello, G.L.; Paulli, M.; et al. A practical approach to the diagnosis of systemic amyloidoses. Blood 2015, 125, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Verga, L.; Concardi, M.; Palladini, G.; Obici, L.; Merlini, G. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid 2002, 9, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Garcia, Y.; Collins, A.B.; Stone, J.R. Abdominal fat pad excisional biopsy for the diagnosis and typing of systemic amyloidosis. Hum. Pathol. 2018, 72, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Westermark, P.; Stenkvist, B. A new method for the diagnosis of systemic amyloidosis. Arch. Intern. Med. 1973, 132, 522–523. [Google Scholar] [CrossRef]
- Fine, N.M.; Arruda-Olson, A.M.; Dispenzieri, A.; Zeldenrust, S.R.; Gertz, M.A.; Kyle, R.A.; Swiecicki, P.L.; Scott, C.G.; Grogan, M. Yield of Noncardiac Biopsy for the Diagnosis of Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2014, 113, 1723–1727. [Google Scholar] [CrossRef]
- Hummel, K.; Meawad, H.; Gunning, W.; Gohara, A. Negative Fat Pad Biopsy in Systemic AL: A Case Report Analyzing the Preferred Amyloidosis Screening Test. Diseases 2021, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; (Histopathology Department, Pisa University Hospital, Pisa, Italy). Histological images. Personal communication.
- Guy, C.D.; Jones, C.K. Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: Specificity, positive predictive value, and diagnostic pitfalls. Diagn. Cytopathol. 2001, 24, 181–185. [Google Scholar] [CrossRef]
- Gilbertson, J.; Botcher, N.; Rowczenio, D.; Whelan, C.; Lachmann, H.; Wechalekar, A.; Gillmore, J.; Hawkins, P. Diagnostic value of fat aspirates for amyloidosis in 950 patients. Orphanet J. Rare Dis. 2015, 10, P50. [Google Scholar] [CrossRef]
- Hasegawa, K.; Uzui, H.; Fukuoka, Y.; Miyanaga, D.; Shiomi, Y.; Tama, N.; Ikeda, H.; Ishida, K.; Miyazaki, S.; Sekijima, Y.; et al. Abdominal Fat Pad Fine-Needle Aspiration for Diagnosis of Cardiac Amyloidosis in Patients with Non-Ischemic Cardiomyopathy. Int. Heart J. 2022, 63, 49–55. [Google Scholar] [CrossRef]
- Rubiś, P.; Rudnicka-Sosin, L.; Jurczyszyn, A.; Janion, M.; Podolec, P. The paramount importance of repeated left ventricular endomyocardial biopsy during the diagnosis of restrictive cardiomyopathy due to AL cardiac amyloidosis. Kardiologia Polska 2016, 74, 796. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kindermann, I.; Kindermann, M.; Mahfoud, F.; Ukena, C.; Athanasiadis, A.; Hill, S.; Mahrholdt, H.; Voehringer, M.; Schieber, M.; et al. Comparative Evaluation of Left and Right Ventricular Endomyocardial Biopsy. Circulation 2010, 122, 900–909. [Google Scholar] [CrossRef]
- Swan, N.; Skinner, M.; O’Hara, C.J. Bone Marrow Core Biopsy Specimens in AL (Primary) Amyloidosis: A Morphologic and Immunohistochemical Study of 100 Cases. Am. J. Clin. Pathol. 2003, 120, 610–616. [Google Scholar] [CrossRef]
- Suzuki, T.; Kusumoto, S.; Yamashita, T.; Masuda, A.; Kinoshita, S.; Yoshida, T.; Takami-Mori, F.; Takino, H.; Ito, A.; Ri, M.; et al. Labial salivary gland biopsy for diagnosing immunoglobulin light chain amyloidosis: A retrospective analysis. Ann. Hematol. 2015, 95, 279–285. [Google Scholar] [CrossRef]
- Freudenthaler, S.; Hegenbart, U.; Schönland, S.; Behrens, H.-M.; Krüger, S.; Röcken, C. Amyloid in biopsies of the gastrointestinal tract—A retrospective observational study on 542 patients. Virchows Arch. 2016, 468, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.D.; Blaney, E. Evaluation and Management Strategies for GI Involvement with Amyloidosis. Am. J. Med. 2022, 135, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Van Gameren, I.I.; Hazenberg, B.P.C.; Bijzet, J.; Van Rijswijk, M.H. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006, 54, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Yakupova, E.I.; Bobyleva, L.G.; Vikhlyantsev, I.M.; Bobylev, A.G. Congo Red and amyloids: History and relationship. Biosci. Rep. 2019, 39, BSR20181415. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.J. Diagnosis of amyloid using Congo red. In Amyloid and Related Disorders; Picken, M.M., Herrera, G.A., Dogan, A., Eds.; Springer: Berlin, Germany, 2012; pp. 167–173. [Google Scholar]
- Stats, M.A.; Stone, J.R. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 2016, 25, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Gustavsson; Hellman, U.; Sletten, K.; Murphy, C.L.; Weiss, D.T.; Solomon, A.; Olofsson, B.-O.; Westermark, P. Amyloid deposits in transthyretin-derived amyloidosis: Cleaved transthyretin is associated with distinct amyloid morphology. J. Pathol. 2005, 206, 224–232. [Google Scholar] [CrossRef]
- Gustafsson, S.; Ihse, E.; Henein, M.Y.; Westermark, P.; Lindqvist, P.; Suhr, O.B. Amyloid Fibril Composition as a Predictor of Development of Cardiomyopathy After Liver Transplantation for Hereditary Transthyretin Amyloidosis. Transplantation 2012, 93, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Howie, A.J. “Green (or apple-green) birefringence” of Congo red-stained amyloid. Amyloid 2015, 22, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Picken, M.M.; Herrera, G.A. The Burden of “Sticky” Amyloid: Typing Challenges. Arch. Pathol. Lab. Med. 2007, 131, 850–851. [Google Scholar] [CrossRef]
- Barreca, A.; Bottasso, E.; Veneziano, F.; Giarin, M.; Nocifora, A.; Martinetti, N.; Attanasio, A.; Biancone, L.; Benevolo, G.; Roccatello, D.; et al. Immunohistochemical typing of amyloid in fixed paraffin-embedded samples by an automatic procedure: Comparison with immunofluorescence data on fresh-frozen tissue. PLoS ONE 2021, 16, e0256306. [Google Scholar] [CrossRef]
- Castellani, C.; Fedrigo, M.; Frigo, A.C.; Della Barbera, M.; Thiene, G.; Valente, M.; Adami, F.; Angelini, A. Application of confocal laser scanning microscopy for the diagnosis of amyloidosis. Virchows Arch. 2017, 470, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Palladini, G. Enlightening light chain deposition disease. Blood 2015, 126, 2770–2771. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Pucci, A.; Bernazzali, S.; Maccherini, M.; Buda, G.; Passino, C.; Merlini, G.; Emdin, M. Cardiac light-chain deposition disease relapsing in the transplanted heart. Amyloid 2017, 24, 135–137. [Google Scholar] [CrossRef]
- Vergaro, G.; Castiglione, V.; Poletti, R.; Buda, G.; Pucci, A.; Musetti, V.; Genovesi, D.; Aimo, A.; Passino, C.; Emdin, M. Biopsy Evidence of Sequential Transthyretin and Immunoglobulin Light-Chain Cardiac Amyloidosis in the Same Patient. JACC Case Rep. 2021, 3, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Pucci, A.; (Histopathology Department, Pisa University Hospital, Pisa, Italy); Genovesi, D.; (Fondazione Toscana G. Monasterio, Pisa, Italy); Giorgetti, A.; (Fondazione Toscana G. Monasterio, Pisa, Italy). Histological and clinical images. Personal communication.
- Vrana, J.A.; Gamez, J.D.; Madden, B.J.; Theis, J.D.; Bergen, H.R.; Dogan, A. Classification of amyloidosis by laser microdissection and mass spectrometry–based proteomic analysis in clinical biopsy specimens. Blood 2009, 114, 4957–4959. [Google Scholar] [CrossRef] [PubMed]
- Lavatelli, F.; Vrana, J.A. Proteomic typing of amyloid deposits in systemic amyloidoses. Amyloid 2011, 18, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.J.; Gamez, J.D.; Vrana, J.A.; Theis, J.D.; Giannini, C.; Scheithauer, B.W.; Parisi, J.E.; Lucchinetti, C.F.; Pendlebury, W.W.; Bergen, H.R.; et al. Immunoglobulin derived depositions in the nervous system: Novel mass spectrometry application for protein characterization in formalin-fixed tissues. Lab. Investig. 2008, 88, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Lavatelli, F.; Valentini, V.; Palladini, G.; Verga, L.; Russo, P.; Foli, A.; Obici, L.; Sarais, G.; Perfetti, V.; Casarini, S.; et al. Mass spectrometry-based proteomics as a diagnostic tool when immunoelectron microscopy fails in typing amyloid deposits. Amyloid 2011, 18, 64–66. [Google Scholar] [CrossRef]
- Dogan, A. Amyloidosis: Insights from Proteomics. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Lavatelli, F.; Merlini, G. Advances in proteomic study of cardiac amyloidosis: Progress and potential. Expert Rev. Proteom. 2016, 13, 1017–1027. [Google Scholar] [CrossRef]
- Benson, M.D.; Berk, J.L.; Dispenzieri, A.; Damy, T.; Gillmore, J.D.; Hazenberg, B.P.; Lavatelli, F.; Picken, M.M.; Röcken, C.; Schönland, S.; et al. Tissue biopsy for the diagnosis of amyloidosis: Experience from some centres. Amyloid 2021, 29, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, F.; Lavatelli, F.; Di Silvestre, D.; Valentini, V.; Rossi, R.; Palladini, G.; Obici, L.; Verga, L.; Mauri, P.; Merlini, G. Reliable typing of systemic amyloidoses through proteomic analysis of subcutaneous adipose tissue. Blood 2012, 119, 1844–1847. [Google Scholar] [CrossRef]
- Sompuram, S.R.; Vani, K.; Messana, E.; Bogen, S.A. A Molecular Mechanism of Formalin Fixation and Antigen Retrieval. Am. J. Clin. Pathol. 2004, 121, 190–199. [Google Scholar] [CrossRef]
- Canetti, D.; Rendell, N.B.; Di Vagno, L.; Gilbertson, J.A.; Rowczenio, D.; Rezk, T.; Gillmore, J.D.; Hawkins, P.N.; Verona, G.; Mangione, P.P.; et al. Misidentification of Transthyretin and Immunoglobulin Variants by Proteomics Due to Methyl Lysine Formation in Formalin-Fixed Paraffin-Embedded Amyloid Tissue. Amyloid 2017, 24, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Angenendt, L.; Schliemann, C.; Hartmann, W.; König, S. Are formalin-fixed and paraffin-embedded tissues fit for proteomic analysis? Biol. Mass Spectrom. 2019, 55, e4347. [Google Scholar] [CrossRef] [PubMed]
- Tanca, A.; Pagnozzi, D.; Falchi, G.; Biosa, G.; Rocca, S.; Foddai, G.; Uzzau, S.; Addis, M.F. Impact of fixation time on GeLC–MS/MS proteomic profiling of formalin-fixed, paraffin-embedded tissues. J. Proteom. 2011, 74, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Lavatelli, F.; Merlini, G. How do we improve treatments for patients with amyloidosis using proteomics? Expert Rev. Proteom. 2017, 14, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Shidham, V.B.; Hunt, B.; Jaradeh, S.S.; Barboi, A.C.; Devata, S.; Hari, P. Performing and Processing FNA of Anterior Fat Pad for Amyloid. J. Vis. Exp. 2010, 44, 1747. [Google Scholar] [CrossRef]
- Lavatelli, F.; Perlman, D.H.; Spencer, B.; Prokaeva, T.; McComb, M.E.; Théberge, R.; Connors, L.; Bellotti, V.; Seldin, D.C.; Merlini, G.; et al. Amyloidogenic and Associated Proteins in Systemic Amyloidosis Proteome of Adipose Tissue. Mol. Cell. Proteom. 2008, 7, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Vrana, J.A.; Theis, J.D.; Dasari, S.; Mereuta, O.M.; Dispenzieri, A.; Zeldenrust, S.R.; Gertz, M.A.; Kurtin, P.J.; Grogg, K.L.; Dogan, A. Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 2014, 99, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Poppi, I.; Cavedagna, T.M.; Zamagni, E.; Leone, O.; Corti, B.; Milandri, A.; Bacci, F.; Ramazzotti, E.; Mancini, R.; et al. A targeted proteomics approach to amyloidosis typing. Clin. Mass Spectrom. 2018, 7, 18–28. [Google Scholar] [CrossRef]
- Palstrøm, N.B.; Rojek, A.M.; Møller, H.E.H.; Hansen, C.T.; Matthiesen, R.; Rasmussen, L.M.; Abildgaard, N.; Beck, H.C. Classification of Amyloidosis by Model-Assisted Mass Spectrometry-Based Proteomics. Int. J. Mol. Sci. 2021, 23, 319. [Google Scholar] [CrossRef] [PubMed]
- Mollee, P.; Boros, S.; Loo, D.; Ruelcke, J.E.; Lakis, V.A.; Cao, K.-A.L.; Renaut, P.; Hill, M.M. Implementation and evaluation of amyloidosis subtyping by laser-capture microdissection and tandem mass spectrometry. Clin. Proteom. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Steen, H.; Mann, M. The abc’s (and xyz’s) of peptide sequencing. Nat. Rev. Mol. Cell Biol. 2004, 5, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, N.; Rojek, A.M.; Møller, H.E.; Palstrøm, N.B.; Nyvold, C.G.; Rasmussen, L.M.; Hansen, C.T.; Beck, H.C.; Marcussen, N. Immunoelectron microscopy and mass spectrometry for classification of amyloid deposits. Amyloid 2019, 27, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Scivetti, M.; Favia, G.; Fatone, L.; Maiorano, E.; Crincoli, V. Concomitant use of Congo red staining and confocal laser scanning microscopy to detect amyloidosis in oral biopsy: A clinicopathological study of 16 patients. Ultrastruct. Pathol. 2016, 40, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Bezard, M.; Petrie, A.; Chacko, L.; Cohen, O.C.; Ravichandran, S.; Ogunbiyi, O.; Kharoubi, M.; Ganeshananthan, S.; Ganeshananthan, S.; et al. Characteristics and natural history of early-stage cardiac transthyretin amyloidosis. Eur. Heart J. 2022, 43, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Lenihan, D.; Merlini, G. Future Developments in Light Chain Amyloidosis Management. Am. J. Med. 2022, 135, S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Morfino, P.; Aimo, A.; Panichella, G.; Rapezzi, C.; Emdin, M. Amyloid seeding as a disease mechanism and treatment target in transthyretin cardiac amyloidosis. Heart Fail. Rev. 2022, 27, 2187–2200. [Google Scholar] [CrossRef]
- Papa, R.; Gilbertson, J.A.; Rendell, N.; Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N.; Lachmann, H.J. Two types of systemic amyloidosis in a single patient. Amyloid 2020, 27, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, J.A.; Theis, J.D.; Vrana, J.A.; Lachmann, H.; Wechalekar, A.; Whelan, C.; Hawkins, P.N.; Dogan, A.; Gillmore, J.D. A Comparison of Immunohistochemistry and Mass Spectrometry for Determining the Amyloid Fibril Protein from Formalin-Fixed Biopsy Tissue. J. Clin. Pathol. 2015, 68, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Theis, J.D.; Vrana, J.A.; Rech, K.L.; Dao, L.N.; Howard, M.T.; Dispenzieri, A.; Gertz, M.A.; Hasadsri, L.; Highsmith, W.E.; et al. Amyloid Typing by Mass Spectrometry in Clinical Practice: A Comprehensive Review of 16,175 Samples. Mayo Clin. Proc. 2020, 95, 1852–1864. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musetti, V.; Greco, F.; Castiglione, V.; Aimo, A.; Palmieri, C.; Genovesi, D.; Giorgetti, A.; Emdin, M.; Vergaro, G.; McDonnell, L.A.; et al. Tissue Characterization in Cardiac Amyloidosis. Biomedicines 2022, 10, 3054. https://doi.org/10.3390/biomedicines10123054
Musetti V, Greco F, Castiglione V, Aimo A, Palmieri C, Genovesi D, Giorgetti A, Emdin M, Vergaro G, McDonnell LA, et al. Tissue Characterization in Cardiac Amyloidosis. Biomedicines. 2022; 10(12):3054. https://doi.org/10.3390/biomedicines10123054
Chicago/Turabian StyleMusetti, Veronica, Francesco Greco, Vincenzo Castiglione, Alberto Aimo, Cataldo Palmieri, Dario Genovesi, Assuero Giorgetti, Michele Emdin, Giuseppe Vergaro, Liam A. McDonnell, and et al. 2022. "Tissue Characterization in Cardiac Amyloidosis" Biomedicines 10, no. 12: 3054. https://doi.org/10.3390/biomedicines10123054
APA StyleMusetti, V., Greco, F., Castiglione, V., Aimo, A., Palmieri, C., Genovesi, D., Giorgetti, A., Emdin, M., Vergaro, G., McDonnell, L. A., & Pucci, A. (2022). Tissue Characterization in Cardiac Amyloidosis. Biomedicines, 10(12), 3054. https://doi.org/10.3390/biomedicines10123054





