The Uremic Toxin Homocysteine Exacerbates the Brain Inflammation Induced by Renal Ischemia-Reperfusion in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of Renal IR Injury
2.3. Biochemical Assays
2.4. Hematoxylin and Eosin (H&E) and Nissel Staining
2.5. Immunohistochemistry (IHC) Staining
2.6. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling (TUNEL) Assay
2.7. Western Blot Analysis
2.8. Reverse Transcription and Quantitative PCR Analysis
2.9. Statistical Analysis
3. Results
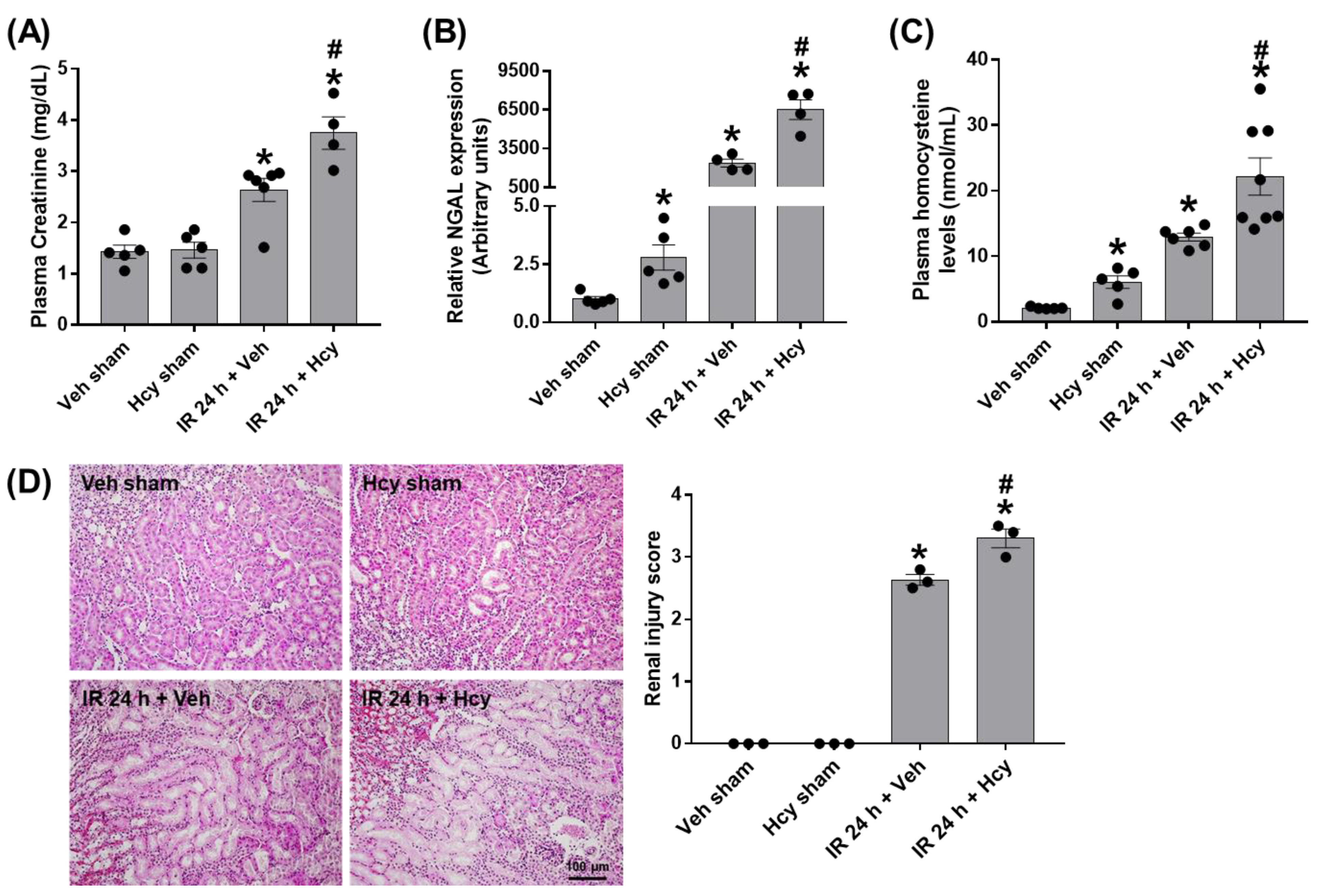
3.1. Homocysteine Exacerbates Renal IR-Induced Renal Damage
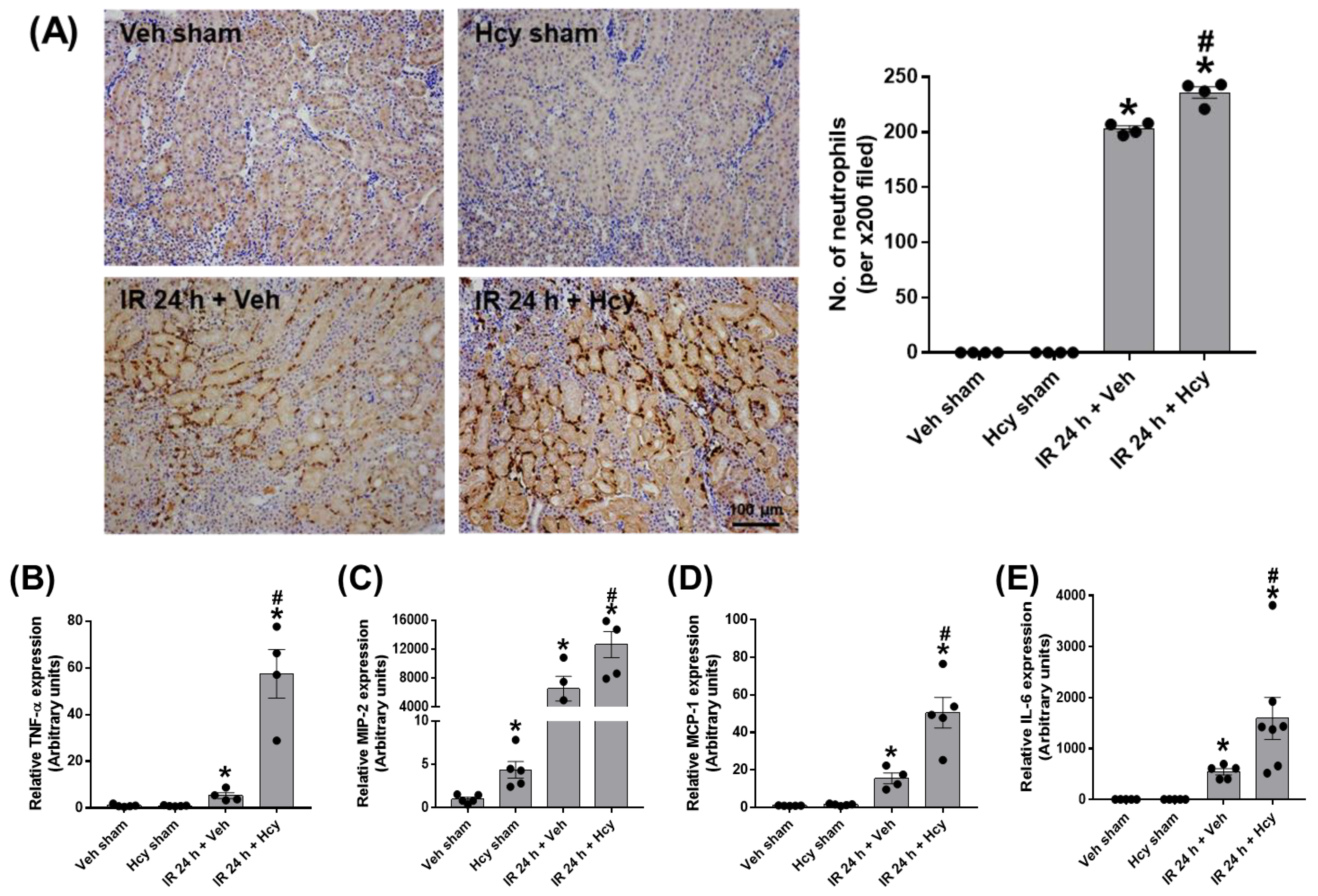
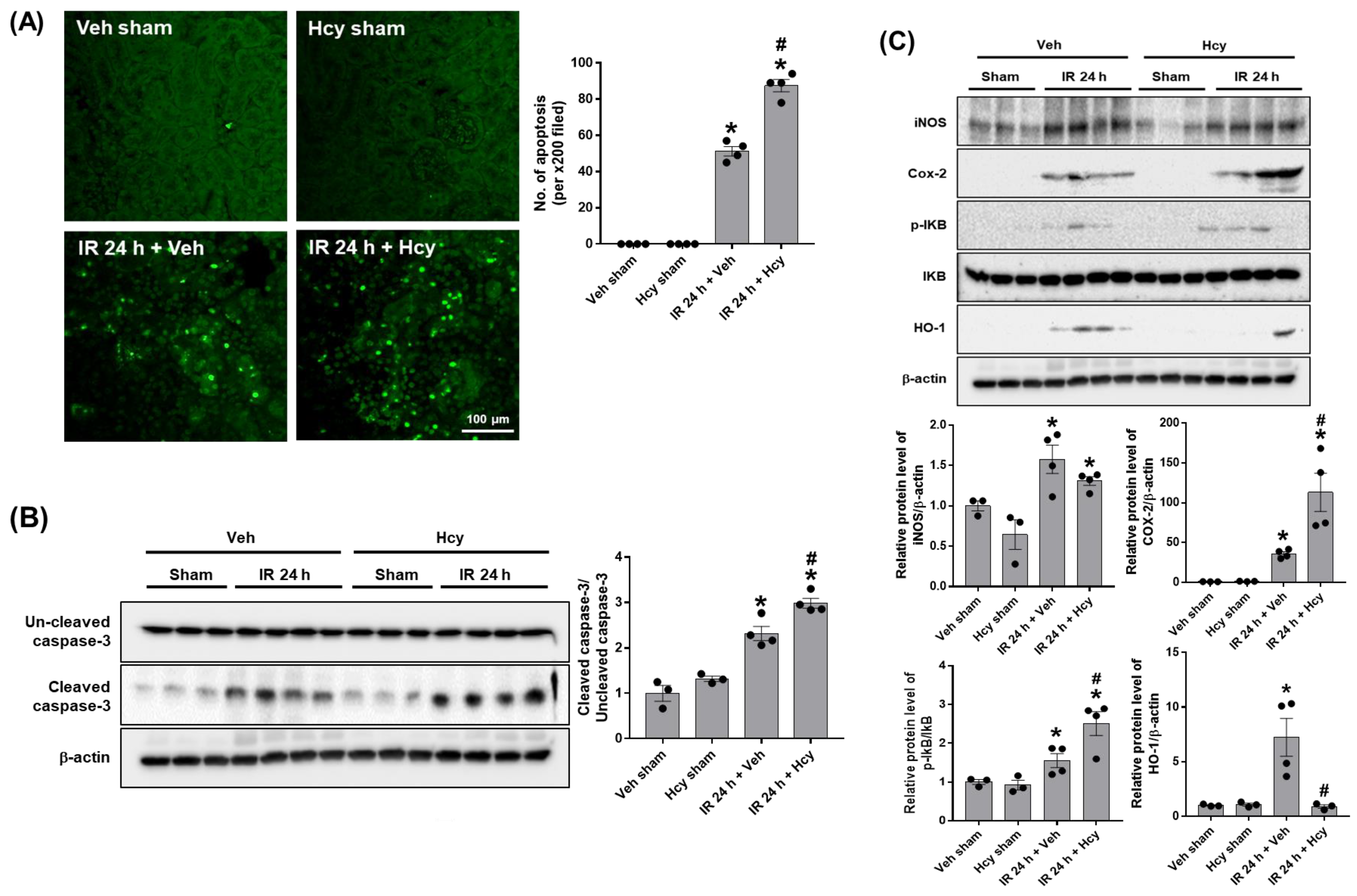
3.2. Hcy Exacerbates Renal IR-Induced Inflammation and Tubular Apoptosis in the Kidney
3.3. Hcy Induces Astrocyte Activation and Inflammation of the Prefrontal Cortex in Renal IR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerda, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Herget-Rosenthal, S.; Glorieux, G.; Jankowski, J.; Jankowski, V. Uremic toxins in acute kidney injury. Semin Dial. 2009, 22, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Falconi, C.A.; Junho, C.; Fogaca-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 2010, 72, 1–11. [Google Scholar]
- Lara-Prado, J.I.; Pazos-Perez, F.; Mendez-Landa, C.E.; Grajales-Garcia, D.P.; Feria-Ramirez, J.A.; Salazar-Gonzalez, J.J.; Cruz-Romero, M.; Trevino-Becerra, A. Acute Kidney Injury and Organ Dysfunction: What Is the Role of Uremic Toxins? Toxins 2021, 13, 551. [Google Scholar] [CrossRef]
- Assem, M.; Lando, M.; Grissi, M.; Kamel, S.; Massy, Z.A.; Chillon, J.M.; Henaut, L. The Impact of Uremic Toxins on Cerebrovascular and Cognitive Disorders. Toxins 2018, 10, 303. [Google Scholar] [CrossRef]
- Liu, M.; Liang, Y.; Chigurupati, S.; Lathia, J.D.; Pletnikov, M.; Sun, Z.; Crow, M.; Ross, C.A.; Mattson, M.P.; Rabb, H. Acute kidney injury leads to inflammation and functional changes in the brain. J. Am. Soc. Nephrol. 2008, 19, 1360–1370. [Google Scholar] [CrossRef]
- Malek, M. Brain consequences of acute kidney injury: Focusing on the hippocampus. Kidney Res. Clin. Pract. 2018, 37, 315–322. [Google Scholar] [CrossRef]
- Massy, Z.A. Importance of homocysteine, lipoprotein (a) and non-classical cardiovascular risk factors (fibrinogen and advanced glycation end-products) for atherogenesis in uraemic patients. Nephrol. Dial. Transplant. 2000, 15 (Suppl. S5), 81–91. [Google Scholar] [CrossRef]
- Zeng, X.; Dai, J.; Remick, D.G.; Wang, X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes. Circ. Res. 2003, 93, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Sibarov, D.A.; Boikov, S.I.; Karelina, T.V.; Antonov, S.M. GluN2 Subunit-Dependent Redox Modulation of NMDA Receptor Activation by Homocysteine. Biomolecules 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S. Elevated plasma homocysteine levels: Risk factor or risk marker for the development of dementia and Alzheimer’s disease? J. Alzheimers Dis. 2006, 9, 393–398. [Google Scholar] [CrossRef]
- Dusabimana, T.; Kim, S.R.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. P2Y2R contributes to the development of diabetic nephropathy by inhibiting autophagy response. Mol. Metab. 2020, 42, 101089. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Dusabimana, T.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Honokiol Protects the Kidney from Renal Ischemia and Reperfusion Injury by Upregulating the Glutathione Biosynthetic Enzymes. Biomedicines 2020, 8, 352. [Google Scholar] [CrossRef]
- Li, S.; Qiu, B.; Lu, H.; Lai, Y.; Liu, J.; Luo, J.; Zhu, F.; Hu, Z.; Zhou, M.; Tian, J.; et al. Hyperhomocysteinemia Accelerates Acute Kidney Injury to Chronic Kidney Disease Progression by Downregulating Heme Oxygenase-1 Expression. Antioxid. Redox Signal 2019, 30, 1635–1650. [Google Scholar] [CrossRef]
- Bobot, M.; Thomas, L.; Moyon, A.; Fernandez, S.; McKay, N.; Balasse, L.; Garrigue, P.; Brige, P.; Chopinet, S.; Poitevin, S.; et al. Uremic Toxic Blood-Brain Barrier Disruption Mediated by AhR Activation Leads to Cognitive Impairment during Experimental Renal Dysfunction. J. Am. Soc. Nephrol. 2020, 31, 1509–1521. [Google Scholar] [CrossRef]
- Faubel, S.; Shah, P.B. Immediate Consequences of Acute Kidney Injury: The Impact of Traditional and Nontraditional Complications on Mortality in Acute Kidney Injury. Adv. Chronic. Kidney Dis. 2016, 23, 179–185. [Google Scholar] [CrossRef]
- Xie, D.; Yuan, Y.; Guo, J.; Yang, S.; Xu, X.; Wang, Q.; Li, Y.; Qin, X.; Tang, G.; Huo, Y.; et al. Hyperhomocysteinemia predicts renal function decline: A prospective study in hypertensive adults. Sci. Rep. 2015, 5, 16268. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lee, H.T. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res. Clin. Pract. 2019, 38, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, J.; Dong, R.; Da, J.; Li, Q.; Hu, Y.; Yu, F.; Ran, Y.; Zha, Y.; Long, Y. Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis. Open Life Sci. 2021, 16, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Prathapasinghe, G.A.; Siow, Y.L.; O, K. Detrimental role of homocysteine in renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2007, 292, F1354–F1363. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kyles, P.; Kalani, A.; Tyagi, N. Hydrogen Sulfide Ameliorates Homocysteine-Induced Alzheimer’s Disease-Like Pathology, Blood-Brain Barrier Disruption, and Synaptic Disorder. Mol. Neurobiol. 2016, 53, 2451–2467. [Google Scholar] [CrossRef]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef]
- Kaushal, V.; Schlichter, L.C. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J. Neurosci. 2008, 28, 2221–2230. [Google Scholar] [CrossRef]
- Tsai, H.H.; Yen, R.F.; Lin, C.L.; Kao, C.H. Increased risk of dementia in patients hospitalized with acute kidney injury: A nationwide population-based cohort study. PLoS ONE 2017, 12, e0171671. [Google Scholar] [CrossRef]

| Genes | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| GAPDH | GTGGCAAAGTGGAGATTGTTG | TTGACTGTGCCGTTGAATTTG |
| GFAP | AGAAAGGTTGAATCGCTGGA | CGGCGATAGTCGTTAGCTTC |
| IL-6 | CCAATTCATCTTGAAATCAC | GGAATGTCCACAAACTGATA |
| MCP-1 | ACCTTTGAATGTGAAGTTGA | CTACAGAAGTGCTTGAGGTG |
| MIP-2 | AGAGGGTGAGTTGGGAACTA | GCCATCCGACTGCATCTATT |
| NGAL | CACCACGGACTACAACCAGTTCGC | TCAGTTGTCAATGCATTGGTCGGTG |
| TNFα | CATATACCTGGGAGGAGTCT | GAGCAATGACTCCAAAGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.J.; Je, J.; Dusabimana, T.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. The Uremic Toxin Homocysteine Exacerbates the Brain Inflammation Induced by Renal Ischemia-Reperfusion in Mice. Biomedicines 2022, 10, 3048. https://doi.org/10.3390/biomedicines10123048
Park EJ, Je J, Dusabimana T, Yun SP, Kim HJ, Kim H, Park SW. The Uremic Toxin Homocysteine Exacerbates the Brain Inflammation Induced by Renal Ischemia-Reperfusion in Mice. Biomedicines. 2022; 10(12):3048. https://doi.org/10.3390/biomedicines10123048
Chicago/Turabian StylePark, Eun Jung, Jihyun Je, Theodomir Dusabimana, Seung Pil Yun, Hye Jung Kim, Hwajin Kim, and Sang Won Park. 2022. "The Uremic Toxin Homocysteine Exacerbates the Brain Inflammation Induced by Renal Ischemia-Reperfusion in Mice" Biomedicines 10, no. 12: 3048. https://doi.org/10.3390/biomedicines10123048
APA StylePark, E. J., Je, J., Dusabimana, T., Yun, S. P., Kim, H. J., Kim, H., & Park, S. W. (2022). The Uremic Toxin Homocysteine Exacerbates the Brain Inflammation Induced by Renal Ischemia-Reperfusion in Mice. Biomedicines, 10(12), 3048. https://doi.org/10.3390/biomedicines10123048








