Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms
Abstract
:1. Introduction
2. Classification of Periodontitis
3. Clinical Features of Periodontitis
4. Diagnosis of Periodontitis
5. Inflammatory Changes Occurring in Periodontitis
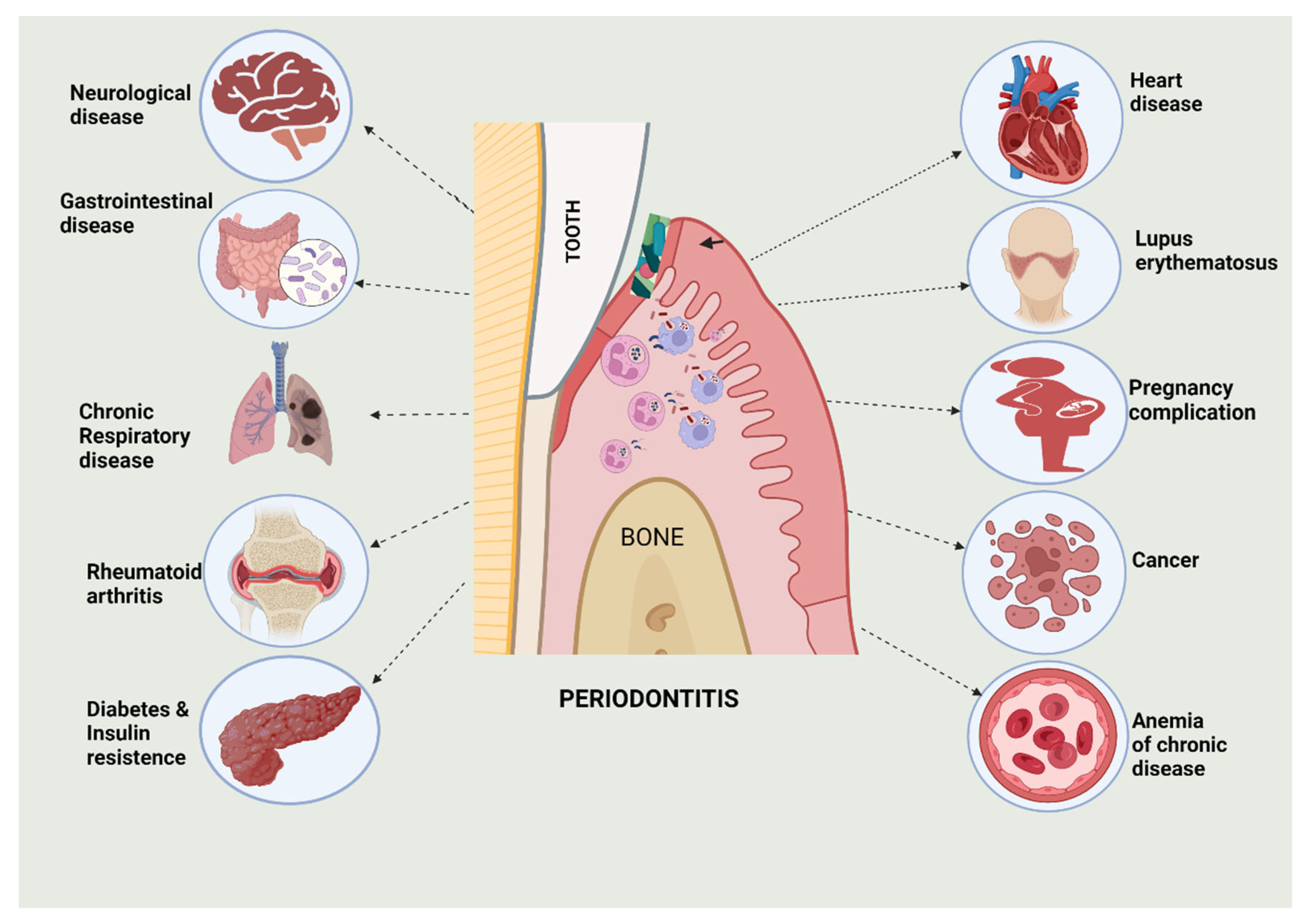
6. Periodontitis Associated with other Systemic Diseases
6.1. Cardiovascular Diseases
6.2. Pneumonia and Respiratory Tract Infections
6.3. Diabetes Mellitus
6.4. Alzheimer’s Disease
6.5. Colorectal Cancer
6.6. Adverse Pregnancy
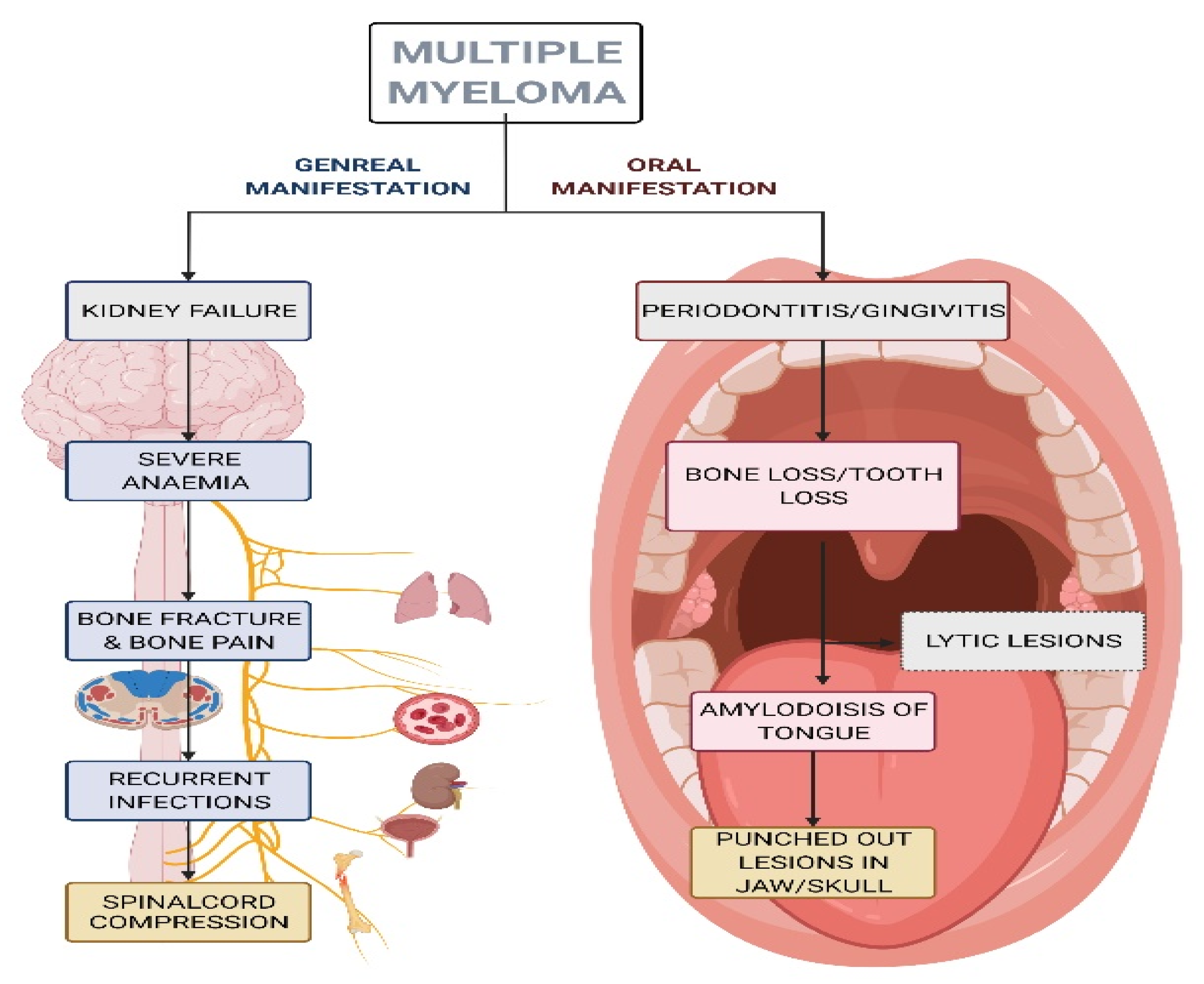
6.7. Multiple Myeloma
6.8. Cancer in Different Parts of the Body
7. Periodontal Microbial Carcinogenesis: Underlying Mechanisms
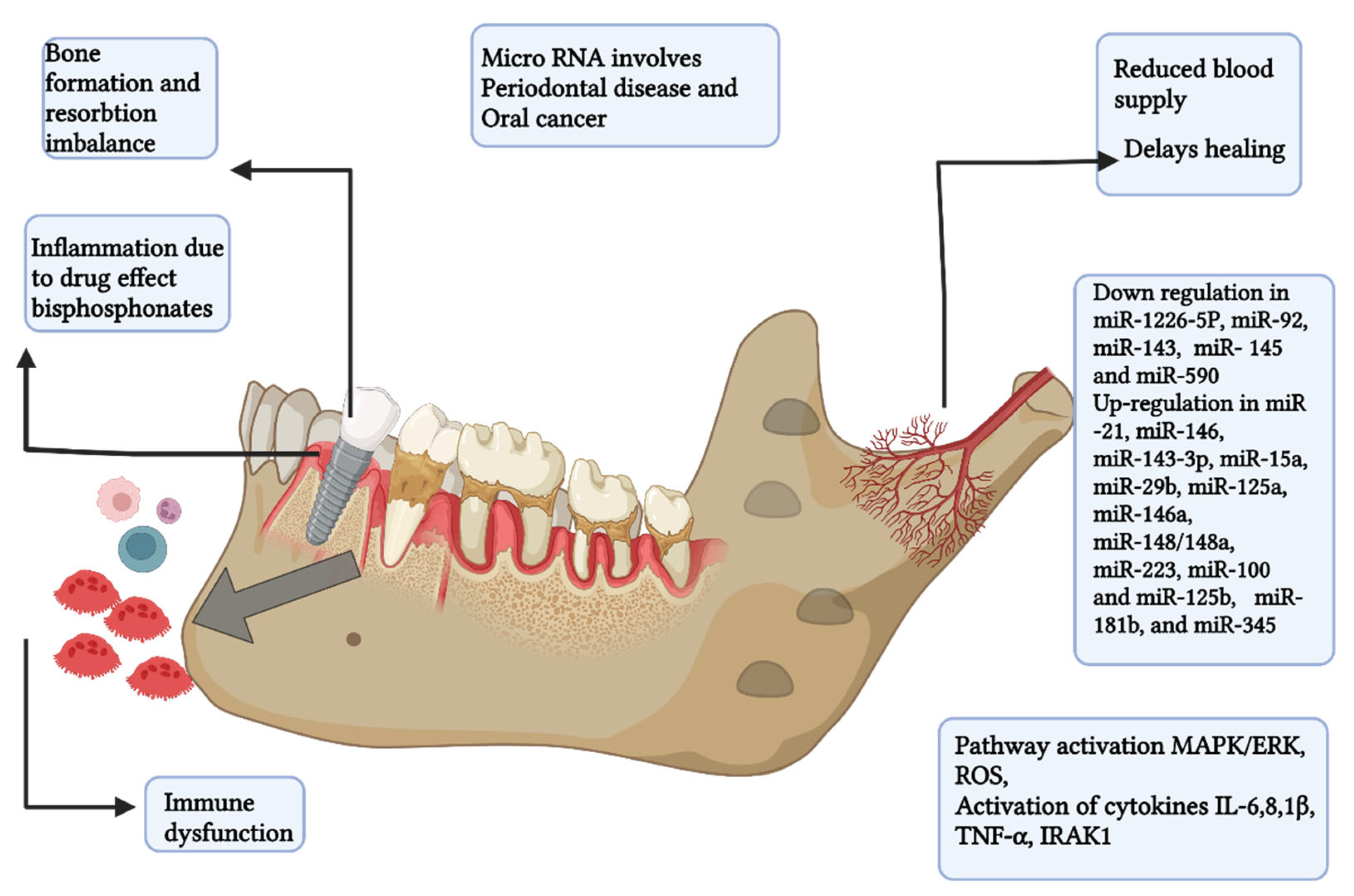
8. Role of microRNA in Periodontitis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Porphyromonas gingivalis | P. gingivalis |
| IL | Interleukin |
| Aggregatibacter actinomycetemcomitans | A. actinomycetemcomitans |
| Prevotella intermedia | P. inter-media |
| Tannerella forsythia | T. forsythia |
| Treponema denticola | T denticola |
| Fusobacterium nucleatum | F. nucleatum |
| Chlamydia pneumoniae | C. pneumoniae |
| mTOR | mammalian target of rapamycin |
| S6K-1 | Ribosomal protein S6 kinase beta-1 |
| BCAAs | branched-chain amino acids |
| PLSCs | periodontal ligament stem cells |
| CRP | C-reactive protein |
| TNFα | Tumor Necrosis Factor α |
| OSCC | Oral squamous cell carcinoma cells |
| TGF | Transforming Growth Factor |
| CRC | Colorectal carcinoma |
| Th | T helper |
| LPS | lipopolysaccharide |
| MM | Multiple myeloma |
| Tannerella forsythia | T. forsythia |
| PKB/Akt | Protein kinase B |
| MMP9 | Serum Matrix metalloproteinase-9 |
| ROS | reactive oxygen species |
| miRNA | microRNA |
| NF-κB | Nuclear factor kappa B |
References
- Jensen, A.; Gronkjaer, L.; Holmstrup, P.; Vilstrup, H.; Kilian, M. Unique subgingival microbiota associated with periodontitis in cirrhosis patients. Sci. Rep. 2018, 8, 10718. [Google Scholar] [CrossRef] [Green Version]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Knight, E.T.; Liu, J.; Seymour, G.J.; Faggion, C.M., Jr.; Cullinan, M.P. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontology 2016, 71, 22–51. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Magalhães, C.B.; Hartenbach, F.A.; do Souto, R.M.; da Silva-Boghossian, C.M. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bascones-Martínez, A.; Figuero-Ruiz, E. Periodontal diseases as bacterial infection. Med. Oral Patol. Oral Cir. Bucal 2004, 9, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrysanthakopoulos, N.A.; Reppas, S.A.; Oikonomou, A.A. Association of periodontal disease indices with risk of gastric adenocarcinoma. Ann. Res. Hosp. 2017, 1, 4. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontology 2000, 83, 213–233. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef]
- Demmer, R.T.; Papapanou, P.N. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontology 2000, 53, 28–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus report: Periodontitis as a manifestation of systemic diseases. Ann. Periodontol. 1999, 4, 64. [Google Scholar] [CrossRef]
- Sanz, M.; Tonetti, M. European Federation of Periodontology. Available online: https://www.efp.org/fileadmin/uploads/efp/Documents/Campaigns/New_Classification/Guidance_Notes/report-02.pdf (accessed on 21 September 2022).
- Gasner, N.S.; Schure, R.S. Periodontal disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554590/ (accessed on 21 September 2022).
- Gaurilcikaite, E.; Renton, T.; Grant, A.D. The paradox of painless periodontal disease. Oral Dis. 2017, 23, 451–463. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk Indicators for periodontitis in US adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, S.; Komiyama, M.; Ozaki, Y.; Yamakage, H.; Satoh-Asahara, N.; Yasoda, A.; Wada, H.; Funamoto, M.; Shimizu, K.; Miyazaki, Y.; et al. Gingival bleeding and pocket depth among smokers and the related changes after short-term smoking cessation. Acta Odontol. Scand. 2022, 80, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Sanz, M. Implementation of the new classification of periodontal diseases: Decision-making algorithms for clinical practice and education. J. Clin. Periodontol. 2019, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Reddahi, S.; Bouziane, A.; Rida, S.; Tligui, H.; Ennibi, O. Salivary biomarkers in periodontitis patients: A pilot study. Int. J. Dent. 2022, 2022, 3664516. [Google Scholar] [CrossRef]
- Preoteasa, C.T.; Dumitrache, A.; Enache, M.; Iosif, L.; Preoteasa, E. Patient’s information on medical aspects. Rom. J. Oral Rehabil. 2018, 10, 36–40. [Google Scholar]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef] [Green Version]
- Gilowski, Ł.; Wiench, R.; Płocica, I.; Krzemiński, T.F. Amount of interleukin-1β and interleukin-1 receptor antagonists in periodontitis and healthy patients. Arch. Oral Biol. 2014, 59, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Isaza-Guzmán, D.M.; Medina-Piedrahíta, V.M.; Gutiérrez-Henao, C.; Tobón-Arroyave, S.I. Salivary levels of NLRP3 inflammasome-related proteins as potential biomarkers of periodontal clinical status. J. Periodontol. 2017, 88, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.R.; Groeger, S.; Johansson, A.; Meyle, J. T helper cells from aggressive periodontitis patients produce higher levels of interleukin-1 beta and interleukin-6 in interaction with Porphyromonas gingivalis. Clin. Oral Investig. 2014, 18, 1835–1843. [Google Scholar] [CrossRef]
- Gum Disease and Heart Disease: The Common Thread. Harvard Health Publishing. Available online: https://www.health.harvard.edu/heart-health/gum-disease-and-heart-disease-the-common-thread#:~:text=People%20with%20gum%20disease%20(also,gum%20disease%20develops%20heart%20problems (accessed on 21 September 2022).
- Periodontitis and Atherosclerotic Cardiovascular Disease. Available online: https://www.bsperio.org.uk/assets/downloads/EFP_cardiovascular-disease-gum-disease.pdf (accessed on 21 September 2022).
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Chukkapalli, S.S.; Rivera, M.F.; Lee, J.Y.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS ONE 2014, 9, e97811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Novak, E.K.; Sojar, H.T.; Swank, R.T.; Kuramitsu, H.K.; Genco, R.J. Porphyromonas gingivalis platelet aggregation activity: Outer membrane vesicles are potent activators of murine platelets. Oral. Microbiol. Immunol. 2000, 15, 393–396. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stănescu, I.; Bulboacă, A.E.; Micu, I.C.; Bolboacă, S.D.; Feștilă, D.G.; Bulboacă, A.C.; Bodizs, G.; Dogaru, G.; Boarescu, P.M.; Popa-Wagner, A.; et al. Gender differences in the levels of periodontal destruction, behavioral risk factors and systemic oxidative stress in ischemic stroke patients: A cohort pilot study. J. Clin. Med. 2020, 9, 1744. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Wang, M.; Bagby, G.J.; Nelson, S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J. Immunol. 2008, 181, 4141–4149. [Google Scholar] [CrossRef] [Green Version]
- Sonti, R.; Fleury, C. Fusobacterium necrophorum presenting as isolated lung nodules. Respir. Med. Case Rep. 2015, 15, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.D.; Kerber, C.A.; Tergin, H.F. Unusual presentation of Lemierre’s syndrome due to Fusobacterium nucleatum. J. Clin. Microbiol. 2003, 41, 3445–3448. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.M.; Sung, R.S.; Scannapieco, F.A.; Haase, E.M. Genetic relationships between Candida albicans strains isolated from dental plaque, trachea, and bronchoalveolar lavage fluid from mechanically ventilated intensive care unit patients. J. Oral Microbiol. 2011, 3, 6362. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Periodontitis: A risk factor to respiratory diseases. Lung India 2010, 27, 189. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Di Pietro, M.; Farcomeni, A.; Schiavoni, G.; Sessa, R. Chlamydia pneumoniae-mediated inflammation in atherosclerosis: A meta-analysis. Mediat. Inflamm. 2015, 2015, 378658. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [Green Version]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Liu, C.; Zheng, X.; Jia, X.; Peng, X.; Yang, R.; Zhou, X.; Xu, X. Porphyromonas gingivalis induces insulin resistance by increasing BCAA levels in mice. J. Dent. Res. 2020, 99, 839–846. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Durazzo, M.; Gruden, G. Novel insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines 2022, 10, 178. [Google Scholar] [CrossRef]
- Kothari, M.; Spin-Neto, R.; Nielsen, J.F. Comprehensive oral-health assessment of individuals with acquired brain injury in the neuro-rehabilitation setting. Brain Inj. 2016, 30, 1103–1108. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Gaur, S.; Agnihotri, R. Alzheimer’s disease and chronic periodontitis: Is there an association? Geriatr. Gerontol. Int. 2015, 15, 391–404. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammation, autotoxicity, and Alzheimer’s disease. Neurobiol. Aging 2001, 22, 799–809. [Google Scholar] [CrossRef]
- Cestari, J.A.; Fabri, G.M.; Kalil, J.; Nitrini, R.; Jacob-Filho, W.; de Siqueira, J.T.; Siqueira, S.R. Oral infections and cytokine levels in patients with Alzheimer’s disease and mild cognitive impairment compared with controls. J. Alzheimer Dis. 2016, 52, 1479–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. periodontitis and cognitive decline in Alzheimer’s disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, R.T.; Sun, Y.; Zhou, X.D.; Liu, S.Y.; Han, Q.; Cheng, L.; Peng, X. Treponema denticola promotes OSCC development via the TGF-β signaling pathway. J. Dent. Res. 2022, 101, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Warren, R.L.; Freeman, D.J.; Pleasance, S.; Watson, P.; Moore, R.A.; Cochrane, K.; Allen-Vercoe, E.; Holt, R.A. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 2013, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum contributes to the carcinogenesis of colorectal cancer by inducing inflammation and suppressing host immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef]
- Kaur, M.; Geisinger, M.L.; Geurs, N.C.; Griffin, R.; Vassilopoulos, P.J.; Vermeulen, L.; Haigh, S.; Reddy, M.S. Effect of intensive oral hygiene regimen during pregnancy on periodontal health, cytokine levels, and pregnancy outcomes: A pilot study. J. Periodontol. 2014, 85, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Moss, K.; Beck, J.D.; Hefti, A.; Offenbacher, S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J. Periodontol. 2007, 78, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Fardini, Y.; Chen, C.; Iacampo, K.G.; Peraino, V.A.; Shamonki, J.M.; Redline, R.W. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet. Gynecol. 2010, 115, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, S.; Tétu, A.; Himaya, E.; Morand, M.; Chandad, F.; Rallu, F.; Bujold, E. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J. Matern. Fetal Neonatal Med. 2011, 24, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Chegini, N.; Shiverick, K.T.; Lamont, R.J. Localization of P. gingivalis in the preterm delivery placenta. J. Dent. Res. 2009, 88, 575–578. [Google Scholar] [CrossRef] [Green Version]
- Reyes, L.; Phillips, P.; Wolfe, B.; Golos, T.G.; Walkenhorst, M.; Progulske-Fox, A.; Brown, M. Porphyromonas gingivalis and adverse pregnancy outcome. J. Oral. Microbiol. 2017, 10, 1374153. [Google Scholar] [CrossRef] [Green Version]
- Kunnen, A.; van Pampus, M.G.; Aarnoudse, J.G.; van der Schans, C.P.; Abbas, F.; Faas, M.M. The effect of Porphyromonas gingivalis lipopolysaccharide on pregnancy in the rat. Oral Dis. 2014, 20, 591–601. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Bradley, J.L.; Purkall, D.B. Anticardiolipin in Porphyromonas gingivalis antisera causes fetal loss in mice. J. Dent. Res. 2013, 92, 814–818. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, A.; Blanlot, C.; Ramirez, V.; Sanz, A.; Quintero, A.; Inostroza, C.; Bittner, M.; Navarro, M.; Illanes, S.E. Porphyromonas gingivalis, Treponema denticola, and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: A case-control study. J. Periodontal Res. 2013, 48, 802–809. [Google Scholar] [CrossRef]
- Mao, S.; Park, Y.; Hasegawa, Y.; Tribble, G.D.; James, C.E.; Handfield, M.; Stavropoulos, M.F.; Yilmaz, Ö.; Lamont, R.J. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell. Microbiol. 2007, 9, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.G.; Smith, M.A.; Arnold, R.R.; Offenbacher, S. Effects of Escherichia coli and Porphyromonas gingivalis lipopolysaccharide on pregnancy outcome in the golden hamster. Infect. Immun. 1994, 62, 4652–4655. [Google Scholar] [CrossRef]
- Paju, S.; Oittinen, J.; Haapala, H.; Asikainen, S.; Paavonen, J.; Pussinen, P.J. Porphyromonas gingivalis may interfere with conception in women. J. Oral Microbiol. 2017, 9, 1330644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namiiro, F.B.; Mugalu, J.; McAdams, R.M.; Ndeezi, G. Poor birth weight recovery among low birth weight/preterm infants following hospital discharge in Kampala, Uganda. BMC Pregnancy Childbirth. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Buekens, P.; Fraser, W.D.; Beck, J.; Offenbacher, S. Periodontal disease and adverse pregnancy outcomes: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Albagoush, S.A.; Shumway, C.; Azevedo, A.M. Multiple Myeloma. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534764/ (accessed on 24 February 2022).
- Das, S.; Juliana, N.; Yazit, N.A.A.; Azmani, S.; Abu, I.F. Multiple myeloma: Challenges encountered and future options for better treatment. Int. J. Mol. Sci. 2022, 23, 1649. [Google Scholar] [CrossRef]
- Dhodapkar, M.V. MGUS to myeloma: A mysterious gammopathy of underexplored significance. Blood 2016, 128, 2599–2606. [Google Scholar] [CrossRef]
- Adeyemo, T.A.; Adeyemo, W.L.; Adediran, A.; Akinbami, A.J.; Akanmu, A.S. Orofacial manifestation of hematological disorders: Hemato-oncologic and immuno-deficiency disorders. Indian J. Dent. Res. 2011, 22, 688–697. [Google Scholar] [CrossRef]
- Zhao, X.J.; Sun, J.; Wang, Y.D.; Wang, L. Maxillary pain is the first indication of the presence of multiple myeloma: A case report. Mol. Clin. Oncol. 2014, 2, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.C.; Gerngross, P.J.; Hofstede, T.M.; Weber, D.M.; Chambers, M.S. The multiple oral presentations of multiple myeloma. Support. Care Cancer 2014, 22, 259–267. [Google Scholar] [CrossRef]
- Viggor, S.F.; Frezzini, C.; Farthing, P.M.; Freeman, C.O.; Yeoman, C.M.; Thornhill, M.H. Amyloidosis: An unusual case of persistent oral ulceration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e46–e50. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Oduncu, F.; Mayr, D.; Ehrenfeld, M.; Pautke, C.; Otto, S. Root resorption caused by jaw infiltration of multiple myeloma: Report of a case and literature review. J. Endod. 2014, 40, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Daneshbod, Y.; Arabi, M.A.; Ramzi, M.; Daneshbod, K. Jaw lesion as the first presentation of multiple myeloma diagnosed by fine needle aspiration. Acta Cytol. 2008, 52, 268–270. [Google Scholar] [CrossRef] [PubMed]
- August, M.; Faquin, W.C.; Ferraro, N.F.; Kaban, L.B. Fine-needle aspiration biopsy of intraosseous jaw lesion. J. Oral Maxillofac. Surg. 1999, 57, 1282–1286. [Google Scholar] [CrossRef]
- Salazar, C.R.; Sun, J.; Li, Y.; Francois, F.; Corby, P.; Perez-Perez, G.; Dasanayake, A.; Pei, Z.; Chen, Y. Association between selected oral pathogens and gastric precancerous lesions. PLoS ONE 2013, 8, e51604. [Google Scholar] [CrossRef] [Green Version]
- Cordero, O.J.; Varela-Calvino, R. Oral hygiene might prevent cancer. Heliyon 2018, 4, 00879. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Luo, T.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Microbial communities associated with primary and metastatic head and neck squamous cell carcinoma—A high Fusobacterial and low Streptococcal signature. Sci. Rep. 2017, 7, 9934. [Google Scholar] [CrossRef] [Green Version]
- Nicolae, F.M.; Didilescu, A.C.; Șurlin, P.; Ungureanu, B.S.; Șurlin, V.M.; Pătrașcu, Ș.; Ramboiu, S.; Jelihovschi, I.; Iancu, L.S.; Ghilusi, M.; et al. Subgingival periopathogens assessment and clinical periodontal evaluation of gastric cancer patients—A cross sectional pilot study. Pathogens 2022, 11, 360. [Google Scholar] [CrossRef]
- Chen, Q.L.; Zeng, X.T.; Luo, Z.X.; Duan, X.L.; Qin, J.; Leng, W.D. Tooth loss is associated with increased risk of esophageal cancer: Evidence from a meta-analysis with dose-response analysis. Sci. Rep. 2016, 6, 18900. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, K.A.; Shingala, J.; Evens, A.; Birmann, B.M.; Giovannucci, E.; Michaud, D.S. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. Int. J. Cancer. 2017, 140, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Abnet, C.C.; Qiao, Y.L.; Dawsey, S.M.; Dong, Z.W.; Taylor, P.R.; Mark, S.D. Tooth loss is associated with increased risk of total death and death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. Int. J. Epidemiol. 2005, 34, 467–474. [Google Scholar] [CrossRef]
- Straif, K.; Weiland, S.K.; Bungers, M.; Holthenrich, D.; Taeger, D.; Yi, S.; Keil, U. Exposure to high concentrations of nitrosamines and cancer mortality among a cohort of rubber workers. Occup. Environ. Med. 2000, 57, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoo, Y.S.; Park, K.; Kwon, J.E.; Kim, J.Y.; Monzon, F.A. Genomic aberrations in salivary duct carcinoma arising in Warthin tumor of the parotid gland: DNA microarray and HER2 fluorescence in situ hybridization. Arch. Pathol. Lab. Med. 2011, 135, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Naito, H.; Ohta, Y.; Tanakna, R.; Maeda, N.; Sasaki, J.; Nord, C.E. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Arch. Oral Biol. 1999, 44, 789–793. [Google Scholar] [CrossRef]
- Olsen, I.; Yilmaz, Ö. Possible role of Porphyromonas gingivalis in orodigestive cancers. J. Oral Microbiol. 2019, 11, 1563410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astuti, L.A.; Hatta, M.; Oktawati, S.; Natzir, R.; Dwiyanti, R. Change of TGF-β1 gene expression and TGF-β1 protein level in gingival crevicular fluid and identification of plaque bacteria in a patient with recurrent localized gingival enlargement before and after gingivectomy. Case Rep. Dent. 2018, 2018, 3670583. [Google Scholar] [CrossRef] [Green Version]
- Rajeev, R.; Choudhary, K.; Panda, S.; Gandhi, N. Role of bacteria in oral carcinogenesis. South Asian J. Cancer 2012, 1, 78–83. [Google Scholar] [CrossRef]
- Tezal, M.; Grossi, S.G.; Genco, R.J. Is periodontitis associated with oral neoplasms? J. Periodontol. 2005, 76, 406–410. [Google Scholar] [CrossRef]
- Whisner, C.M.; Athena Aktipis, C. The role of the microbiome in cancer initiation and progression: How microbes and cancer cells utilize excess energy and promote one another’s growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Niture, S.; Dong, X.; Arthur, E.; Chimeh, U.; Nature, S.S.; Zheng, W.; Kumar, D. Oncogenic role of tumor necrosis factor α-induced protein 8 (TNFAIP8). Cells 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Li, L.; Zhao, G.D.; Shi, Z.; Qi, L.L.; Zhou, L.Y.; Fu, Z.X. The Ras/Raf/MEK/ERK signalling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Hermouet, S.; Bigot-Corbel, E.; Gardie, B. Pathogenesis of myeloproliferative neoplasms: Role and mechanisms of chronic inflammation. Mediat. Inflamm. 2015, 2015, 145293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgillo, F.; Dalio, M.; Della Corte, C.M.; Gravina, A.G.; Viscardi, G.; Loguercio, C.; Ciardiello, F.; Federico, A. Carcinogenesis as a result of multiple inflammatory and oxidative hits: A comprehensive review from tumor microenvironment to gut microbiota. Neoplasia 2018, 20, 721. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Wang, Q.; Li, C.; Liu, J.; Zhang, D.; Zhang, S.; Pan, Y. Identification of potential candidate genes of oral cancer in response to chronic infection with Porphyromonas gingivalis using bioinformatical analyses. Front. Oncol. 2019, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 beta-A friend or foe in malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [Green Version]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.S.; Tang, Y.L.; Pang, X.; Zheng, M.; Tang, Y.J.; Liang, X.H. The maintenance of an oral epithelial barrier. Life Sci. 2019, 227, 129–136. [Google Scholar] [CrossRef]
- Barros, M.R., Jr.; de Oliveira, T.H.A.; de Melo, C.M.L. Viral modulation of TLRs and cytokines and the related immunotherapies for HPV-associated cancers. J. Immunol. Res. 2018, 2018, 2912671. [Google Scholar] [CrossRef]
- Ortiz, A.P.; González, D.; Vivaldi-Oliver, J.; Castañeda, M.; Rivera, V.; Díaz, E.; Centeno, H.; Muñoz, C.; Palefsky, J.; Joshipura, K.; et al. Periodontitis and oral human papillomavirus infection among Hispanic adults. Papillomavirus Res. 2018, 5, 128–133. [Google Scholar] [CrossRef]
- Gao, Z.; Lv, J.; Wang, M. Epstein—Barr virus is associated with periodontal diseases: A meta-analysis based on 21 case-control studies. Medicine 2017, 96, e5980. [Google Scholar] [CrossRef]
- Kazi, M.M.; Bharadwaj, R. Role of herpesviruses in chronic periodontitis and their association with clinical parameters and increasing severity of the disease. Eur. J. Dent. 2017, 11, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Shipilova, A.; Dayakar, M.M.; Gupta, D. High-risk human papillomavirus in the periodontium: A case-control study. J. Indian Soc. Periodontol. 2017, 21, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Cervigne, N.K.; Reis, P.P.; Machado, J.; Sadikovic, B.; Bradley, G.; Galloni, N.N.; Pintilie, M.; Jurisica, I.; Perez-Ordonez, B.; Gilbert, R.; et al. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum. Mol. Genet. 2009, 18, 4818–4829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Gong, F.; Zhang, S.; Zhang, C.Y.; Zen, K.; Chen, X. The origin, function and diagnostic potential of extracellular microRNA in human body fluids. Wiley Interdiscip. Rev. RNA 2014, 5, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.M.; Gimenez, J.L.; Cervera, M.S.; Roldán, A.L.; Pastor, P.J.; Illueca, F.M.; Calatayud, F.V. miR-1226 detection in GCF as potential biomarker of chronic periodontitis: A pilot study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, 8. [Google Scholar] [CrossRef]
- Luan, X.; Zhou, X.; Trombetta-Silva, J.; Francis, M.; Gaharwar, A.K.; Atsawasuwan, P. MicroRNAs and periodontal homeostasis. J. Dent. Res. 2017, 96, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018, 101, 608–614. [Google Scholar] [CrossRef]
- Xie, Y.F.; Shu, R.; Jiang, S.Y.; Liu, D.L.; Ni, J.; Zhang, X.L. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J. Inflamm. 2013, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-M.; Splinter, P.L.; O’Hara, S.P.; LaRusso, N.F. A cellular micro-RNA, let-7i, regulates toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J. Biol. Chem. 2007, 282, 28929–28938. [Google Scholar] [CrossRef] [Green Version]
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and immunity in periodontal health and disease. Int. J. Oral Sci. 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Singhrao, S.K.; Osmundsen, H. Periodontitis, pathogenesis and progression: miRNA-mediated cellular responses to Porphyromonas gingivalis. J. Oral Microbiol. 2017, 9, 1333396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazit, N.A.A.; Juliana, N.; Das, S.; Teng, N.I.M.F.; Fahmy, N.M.; Azmani, S.; Kadiman, S. Association of micro RNA and postoperative cognitive dysfunction: A review. Mini Rev. Med. Chem. 2020, 20, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. https://doi.org/10.3390/biomedicines10102659
Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines. 2022; 10(10):2659. https://doi.org/10.3390/biomedicines10102659
Chicago/Turabian StyleBhuyan, Ruchi, Sanat Kumar Bhuyan, Jatindra Nath Mohanty, Srijit Das, Norsham Juliana, and Izuddin Fahmy Juliana. 2022. "Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms" Biomedicines 10, no. 10: 2659. https://doi.org/10.3390/biomedicines10102659
APA StyleBhuyan, R., Bhuyan, S. K., Mohanty, J. N., Das, S., Juliana, N., & Juliana, I. F. (2022). Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines, 10(10), 2659. https://doi.org/10.3390/biomedicines10102659





