Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications
Abstract
:1. Introduction
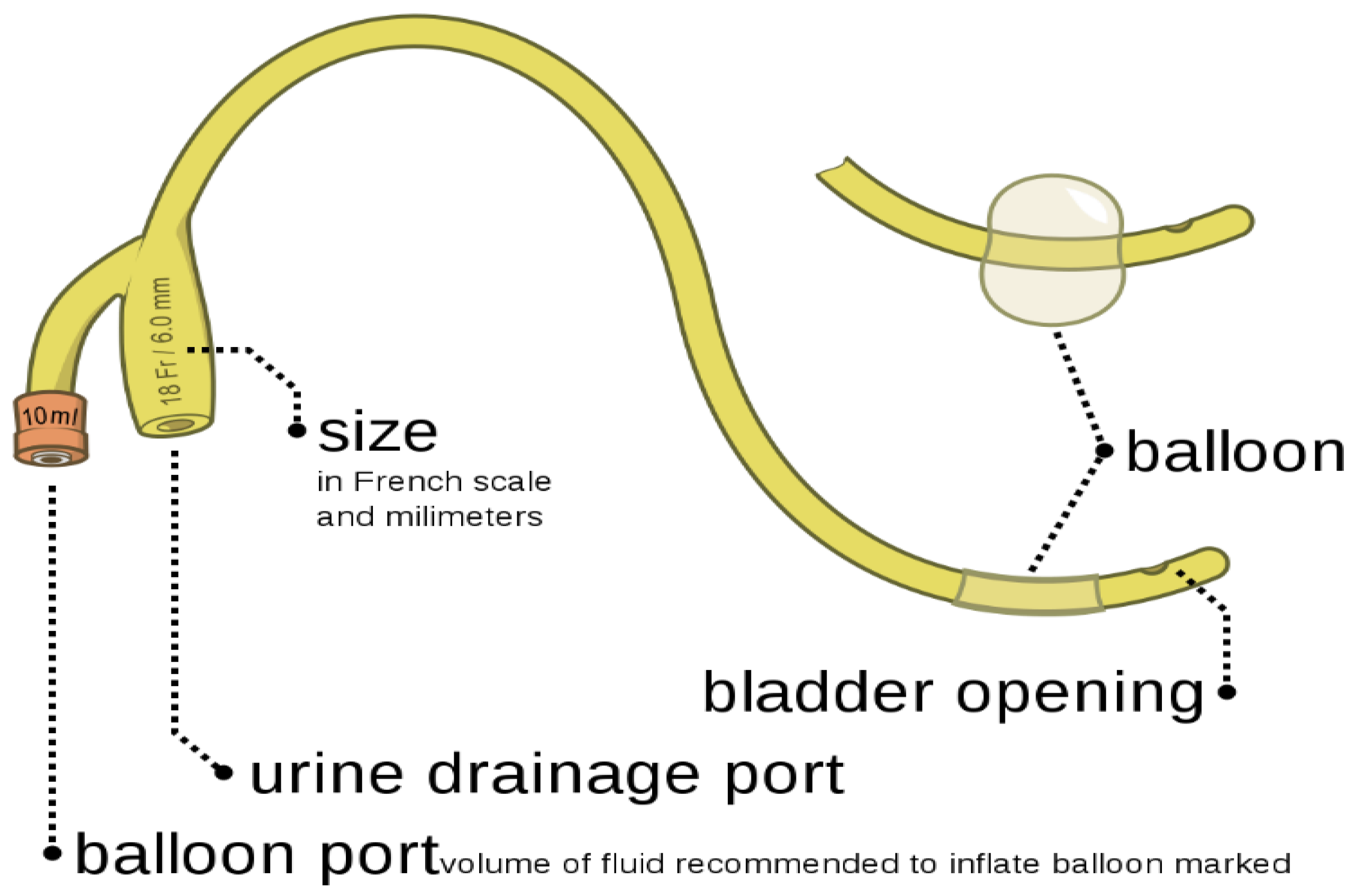
1.1. Classification of Urinary Catheters [7]
- Class I, if they are intended for transient use (intended for continuous use for less than 60 min);
- Class IIa, if they are intended for short-term use (intended for continuous use for between 60 min and 30 days) and;
- Class IIb, if they are intended for long-term use (intended for continuous use for more than 30 days).
1.2. Types of Urinary Catheters
1.2.1. Indwelling Catheters/Foley Catheters
1.2.2. Intermittent Catheters
1.2.3. Suprapubic Catheters
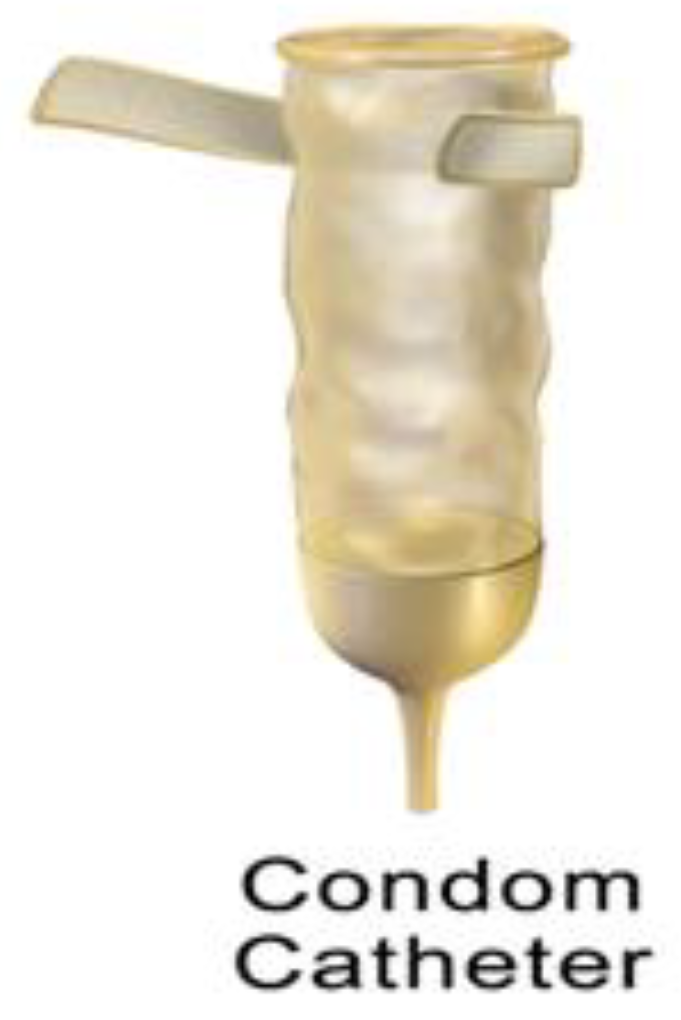
1.2.4. Condom Catheters
2. Urinary Incontinence
2.1. Vesicoureteral Reflux Caused by Urinary Incontinence
2.2. Bladder Stones
2.3. High Blood Pressure
2.4. Urinary Tract Infection
3. Complications Associated with Catheterization
3.1. Bacteriuria
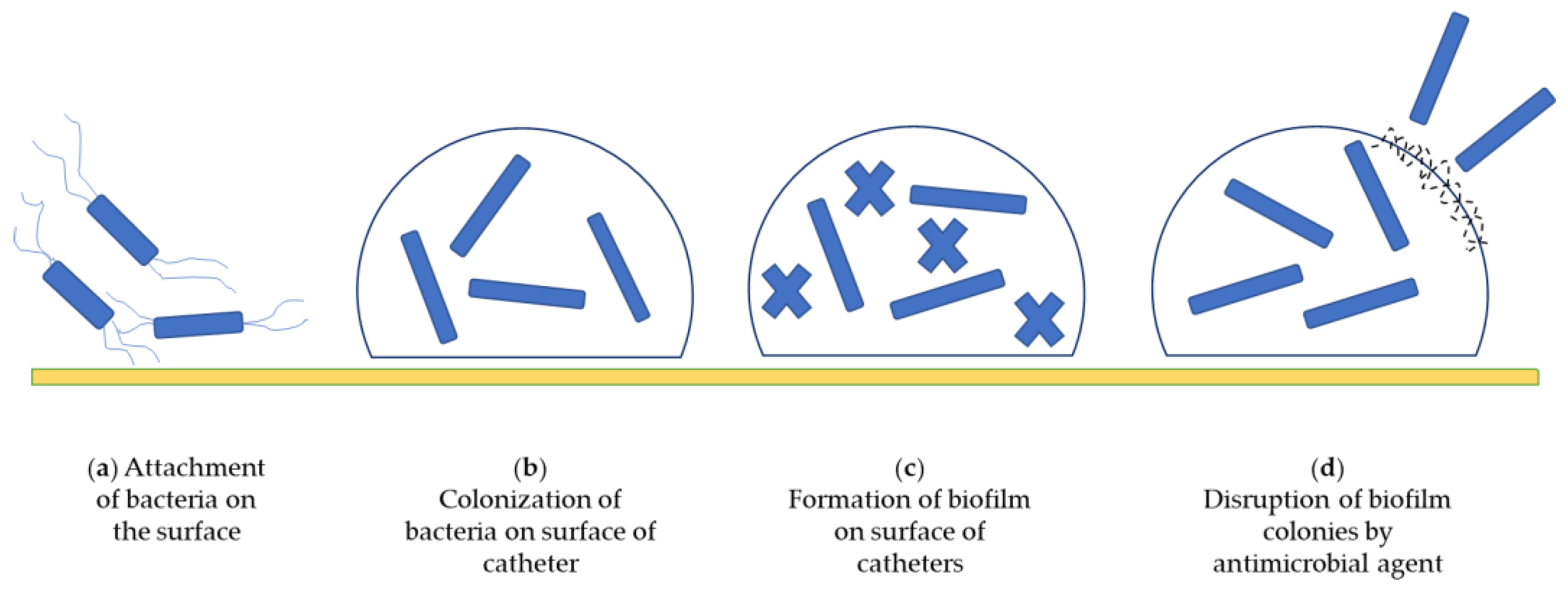
3.2. Catheter-Associated Biofilms
3.3. Encrustations
3.4. Urosepsis
3.5. Urethral Damage
3.6. CAUTI (Catheter-Associated Urinary Tract Infection)
4. Prevention and Management of CAUTIs
- Applying the antimicrobial coating on the surface of urinary catheters, such as metal ions, antibiotics, nitric oxide, antimicrobial peptides, and bacteriophages.
4.1. Approaches to Prevent CAUTIs by Modifying Materials
4.1.1. Hydrogels
4.1.2. Poly(Tetrafluoroethylene) (PTFE) Coating
4.1.3. Polyethylene Glycol (PEG Coatings)
4.1.4. Polyzwitterions
4.1.5. Enzymes
- (a)
- Acyclase—Acyclase, in combination with alpha-amylase, was tested for quorum quenching. Quorum quenching activity is responsible for inhibiting biofilm formation, which is why the risk of developing drug resistance is minimal in this case [53]. An in vitro study has been carried out with the coating of acylase and alpha-amylase, and the results show that the coating was successful in significantly reducing the biofilm formation by P. aeruginosa by 40%, and by S. aureus by 30%. In in vivo studies, the biofilm formation was decreased by 90% on the catheter balloon section [72].
- (b)
- ()— is a serine endopeptidase which attacks the unreactive carbonyl group and breaks the peptide bonds [73]. Based on this property of , its ability to disrupt biofilm formation has been studied in an in vitro study as the matrix includes proteins, polysaccharides, and extracellular DNA [74,75]. In the in vitro study, was immobilized on a low-density polyethylene and nurtured with LB media containing E. coli MG1655 in a CDC biofilm reactor performing continuous stirring. The study showed a reduction in the no. of adherent cells, and in biofilm thickness, roughness, and coverage [75].
- (c)
- Exopolysaccharide-specific Glycoside hydrolase—Exopolysaccharides are a major component of bacterial biofilm development, resulting in protection against antibacterial agents [74]. Glycoside hydrolases target and hydrolyse the glycosidic bonds of exopolysaccharide components of the biofilm matrix. Glycosides have properties of anti-biofilm agents [76,77]. Baker and collaborators investigated the glycoside hydrolase activity in a treatment against Pseudomonas aeruginosa biofilm development [78]. P. aeruginosa cultures were diluted in Luria–Bertani media (LB), and then, these diluted cultures were added to sterile 96-well polystyrene microtiter plates and nurtured for 24 h under controlled conditions. Glycoside hydrolase was added in different concentrations at different times at 0, or in developed biofilm conditions. The results show a significant reduction and disruption in biofilms [76].
5. Approaches to Prevent CAUTIs by Antimicrobial Coatings
5.1. Antibiotics
5.1.1. Nitrofurazone
5.1.2. Gentamicin
5.1.3. Norfloxacin
5.1.4. Ciprofloxacin
5.1.5. Sparfloxacin
5.1.6. Triclosan
5.1.7. Chlorhexidine
5.2. Metal-Based Approaches
5.2.1. Silver Ions
5.2.2. Nanoparticles
5.3. Nitric Oxide (NO)
5.4. Bacteriophages
- (a)
- They can act as biofilm-controlling agents because of their property to target specific pathogens.
- (b)
- They have self-replicating properties in the presence of their host cells.
- (c)
- They are used effectively against the bacteria, which are multidrug resistant.
- (d)
- If multiple phages are combined, they show better results in the treatment.
5.5. Antimicrobial Peptides (AMPs)
6. Authors’ Perspective
6.1. Ambiguities in Testing New Coatings
6.2. 3D Printing and Regulatory Aspect
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lachance, C.C.; Grobelna, A. Management of Patients with Long-Term Indwelling Urinary Catheters: A Review of Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release 2010, 148, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Field Epidemiology Manual Wiki. 2016. Available online: https://wiki.ecdc.europa.eu/fem/Pages/CAUTI.aspx (accessed on 22 February 2022).
- Al-Qahtani, M.; Safan, A.; Jassim, G.; Abadla, S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates. J. Infect. Public Health 2019, 12, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Medical Device Coordination Group (EU). MDCG 2021–2024 Guidance on Classification of Medical Devices. 2021. Available online: https://ec.europa.eu/health/system/files/2021-10/mdcg_2021-24_en_0.pdf (accessed on 19 March 2022).
- Antich-Isern, P.; Caro-Barri, J.; Aparicio-Blanco, J. The combination of medical devices and medicinal products revisited from the new European legal framework. Int. J. Pharm. 2021, 607, 120992. [Google Scholar] [CrossRef] [PubMed]
- Steve, B. Incontinence and Overactive Bladder WebMD. 2020. Available online: https://www.webmd.com/urinary-incontinence-oab/causes-overative-bladder (accessed on 19 March 2022).
- Medline, P. Urinary Catheters National Library of Medicine. Available online: https://medlineplus.gov/ency/patientinstructions/000140.htm (accessed on 19 March 2022).
- Bio Render. Available online: https://app.biorender.com/illustrations/6346ad043936f6adc3f8307d (accessed on 10 August 2022).
- National Health Service (UK). Types of Urinary Catheter National Health Service. Available online: https://www.nhs.uk/conditions/urinary-catheters/types/ (accessed on 18 March 2022).
- Urinary Catheters. Available online: https://commons.wikimedia.org/wiki/File:Urinary_catheter.JPG (accessed on 10 August 2022).
- Foley catheter inflated and deflated. Available online: https://commons.wikimedia.org/wiki/File:Foley_catheter_inflated_and_deflated_EN.svg (accessed on 10 August 2022).
- Steve, B. Catheters WebMD. 2020. Available online: https://www.webmd.com/urinary-incontinence-oab/catheter-types (accessed on 19 March 2022).
- Urinary Catheterization. Available online: https://en.wikipedia.org/wiki/Urinary_catheterization (accessed on 10 August 2022).
- Diane, K.N. Indwelling Urinary Catheters: Types Uro Today. Available online: https://www.urotoday.com/urinary-catheters-home/indwelling-catheters/description/types.html (accessed on 18 March 2022).
- Jahn, P.; Kernig, A.; Langer, G.; Preuss, M.; Seifert-Hühmer, A. Types of urinary catheters for management of long-term voiding problems in adults. In Cochrane Database of Systematic Reviews Chichester; Jahn, P., Ed.; John Wiley & Sons, Ltd.: London, UK, 2004. [Google Scholar]
- Newman, D.K.; Wilson, M.M. Review of Intermittent Catheterization and Current Best Practices. Urol. Nurs. 2011, 31, 12. [Google Scholar] [CrossRef] [PubMed]
- Byram Healthcare Product Catalogue. Available online: https://www.byramhealthcare.com/product-and-services/catalog/63113-urology?mpp=15 (accessed on 10 August 2022).
- What is Urinary Incontinence? Urology Care Foundation. Available online: https://www.urologyhealth.org/urology-a-z/u/urinary-incontinence (accessed on 18 March 2022).
- Urinary Incontinence Mayo Clinic Press. Available online: https://www.mayoclinic.org/diseases-conditions/urinary-incontinence/symptoms-causes/syc-20352808 (accessed on 18 March 2022).
- What Is Vesicoureteral Reflux? WebMD. 2021. Available online: https://www.webmd.com/children/vesicoureteral-reflux#:~:text=Vesicoureteral%20reflux%20(VUR)%20is%20when,gets%20stored%20in%20your%20bladder (accessed on 17 March 2022).
- Mattoo, T.K. Vesicoureteral Reflux and Reflux Nephropathy. Adv. Chronic Kidney Dis. 2011, 18, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jody, A.C. New Stone Risk Found in VUR Patients Renal and Urology News. 2012. Available online: https://www.renalandurologynews.com/home/news/urology/kidney-stones/new-stone-risk-found-in-vur-patients/ (accessed on 17 May 2022).
- García-Perdomo, H.A.; Solarte, P.B.; España, P.P. Pathophysiology associated with forming urinary stones. Urol. Colomb. 2016, 25, 118–125. [Google Scholar] [CrossRef]
- Shah, A.; Keir, M.; Ducas, R.; Crean, A.M. Uric acid bladder stones in congenital cyanotic heart disease. Lancet 2016, 388, 1921. [Google Scholar] [CrossRef]
- Hammad, F.T.; Kaya, M.; Kazim, E. Bladder calculi: Did the clinical picture change? Urology 2006, 67, 1154–1158. [Google Scholar] [CrossRef]
- Douenias, R.; Rich, M.; Badlani, G.; Mazor, D.; Smith, A. Predisposing factors in bladder calculi: Review of 100 cases. Urology 1991, 37, 240–243. [Google Scholar] [CrossRef]
- Tim, N. Bladder Stones Medical News Today. 2022. Available online: https://www.medicalnewstoday.com/articles/184998 (accessed on 19 March 2022).
- Badejoko, O.O.; Salako, A.A.; Egharevba, P. Overflow urinary incontinence due to bladder stones. Int. Urogynecol. J. 2014, 25, 425–427. [Google Scholar] [CrossRef]
- Bladder stones. Available online: https://en.wikipedia.org/wiki/Bladder_stone (accessed on 10 August 2022).
- High Blood Pressure Symptoms and Causes Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/bloodpressure/about.htm#:~:text=serious%20health%20problems.-,Heart%20Attack%20and%20Heart%20Disease,Chest%20pain%2C%20also%20called%20angina (accessed on 19 May 2022).
- Torimoto, K.; Matsumoto, Y.; Gotoh, D.; Morizawa, Y.; Miyake, M.; Samma, S.; Nobumichi, T.; Akihide, H.; Kiyohide, F. Overactive bladder induces transient hypertension. BMC Res. Notes 2018, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Can Urinary Incontinence Cause UTI? National Association for Continence. Available online: https://liveutifree.com/urinary-incontinence/#incontinenceimpact (accessed on 19 March 2022).
- Albu, S.; Voidazan, S.; Bilca, D.; Badiu, M.; Trut, A.; Ciorea, M.; Ichim, A.; Luca, D.; Moldovan, G. Bacteriuria and Asymptomatic Infection in Chronic Patients with Indwelling Urinary Catheter The Incidence of ESBL Bacteria. Medicine 2018, 97, e11796. [Google Scholar] [CrossRef]
- Wilde, M.H.; McDonald, M.V.; Brasch, J.; McMahon, J.; Fairbanks, E.; Shah, S.; Tang, W.; Scheid, E. Long-term urinary catheter users self-care practices and problems. J. Clin. Nurs. 2013, 22, 356–367. [Google Scholar] [CrossRef]
- Kidd, E.A.; Stewart, F.; Kassis, N.C.; Hom, E.; Omar, M.I. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2015, 2015, CD004203. [Google Scholar] [CrossRef]
- Choong, S.K.S.; Whitfield, H.N. Urinary Encrustation of Alloplastic Materials. J. Endourol. 2000, 14, 19–23. [Google Scholar] [CrossRef]
- Maiti, P.; Chatterjee, S.; Dey, R.; Kundu, A.; Dey, R. Biofilms on indwelling urologic devices: Microbes and antimicrobial management prospect. Ann. Med. Health Sci. Res. 2014, 4, 100. [Google Scholar] [CrossRef] [Green Version]
- Norsworthy, A.N.; Pearson, M.M. From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis. Trends Microbiol. 2017, 25, 304–315. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, N.R.; Davis, N.F.; MacCraith, E.; Manecksha, R.P.; Flood, H.D.; Mooney, R.; Leonard, G.; Walsh, M.T. Long-term outcomes of urethral catheterisation injuries: A prospective multi-institutional study. Eur. Urol. Suppl. 2019, 18, e2549. [Google Scholar] [CrossRef]
- Hollingsworth, J.M.; Rogers, M.A.M.; Krein, S.L.; Hickner, A.; Kuhn, L.; Cheng, A.; Saint, S. Determining the Noninfectious Complications of Indwelling Urethral Catheters. Ann. Intern. Med. 2013, 159, 401. [Google Scholar] [CrossRef]
- Alexaitis, I.; Broome, B. Implementation of a Nurse-Driven Protocol to Prevent Catheter-Associated Urinary Tract Infections. J. Nurs. Care Qual. 2014, 29, 245–252. [Google Scholar] [CrossRef]
- Cole, S.J.; Records, A.R.; Orr, M.W.; Linden, S.B.; Lee, V.T. Catheter-Associated Urinary Tract Infection by Pseudomonas aeruginosa Is Mediated by Exopolysaccharide-Independent Biofilms. Infect. Immun. 2014, 82, 2048–2058. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.K.; Strauss, R. Preventing Catheter-Associated Urinary Tract Infections. UroToday Int. J. 2013, 6, 19–32. [Google Scholar] [CrossRef]
- Menegueti, M.G.; Ciol, M.A.; Bellissimo-Rodrigues, F.; Auxiliadora-Martins, M.; Gaspar, G.G.; Canini, S.R.M.D.S.; Basile-Filho, A.; Laus, A.M. Long-term prevention of catheter-associated urinary tract infections among critically ill patients through the implementation of an educational program and a daily checklist for maintenance of indwelling urinary catheters. Medicine 2019, 98, e14417. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Keatch, R.; Corner, G.; Nabi, G.; Murdoch, S.; Davidson, F.; Zhao, Q. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J. Hosp. Infect. 2019, 103, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gilles, K.; McDonald, A.; Walton, K. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet 2012, 380, 1927–1935. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.B.; Omar, M.I.; Fisher, E.; Gillies, K.; MacLennan, S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2014, 9, CD004013. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Li, S.; Yuan, X. Antimicrobial strategies for urinary catheters. J. Biomed. Mater. Res. A 2019, 107, 445–467. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Yang, C.; Ding, X.; Ono, R.J.; Lee, H.; Hsu, L.Y.; Tong, Y.W.; Hedrick, J.; Yan Yang, Y. Brush-Like Polycarbonates Containing Dopamine, Cations, and PEG Providing a Broad-Spectrum, Antibacterial, and Antifouling Surface via One-Step Coating. Adv. Mater. 2014, 26, 7346–7351. [Google Scholar] [CrossRef] [PubMed]
- Kazmierska, K.A.; Thompson, R.; Morris, N.; Long, A.; Ciach, T. In Vitro Multicompartmental Bladder Model for Assessing Blockage of Urinary Catheters: Effect of Hydrogel Coating on Dynamics of Proteus mirabilis Growth. Urology 2010, 76, 515.e15–515.e20. [Google Scholar] [CrossRef] [PubMed]
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an Antifouling Zwitterionic Coating on Urinary Catheters Using an Enzymatically Triggered Bottom-Up Approach. ACS Appl. Mater. Interfaces 2014, 6, 11385–11393. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
- McCloskey, A.; Gilmore, B.; Laverty, G. Evolution of Antimicrobial Peptides to Self-Assembled Peptides for Biomaterial Applications. Pathogens 2014, 3, 791–821. [Google Scholar] [CrossRef] [Green Version]
- Talja, M.; Korpela, A.; Järvi, K. Comparison of Urethral Reaction to Full Silicone, Hydrogen-coated and Siliconised Latex Catheters. Br. J. Urol. 1990, 66, 652–657. [Google Scholar] [CrossRef]
- Kunin, C.M.; Chin, Q.F.; Chambers, S. Formation of Encrustations on Indwelling Urinary Catheters in the Elderly: A Comparison of Different types of Catheter Materials in “Blockers” and “Nonblockers”. J. Urol. 1987, 138, 899–902. [Google Scholar] [CrossRef]
- Kilonzo, M.; Vale, L.; Pickard, R.; Lam, T.; N’Dow, J. Cost Effectiveness of Antimicrobial Catheters for Adults Requiring Short-term Catheterisation in Hospital. Eur. Urol. 2014, 66, 615–618. [Google Scholar] [CrossRef]
- Razatos, A.; Ong, Y.L.; Boulay, F.; Elbert, D.L.; Hubbell, J.A.; Sharma, M.M.; Georgiou, G. Force Measurements between Bacteria and Poly(ethylene glycol)-Coated Surfaces. Langmuir 2000, 16, 9155–9158. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.B.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Segev, G.; Bankirer, T.; Steinberg, D.; Duvdevani, M.; Shapur, N.K.; Friedman, M.; Lavy, E. Evaluation of Urinary Catheters Coated with Sustained-Release Varnish of Chlorhexidine in Mitigating Biofilm Formation on Urinary Catheters in Dogs. J. Vet. Intern. Med. 2013, 27, 39–46. [Google Scholar] [CrossRef]
- Vaterrodt, A.; Thallinger, B.; Daumann, K.; Koch, D.; Guebitz, G.M.; Ulbricht, M. Antifouling and Antibacterial Multifunctional Polyzwitterion/Enzyme Coating on Silicone Catheter Material Prepared by Electrostatic Layer-by-Layer Assembly. Langmuir 2016, 32, 1347–1359. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, J.; Yu, Y.; Yu, L.; Wang, Y.X.; Ren, K.F.; Ji, J. A polyzwitterion-based antifouling and flexible bilayer hydrogel coating. Compos. B Eng. 2022, 244, 110164. [Google Scholar] [CrossRef]
- Paschke, S.; Lienkamp, K. Polyzwitterions: From Surface Properties and Bioactivity Profiles to Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 129–151. [Google Scholar] [CrossRef]
- Stickler, D.J.; Evans, A.; Morris, N.; Hughes, G. Strategies for the control of catheter encrustation. Int. J. Antimicrob. Agents 2002, 19, 499–506. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, Q.; Long, Z.; Huo, Q.; Ge, Y.; Vianney, N.; Daliko, N.A.; Meng, Y.; Qu, J.; Chen, H.; et al. Polydopamine/poly(sulfobetaine methacrylate) Co-deposition coatings triggered by CuSO4/H2O2 on implants for improved surface hemocompatibility and antibacterial activity. Bioact. Mater. 2021, 6, 2546–2556. [Google Scholar] [CrossRef]
- Mandakhalikar, K.D.; Wang, R.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Tambyah, P.A. Restriction of in vivo infection by antifouling coating on urinary catheter with controllable and sustained silver release: A proof of concept study. BMC Infect. Dis. 2018, 18, 370. [Google Scholar] [CrossRef]
- Ivanova, K.; Fernandes, M.M.; Mendoza, E.; Tzanov, T. Enzyme multilayer coatings inhibit Pseudomonas aeruginosa biofilm formation on urinary catheters. Appl. Microbiol. Biotechnol. 2015, 99, 4373–4385. [Google Scholar] [CrossRef]
- Appel, W. Chymotrypsin: Molecular and catalytic properties. Clin. Biochem. 1986, 19, 317–322. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Cattò, C.; Secundo, F.; James, G.; Villa, F.; Cappitelli, F. α-Chymotrypsin Immobilized on a Low-Density Polyethylene Surface Successfully Weakens Escherichia coli Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 4003. [Google Scholar] [CrossRef] [Green Version]
- Snarr, B.D.; Baker, P.; Bamford, N.C.; Sato, Y.; Liu, H.; Lehoux, M.; Gravelat, F.N.; Ostapska, H.; Baistrocchi, S.R.; Cerone, R.P.; et al. Microbial glycoside hydrolases as antibiofilm agents with cross-kingdom activity. Proc. Natl. Acad. Sci. USA 2017, 114, 7124–7129. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef] [Green Version]
- Asker, D.; Awad, T.S.; Baker, P.; Howell, P.L.; Hatton, B.D. Non-eluting, surface-bound enzymes disrupt surface attachment of bacteria by continuous biofilm polysaccharide degradation. Biomaterials 2018, 167, 168–176. [Google Scholar] [CrossRef]
- Palugan, L.; Cerea, M.; Cirilli, M.; Moutaharrik, S.; Maroni, A.; Zema, L. Intravesical drug delivery approaches for improved therapy of urinary bladder diseases. Int. J. Pharm. X 2021, 3, 100100. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Mansouri, M.D.; Gawande, P.V.; Madhyastha, S. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J. Antimicrob. Chemother. 2008, 61, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Berggren, T.; Conway, A.J. Activity of a nitrofurazone matrix urinary catheter against catheter-associated uropathogens. Antimicrob. Agents Chemother. 1993, 37, 2033–2036. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Delavari, P.; Azar, M. Activities of a Nitrofurazone-Containing Urinary Catheter and a Silver Hydrogel Catheter against Multidrug-Resistant Bacteria Characteristic of Catheter-Associated Urinary Tract Infection. Antimicrob. Agents Chemother. 1999, 43, 2990–2995. [Google Scholar] [CrossRef]
- Menezes, F.G.; Corrêa, L.; Medina-Pestana, J.O.; Aguiar, W.F.; Camargo, L.F.A. A randomized clinical trial comparing Nitrofurazone-coated and uncoated urinary catheters in kidney transplant recipients: Results from a pilot study. Transpl. Infect. Dis. 2019, 21, e13031. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.W.; Cho, Y.H.; Shin, W.S.; Lee, S.E.; Kim, C.S.; Hong, S.J.; Chung, B.H.; Kim, J.J.; Yoon, M.S. A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazone-coated and silicone catheters. Int. J. Antimicrob. Agents 2004, 24 (Suppl. 1), 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.E.; Sarre, S.G. Mechanism of Action of Gentamicin. J. Infect. Dis. 1969, 119, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Tangy, F.; Moukkadem, M.; Vindimian, E.; Capmau, M.L.; Goffic, F. Mechanism of action of gentamicin components. Characteristics of their binding to Escherichia coli ribosomes. Eur. J. Biochem. 1985, 147, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [Green Version]
- Rafienia, M.; Zarinmehr, B.; Poursamar, S.A.; Bonakdar, S.; Ghavami, M.; Janmaleki, M. Coated urinary catheter by PEG/PVA/gentamicin with drug delivery capability against hospital infection. Iran. Polym. J. 2013, 22, 75–83. [Google Scholar] [CrossRef]
- Reid, G.; Sharma, S.; Advikolanu, K.; Tieszer, C.; Martin, R.A.; Bruce, A.W. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrob. Agents Chemother. 1994, 38, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Cho, Y.W.; Cho, Y.H.; Choi, J.M.; Shin, H.J.; Bae, Y.H.; Chung, H.; Jeong, S.Y.; Kwon, I.C. Norfloxacin-releasing urethral catheter for long-term catheterization. J. Biomater. Sci. Polym. Ed. 2003, 14, 951–962. [Google Scholar] [CrossRef]
- Saini, H.; Chhibber, S.; Harjai, K. Antimicrobial and antifouling efficacy of urinary catheters impregnated with a combination of macrolide and fluoroquinolone antibiotics against Pseudomonas aeruginosa. Biofouling 2016, 32, 511–522. [Google Scholar] [CrossRef]
- Vidyavathi, M.; Srividya, G. A Review On Ciprofloxacin: Dosage Form Perspective. Int. J. Appl. Pharm. 2018, 10, 6. [Google Scholar] [CrossRef]
- Pugach, J.L.; Ditizio, V.; Mittelman, M.W.; Bruce, A.W.; Dicosmo, F.; Khoury, A.E. Antibiotic Hydrogel Coated Foley Catheters For Prevention Of Urinary Tract Infection In A Rabbit Model. J. Urol. 1999, 162, 883–887. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Ginalska, G.; Piersiak, T.; Miazga-Karska, M. Prevention of biofilm formation on urinary catheters: Comparison of the sparfloxacin-treated long-term antimicrobial catheters with silver-coated ones. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1874–1882. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J. The impact of triclosan on the spread of antibiotic resistance in the environment. Front. Microbiol. 2015, 5, 780. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D. Whither triclosan? J. Antimicrob. Chemother. 2004, 53, 693–695. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. 2017. Available online: https://www.federalregister.gov/documents/2017/12/20/2017-27317/safety-and-effectiveness-of-health-care-antiseptics-topical-antimicrobial-drug-products-for (accessed on 17 April 2022).
- Petersen, R.C. Triclosan antimicrobial polymers. AIMS Mol. Sci. 2016, 3, 88–103. [Google Scholar] [CrossRef]
- Westfall, C.; Flores-Mireles, A.L.; Robinson, J.I.; Lynch, A.J.L.; Hultgren, S.; Henderson, J.P.; Levin, P.A. The Widely Used Antimicrobial Triclosan Induces High Levels of Antibiotic Tolerance In Vitro and Reduces Antibiotic Efficacy up to 100-Fold In Vivo. Antimicrob. Agents Chemother. 2019, 63, e02312-18. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.G.; Fasugba, O.; Cheng, A.C.; Gregory, V.; Koerner, J.; Collignon, P.; Gardner, A.; Graves, N. Chlorhexidine versus saline in reducing the risk of catheter associated urinary tract infection: A cost-effectiveness analysis. Int. J. Nurs. Stud. 2019, 97, 1–6. [Google Scholar] [CrossRef]
- Gefter (Shenderovich), J.; Zaks, B.; Kirmayer, D.; Lavy, E.; Steinberg, D.; Friedman, M. Chlorhexidine sustained-release varnishes for catheter coating—Dissolution kinetics and antibiofilm properties. Eur. J. Pharm. Sci. 2018, 112, 1–7. [Google Scholar] [CrossRef]
- Gaonkar, T.A.; Caraos, L.; Modak, S. Efficacy of a Silicone Urinary Catheter Impregnated with Chlorhexidine and Triclosan Against Colonization With Proteus mirabilis and Other Uropathogens. Infect. Control Hosp. Epidemiol. 2007, 28, 596–598. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Send Orders for Reprints to reprints@benthamscience.ae The Open Microbiology Journal Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 1945–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshehri, S.M.; Aldalbahi, A.; Al-hajji, A.B.; Chaudhary, A.A.; Panhuis, M.; Alhokbany, N.; Ahamad, T. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bologna, R.A.; Tu, L.M.; Polansky, M.; Fraimow, H.D.; Gordon, D.A.; Whitmore, K.E. Hydrogel/silver ion-coated urinary catheter reduces nosocomial urinary tract infection rates in intensive care unit patients: A multicenter study. Urology 1999, 54, 982–987. [Google Scholar] [CrossRef]
- Funao, H.; Nagai, S.; Sasaki, A.; Hoshikawa, T.; Tsuji, T.; Okada, Y.; Koyasu, S.; Toyama, Y.; Nakamura, M.; Aizawa, M.; et al. A novel hydroxyapatite film coated with ionic silver via inositol hexaphosphate chelation prevents implant-associated infection OPEN. Sci. Rep. 2016, 6, 23238. [Google Scholar] [CrossRef] [Green Version]
- Leuck, A.M.; Johnson, J.R.; Hunt, M.A.; Dhody, K.; Kazempour, K.; Ferrieri, P.; Kline, S. Safety and efficacy of a novel silver-impregnated urinary catheter system for preventing catheter-associated bacteriuria: A pilot randomized clinical trial. Am. J. Infect. Control 2015, 43, 260–265. [Google Scholar] [CrossRef] [Green Version]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial Surface Functionalization of Plastic Catheters by Silver Nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar]
- Mala, R.; Aglin, A.A.; Selvaraj, A.; Celsia, R.; Geerthika, S.; Kiruthika, N. Foley catheters functionalised with a synergistic combination of antibiotics and silver nanoparticles resist biofilm formation. IET Nanobiotechnology Res. Artic. 2017, 11, 612–620. [Google Scholar] [CrossRef]
- Thibon, P.; le Coutour, X.; Leroyer, R.; Fabry, J. Randomized multi-centre trial of the effects of a catheter coated with hydrogel and silver salts on the incidence of hospital-acquired urinary tract infections. J. Hosp. Infect. 2000, 45, 117–124. [Google Scholar] [CrossRef]
- Desai, D.G.; Liao, K.S.; Cevallos, M.E.; Trautner, B.W. Silver or Nitrofurazone Impregnation of Urinary Catheters Has a Minimal Effect on Uropathogen Adherence. J. Urol. 2010, 184, 2565–2571. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, A.T.; Brisson, B.A.; Gow, W.R.; Wainberg, S.; Singh, A.; Weese, J.S. Effects of the use of silver-coated urinary catheters on the incidence of catheter-associated bacteriuria and urinary tract infection in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 1289–1293. [Google Scholar] [CrossRef]
- Karchmer, T.B.; Giannetta, E.T.; Muto, C.A.; Strain, B.A.; Farr, B.M. A Randomized Crossover Study of Silver-Coated Urinary Catheters in Hospitalized Patients. Arch. Intern. Med. 2000, 160, 3294–3298. [Google Scholar] [CrossRef]
- Chung, P.H.; Wong, C.W.; Lai, C.K.; Siu, H.; Tsang, D.N.; Yeung, K. A prospective interventional study to examine the effect of a silver alloy and hydrogel—Coated catheter on the incidence of catheter-associated urinary tract infection. Hong Kong Med. J. 2017, 23, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Lederer, J.W.; Jarvis, W.R.; Thomas, L.; Ritter, J. Multicenter Cohort Study to Assess the Impact of a Silver-Alloy and Hydrogel-Coated Urinary Catheter on Symptomatic Catheter-Associated Urinary Tract Infections. J. Wound Ostomy Cont. Nurs. 2014, 41, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Burdus, A.C.; Gherasim, O.; Mihai Grumezescu, A.; Mogoantă iu Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar]
- Speruda, M.; Krzy, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskónska, G. Molecular Sciences Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Putting copper into action: Copper-impregnated products with potent biocidal activities. FASEB J. 2004, 18, 1728–1730. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Kitamoto, K.; Anraku, Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 1988, 170, 2676–2682. [Google Scholar] [CrossRef] [Green Version]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Quasi-Instantaneous Bacterial Inactivation on Cu–Ag Nanoparticulate 3D Catheters in the Dark and Under Light: Mechanism and Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 47–55. [Google Scholar] [CrossRef]
- Mihut, D.M.; Afshar, A.; Lackey, L.W.; Le, K.N. Antibacterial effectiveness of metallic nanoparticles deposited on water filter paper by magnetron sputtering. Surf. Coat. Technol. 2019, 368, 59–66. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Ko, M.; Miller, C.; Av-Gay, Y. Slow Release of Nitric Oxide from Charged Catheters and Its Effect on Biofilm Formation by Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, S.; Weitzberg, E.; Wiklund, P.; Lundberg, J.O. Intravesical Nitric Oxide Delivery for Prevention of Catheter-Associated Urinary Tract Infections. Antimicrob. Agents Chemother. 2005, 49, 2352–2355. [Google Scholar] [CrossRef] [Green Version]
- Lehman, S.M.; Donlan, R.M. Bacteriophage-Mediated Control of a Two-Species Biofilm Formed by Microorganisms Causing Catheter-Associated Urinary Tract Infections in an In Vitro Urinary Catheter Model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- Curtin, J.J.; Donlan, R.M. Using Bacteriophages To Reduce Formation of Catheter-Associated Biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2006, 50, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Carson, L.; Gorman, S.P.; Gilmore, B.F. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol. Med. Microbiol. 2010, 59, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S.J. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative Mode of Action of the Antimicrobial Peptide Melimine and Its Derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar] [CrossRef] [Green Version]
- Strempel, N.; Strehmel, J.; Overhage, J. Potential Application of Antimicrobial Peptides in the Treatment of Bacterial Biofilm Infections. Curr. Pharm. Des. 2014, 21, 67–84. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.Y.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.W.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Flores-Mireles, A.L.; Cusumano, Z.T.; Takagi, E.; Hultgren, S.J.; Caparon, M.G. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 2017, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colomer-Winter, C.; Lemos, J.; Flores-Mireles, A. Biofilm Assays on Fibrinogen-coated Silicone Catheters and 96-well Polystyrene Plates. Bio. Protoc. 2019, 9, e3196. [Google Scholar] [CrossRef] [PubMed]
- Beebout, C.J.; Eberly, A.R.; Werby, S.H.; Reasoner, S.A.; Brannon, J.R.; De, S.; Fitzgerald, M.J.; Huggins, M.M.; Clayton, D.B.; Cegelski, L.; et al. Respiratory Heterogeneity Shapes Biofilm Formation and Host Colonization in Uropathogenic Escherichia coli. mBio 2019, 10, e02400-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakopoulos, X.; Evangelou, A.; Kalfakakou, V.; Grammeniatis, E.; Papandropoulos, I.; Charalambopoulos, K. Human bladder urine oxygen content: Implications for urinary tract diseases. Int. Urol. Nephrol. 1997, 29, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Hannan, T.J.; Ford, B.; Caparon, M.G.; Hultgren, S.J. Enterococcus faecalis Overcomes Foreign Body-Mediated Inflammation To Establish Urinary Tract Infections. Infect. Immun. 2013, 81, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Potretzke, A.; Schreiber, H.L.; Pinkner, J.S.; Bauman, T.M.; Park, A.M.; Desai, A.; Hultgren, S.J.; Caparon, M.G. Antibody-Based Therapy for Enterococcal Catheter-Associated Urinary Tract Infections. mBio 2016, 7, e01653-16. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.N.; Flores-Mireles, A.L.; Pinkner, C.L.; Schreiber Iv, H.L.; Joens, M.S.; Park, A.M.; Potretzke, A.M.; Bauman, T.M.; Pinkner, J.S.; Fitzpatrick, J.A.J.; et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl. Acad. Sci. USA 2017, 114, E8721–E8730. [Google Scholar] [CrossRef] [Green Version]
- Domsta, V.; Seidlitz, A. Molecules 3D-Printing of Drug-Eluting Implants: An Overview of the Current Developments Described in the Literature. Molecules 2021, 26, 4066. [Google Scholar] [CrossRef]
- Mathew, E.; Domínguez-Robles, J.; Larrañeta, E.; Lamprou, D.A. Fused deposition modelling as a potential tool for antimicrobial dialysis catheters manufacturing: New trends vs. conventional approaches. Coatings 2019, 9, 515. [Google Scholar] [CrossRef]


| Type of Coatings for Catheter | Materials for Catheter | Size Available in Fr | Target Population | Tip of Catheter Available |
|---|---|---|---|---|
| (Paeds—6–10 Fr) | ||||
| (Female—10–12 Fr) | ||||
| (Male—14–18 Fr) | ||||
| (Clot Retention—20–26 Fr) | ||||
| Hydrogel-coated catheters | Latex, polyvinyl chloride (PVC), red rubber, silicone | 8,6,10,16,18 | Paediatrics, males, and females | Straight and coudé tip |
| Silver-coated catheters | Latex, PVC, silicone | 6,10,12,16,17 | Paediatrics, Males, and Females | Straight and coudé tip |
| Hydrophilic-coated catheters | Silicone, vinyl, polyurethane, polyolefin-based elastomer (POBE), PVC, red rubber | 5,6,8,10,12,14,16,18, 19,20,22 | Paediatrics, Males, and Females | Straight and coudé tip |
| Pre-lubricated catheters | PVC, red rubber | 12,14,16 | Males and Females | Straight and coudé tip |
| Polytetrafluoroethylene (PTFE)-coated catheters | Latex | 16 | Males | Coudé tip |
| Uncoated catheters | Silicone, latex, PVC | 6,8,10,12,14,16,18,22 | Males and Females | Straight and coudé tip |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanti, S.P.Y.; Csóka, I.; Jójárt-Laczkovich, O.; Adalbert, L. Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines 2022, 10, 2580. https://doi.org/10.3390/biomedicines10102580
Kanti SPY, Csóka I, Jójárt-Laczkovich O, Adalbert L. Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines. 2022; 10(10):2580. https://doi.org/10.3390/biomedicines10102580
Chicago/Turabian StyleKanti, S. P. Yamini, Ildikó Csóka, Orsolya Jójárt-Laczkovich, and Lívia Adalbert. 2022. "Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications" Biomedicines 10, no. 10: 2580. https://doi.org/10.3390/biomedicines10102580
APA StyleKanti, S. P. Y., Csóka, I., Jójárt-Laczkovich, O., & Adalbert, L. (2022). Recent Advances in Antimicrobial Coatings and Material Modification Strategies for Preventing Urinary Catheter-Associated Complications. Biomedicines, 10(10), 2580. https://doi.org/10.3390/biomedicines10102580








