Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders
Abstract
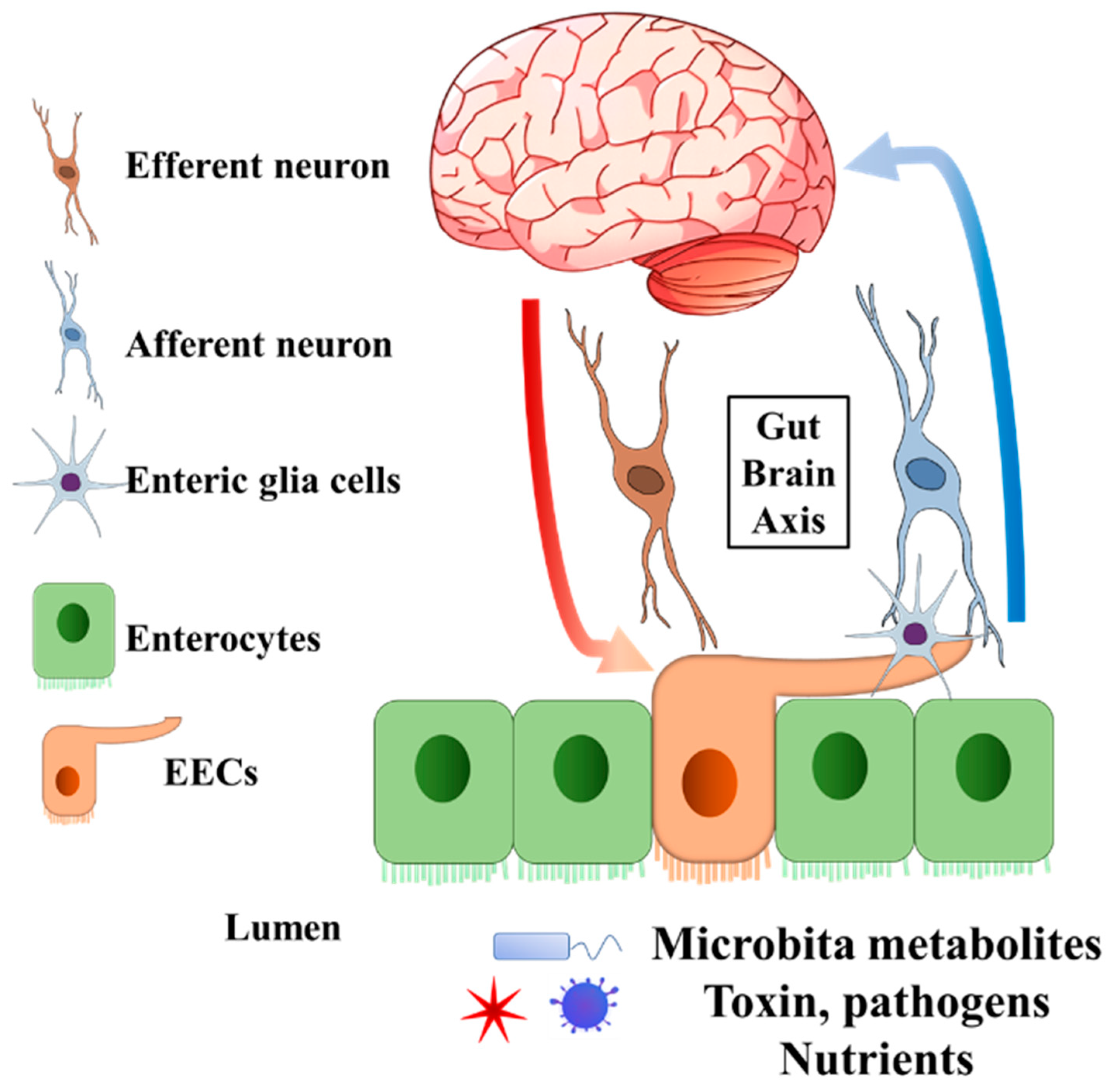
:1. Introduction
2. Enteroendocrine Cell Functions That Might Be Related to Neurological and Psychiatric Disorders
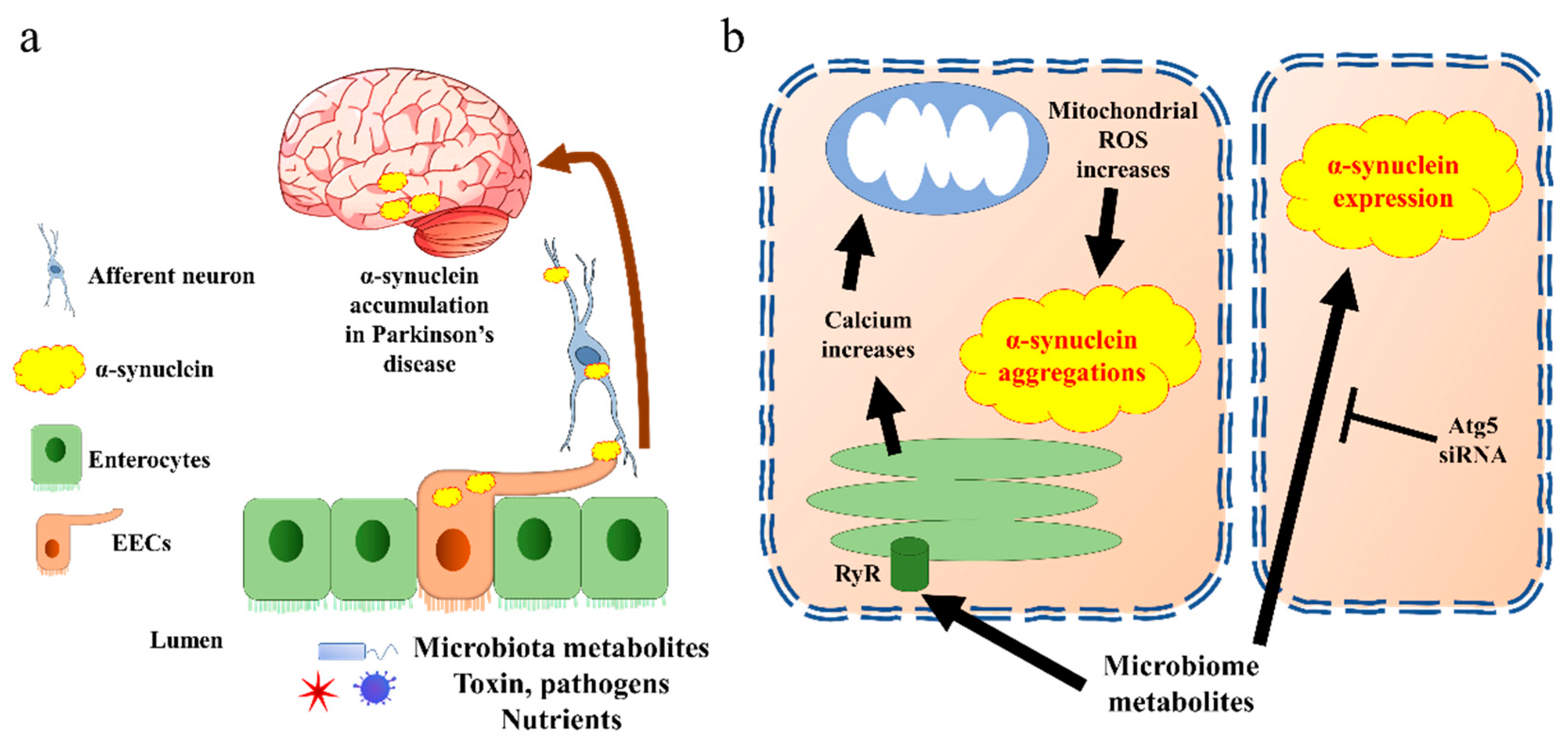
3. Enteroendocrine Cells in Parkinson’s Disease
4. Enteroendocrine Cells in Schizophrenia
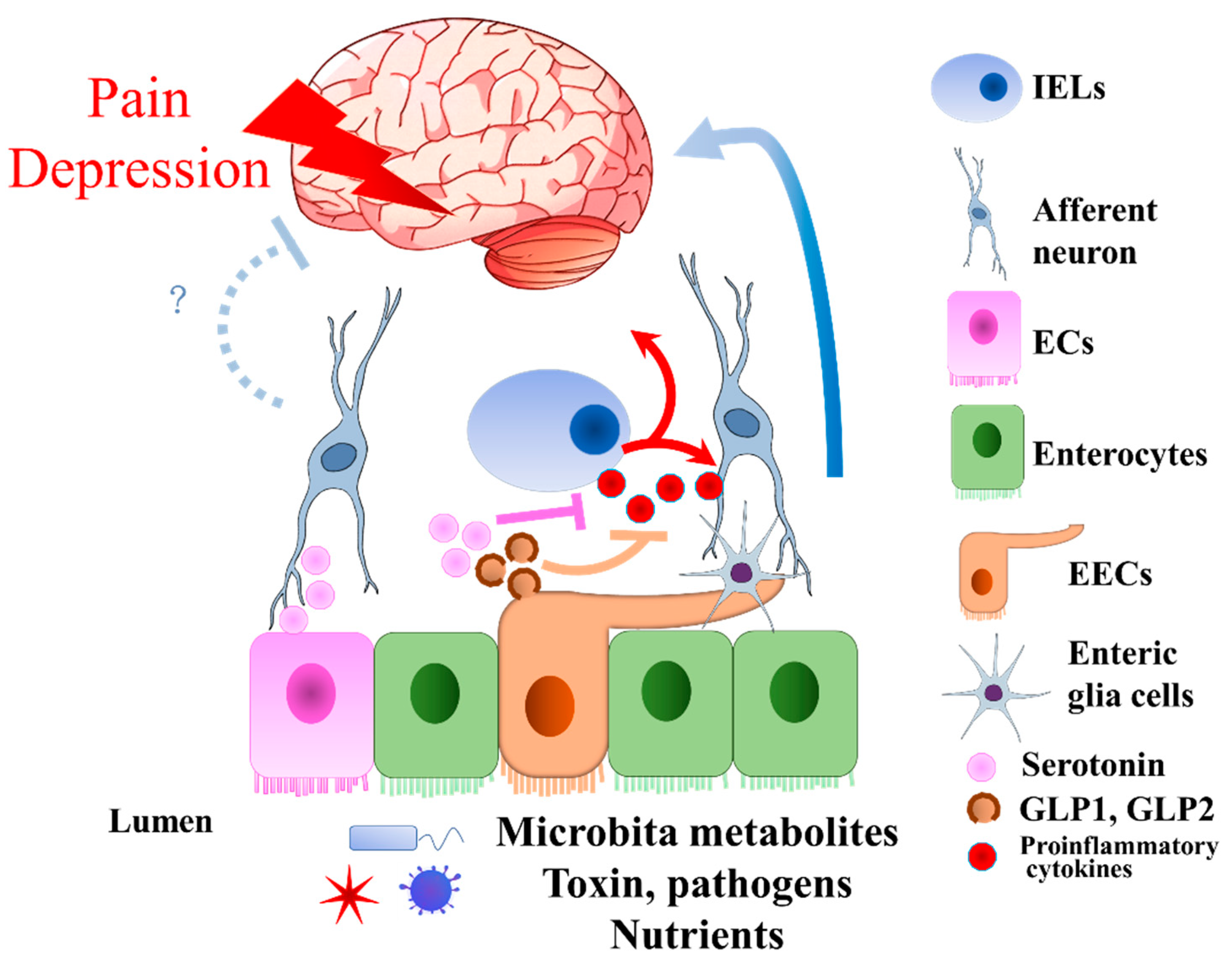
5. Enteroendocrine Cells in Visceral Pain and Neuropathic Pain
6. Enteroendocrine Cells in Depression
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 16 May 2022).
- WHO. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 16 May 2022).
- WHO. Nearly One Billion People Have a Mental Disorder: WHO. UN News, 17 June 2022. [Google Scholar]
- Hanson, K.A.; Loftus, E.V., Jr.; Harmsen, W.S.; Diehl, N.N.; Zinsmeister, A.R.; Sandborn, W.J. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: A case-control study. Inflamm. Bowel Dis. 2009, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A.; Morris, C.B.; Edwards, H.; Wrennall, C.E.; Weinland, S.R.; Aderoju, A.O.; Kulkarni-Kelapure, R.R.; Hu, Y.J.; Dalton, C.; Bouma, M.H.; et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am. J. Gastroenterol. 2012, 107, 1426–1440. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.I.; Leon, A.C.; Keller, M.B.; Solomon, D.A.; Endicott, J.; Coryell, W.; Warshaw, M.; Maser, J.D. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am. J. Psychiatry 1999, 156, 1000–1006. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M.; et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohorquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881. [Google Scholar] [CrossRef] [Green Version]
- Bohorquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. The role of the gut microbiome in the development of schizophrenia. Schizophr. Res. 2021, 234, 4–23. [Google Scholar] [CrossRef]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jones, K.L.; Rayner, C.K.; Wu, T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics 2020, 12, 790. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Keating, D.J. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J. Endocrinol. 2019, 244, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Upchurch, B.H.; Fung, B.P.; Rindi, G.; Ronco, A.; Leiter, A.B. Peptide YY expression is an early event in colonic endocrine cell differentiation: Evidence from normal and transgenic mice. Development 1996, 122, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Vianna, A.R.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Beneficial effects of liraglutide (GLP1 analog) in the hippocampal inflammation. Metab. Brain Dis. 2017, 32, 1735–1745. [Google Scholar] [CrossRef]
- Solmaz, V.; Cinar, B.P.; Yigitturk, G.; Cavusoglu, T.; Taskiran, D.; Erbas, O. Exenatide reduces TNF-alpha expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur. J. Pharmacol. 2015, 765, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Manneras-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Hakansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Backhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Lahiri, D.K.; Chen, D.; Zhou, J.; Shaw, K.T.; Egan, J.M.; Greig, N.H. A novel neurotrophic property of glucagon-like peptide 1: A promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharm. Exp. Ther. 2002, 300, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharm. Exp. Ther. 2002, 302, 881–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Voss, U.; Sand, E.; Hellstrom, P.M.; Ekblad, E. Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol. 2012, 12, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, L.; Li, Y.; Li, L.; Melchiorsen, J.U.; Rosenkilde, M.; Holscher, C. The Novel Dual GLP-1/GIP Receptor Agonist DA-CH5 Is Superior to Single GLP-1 Receptor Agonists in the MPTP Model of Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Erdogan, M.A.; Erdogan, A.; Erbas, O. The Anti-Seizure Effect of Liraglutide on Ptz-Induced Convulsions Through its Anti-Oxidant and Anti-Inflammatory Properties. Neurochem. Res. 2022. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of Glucagon-Like Peptide-1 (GLP-1) with the Blood-Brain Barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Hunter, K.; Holscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuzzo, D.; Baldassano, S.; Amato, A.; Picone, P.; Galizzi, G.; Caldara, G.F.; Di Carlo, M.; Mule, F. Glucagon-like peptide-2 reduces the obesity-associated inflammation in the brain. Neurobiol. Dis. 2019, 121, 296–304. [Google Scholar] [CrossRef]
- Xie, S.; Liu, B.; Fu, S.; Wang, W.; Yin, Y.; Li, N.; Chen, W.; Liu, J.; Liu, D. GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-kappaB activation. Cell Physiol. Biochem. 2014, 34, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Kvidera, S.K.; Horst, E.A.; Sanz Fernandez, M.V.; Abuajamieh, M.; Ganesan, S.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; Trout, W.E.; Keating, A.F.; et al. Characterizing effects of feed restriction and glucagon-like peptide 2 administration on biomarkers of inflammation and intestinal morphology. J. Dairy Sci. 2017, 100, 9402–9417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Liu, B.W.; Ren, W.Z.; Liu, J.X.; Li, S.N.; Fu, S.P.; Zeng, Y.L.; Xu, S.Y.; Yan, X.; Gao, Y.J.; et al. GLP-2 Attenuates LPS-Induced Inflammation in BV-2 Cells by Inhibiting ERK1/2, JNK1/2 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Hao, L.; Shi, M.; Yu, Z.; Shao, S.; Yuan, Y.; Zhang, Z.; Holscher, C. Neuroprotective Effects of a GLP-2 Analogue in the MPTP Parkinson’s Disease Mouse Model. J. Parkinsons Dis. 2021, 11, 529–543. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Li, H.; Ma, J.; Sun, L.; Shao, S.; Zhang, Z.; Holscher, C. A GLP-2 Analogue Protects SH-SY5Y and Neuro-2a Cells Against Mitochondrial Damage, Autophagy Impairments and Apoptosis in a Parkinson Model. Drug Res. 2021, 71, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kuang, X.; Chen, T.; Shen, T.; Wu, J. Peptide YY 3-36 attenuates trinitrobenzene sulfonic acid-induced colitis in mice by modulating Th1/Th2 differentiation. Bioengineered 2022, 13, 10144–10158. [Google Scholar] [CrossRef] [PubMed]
- Guzzardi, M.A.; La Rosa, F.; Campani, D.; Cacciato Insilla, A.; Nannipieri, M.; Brunetto, M.R.; Bonino, F.; Iozzo, P. Evidence of a Gastro-Duodenal Effect on Adipose Tissue and Brain Metabolism, Potentially Mediated by Gut-Liver Inflammation: A Study with Positron Emission Tomography and Oral (18)FDG in Mice. Int. J. Mol. Sci. 2022, 23, 2695. [Google Scholar] [CrossRef]
- Mota, C.M.D.; Rodrigues-Santos, C.; Fernandez, R.A.R.; Carolino, R.O.G.; Antunes-Rodrigues, J.; Anselmo-Franci, J.A.; Branco, L.G.S. Central serotonin attenuates LPS-induced systemic inflammation. Brain Behav. Immun. 2017, 66, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.R.; Carnevale, L.N.; Xie, Z.; Baylon, J.L.; Tajkhorshid, E.; Hu, H.; Das, A. Anti-inflammatory dopamine- and serotonin-based endocannabinoid epoxides reciprocally regulate cannabinoid receptors and the TRPV1 channel. Nat. Commun. 2021, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A.; Schafer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Hirayama, M.; Ohno, K. Parkinson’s Disease and Gut Microbiota. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 28–35. [Google Scholar] [CrossRef]
- Lin, J.C.; Lin, C.S.; Hsu, C.W.; Lin, C.L.; Kao, C.H. Association Between Parkinson’s Disease and Inflammatory Bowel Disease: A Nationwide Taiwanese Retrospective Cohort Study. Inflamm. Bowel Dis. 2016, 22, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Braak, H.; Rub, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Del Tredici, K.; Rub, U.; De Vos, R.A.; Bohl, J.R.; Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002, 61, 413–426. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Mutlu, E.; Dodiya, H.B.; Daian, D.; Jaglin, J.A.; Kordower, J.H. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 2012, 27, 709–715. [Google Scholar] [CrossRef]
- Sanchez-Ferro, A.; Rabano, A.; Catalan, M.J.; Rodriguez-Valcarcel, F.C.; Fernandez Diez, S.; Herreros-Rodriguez, J.; Garcia-Cobos, E.; Alvarez-Santullano, M.M.; Lopez-Manzanares, L.; Mosqueira, A.J.; et al. In vivo gastric detection of alpha-synuclein inclusions in Parkinson’s disease. Mov. Disord. 2015, 30, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Hiniker, A.; Kuo, Y.M.; Nussbaum, R.L.; Liddle, R.A. alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2017, 2, e92295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casini, A.; Mancinelli, R.; Mammola, C.L.; Pannarale, L.; Chirletti, P.; Onori, P.; Vaccaro, R. Distribution of alpha-synuclein in normal human jejunum and its relations with the chemosensory and neuroendocrine system. Eur. J. Histochem. EJH 2021, 65, 3310. [Google Scholar] [CrossRef]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; Gonzalez-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila induces mitochondrial calcium overload and alpha -synuclein aggregation in an enteroendocrine cell line. iScience 2022, 25, 103908. [Google Scholar] [CrossRef]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes alpha-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell Res. 2020, 387, 111772. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Bjorklund, T.; Wang, Z.Y.; Roybon, L.; Melki, R.; Li, J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, P.V.; de Godoy, J.V.P.; Bosque, B.P.; Amorim Neto, D.P.; Tostes, K.; Palameta, S.; Garcia-Rosa, S.; Tonoli, C.C.C.; de Carvalho, H.F.; de Castro Fonseca, M. Transcellular propagation of fibrillar alpha-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent. Sci. Rep. 2022, 12, 4168. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022. [Google Scholar] [CrossRef]
- Boyle, J.G.; Livingstone, R.; Petrie, J.R. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: A comparative review. Clin. Sci. 2018, 132, 1699–1709. [Google Scholar] [CrossRef]
- Drobny, A.; Ngo, P.A.; Neurath, M.F.; Zunke, F.; Lopez-Posadas, R. Molecular Communication Between Neuronal Networks and Intestinal Epithelial Cells in Gut Inflammation and Parkinson’s Disease. Front. Med. 2021, 8, 655123. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Couch, Y.; Richardson, J.; Cooper, J.M.; Wood, M.J. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci. Res. 2011, 69, 337–342. [Google Scholar] [CrossRef]
- Beraud, D.; Hathaway, H.A.; Trecki, J.; Chasovskikh, S.; Johnson, D.A.; Johnson, J.A.; Federoff, H.J.; Shimoji, M.; Mhyre, T.R.; Maguire-Zeiss, K.A. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein alpha-synuclein. J. Neuroimmune Pharm. 2013, 8, 94–117. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habela, C.W.; Song, H.; Ming, G.L. Modeling synaptogenesis in schizophrenia and autism using human iPSC derived neurons. Mol. Cell Neurosci. 2016, 73, 52–62. [Google Scholar] [CrossRef]
- Faludi, G.; Mirnics, K. Synaptic changes in the brain of subjects with schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Depp, C.; Shih, P.B.; Cadenhead, K.S.; Schmid-Schonbein, G. Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front Neurosci. 2017, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Uellendahl-Werth, F.; Maj, C.; Borisov, O.; Juzenas, S.; Wacker, E.M.; Jorgensen, I.F.; Steiert, T.A.; Bej, S.; Krawitz, P.; Hoffmann, P.; et al. Cross-tissue transcriptome-wide association studies identify susceptibility genes shared between schizophrenia and inflammatory bowel disease. Commun. Biol. 2022, 5, 80. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Amad, A. GLP-1 agonists for metabolic disorders in schizophrenia. Schizophr. Res. 2019, 204, 448–449. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Powell, A.M.; Kaidanovich-Beilin, O.; Soczynska, J.K.; Alsuwaidan, M.; Woldeyohannes, H.O.; Kim, A.S.; Gallaugher, L.A. The neuroprotective effects of GLP-1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav. Brain Res. 2013, 237, 164–171. [Google Scholar] [CrossRef]
- Camkurt, M.A.; Lavagnino, L.; Zhang, X.Y.; Teixeira, A.L. Liraglutide for psychiatric disorders: Clinical evidence and challenges. Horm. Mol. Biol. Clin. Investig. 2018, 36, 1–6. [Google Scholar] [CrossRef]
- Ishoy, P.L.; Fagerlund, B.; Broberg, B.V.; Bak, N.; Knop, F.K.; Glenthoj, B.Y.; Ebdrup, B.H. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatr. Scand. 2017, 136, 52–62. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, J.N. Visceral pain: The neurophysiological mechanism. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2009; pp. 31–74. [Google Scholar] [CrossRef] [Green Version]
- Katz, B.L.; Van Houten, T.; Sabouri, A.S. Neuroanatomy and Mechanisms of Visceral Pain. In Interventional Management of Chronic Visceral Pain Syndromes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 5–15. [Google Scholar] [CrossRef]
- Ohlmann, H.; Koenen, L.R.; Labrenz, F.; Engler, H.; Theysohn, N.; Langhorst, J.; Elsenbruch, S. Altered Brain Structure in Chronic Visceral Pain: Specific Differences in Gray Matter Volume and Associations With Visceral Symptoms and Chronic Stress. Front. Neurol. 2021, 12, 733035. [Google Scholar] [CrossRef] [PubMed]
- Cortelli, P.; Montagna, P. Migraine as a visceral pain. Neurol. Sci. 2009, 30 (Suppl. 1.), S19–S22. [Google Scholar] [CrossRef]
- Moshiree, B.; Zhou, Q.; Price, D.D.; Verne, G.N. Central sensitisation in visceral pain disorders. Gut 2006, 55, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Davis, B.; Binion, D.G. Pain and inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 778–788. [Google Scholar] [CrossRef]
- Balmus, I.M.; Ciobica, A.; Cojocariu, R.; Luca, A.C.; Gorgan, L. Irritable Bowel Syndrome and Neurological Deficiencies: Is There A Relationship? The Possible Relevance of the Oxidative Stress Status. Medicina 2020, 56, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moloney, R.D.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front. Psychiatry 2015, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Greenwood-Van Meerveld, B.; Johnson, A.C. Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin. Front. Syst. Neurosci. 2017, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Lomax, A.E.; Pradhananga, S.; Sessenwein, J.L.; O’Malley, D. Bacterial modulation of visceral sensation: Mediators and mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G363–G372. [Google Scholar] [CrossRef] [PubMed]
- Morreale, C.; Bresesti, I.; Bosi, A.; Baj, A.; Giaroni, C.; Agosti, M.; Salvatore, S. Microbiota and Pain: Save Your Gut Feeling. Cells 2022, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlop, S.P.; Jenkins, D.; Neal, K.R.; Spiller, R.C. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003, 125, 1651–1659. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Taiwo, Y.O.; Levine, J.D. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience 1992, 48, 485–490. [Google Scholar] [CrossRef]
- Sufka, K.J.; Schomburg, F.M.; Giordano, J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol. Biochem. Behav. 1992, 41, 53–56. [Google Scholar] [CrossRef]
- Zeitz, K.P.; Guy, N.; Malmberg, A.B.; Dirajlal, S.; Martin, W.J.; Sun, L.; Bonhaus, D.W.; Stucky, C.L.; Julius, D.; Basbaum, A.I. The 5-HT3Subtype of Serotonin Receptor Contributes to Nociceptive Processing via a Novel Subset of Myelinated and Unmyelinated Nociceptors. J. Neurosci. 2002, 22, 1010–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaga, M.; Binienda, A.; Piscitelli, F.; Mokrowiecka, A.; Cygankiewicz, A.I.; Verde, R.; Malecka-Panas, E.; Kordek, R.; Krajewska, W.M.; Di Marzo, V.; et al. Systemic administration of serotonin exacerbates abdominal pain and colitis via interaction with the endocannabinoid system. Biochem. Pharm. 2019, 161, 37–51. [Google Scholar] [CrossRef]
- Diatchenko, L.; Slade, G.D.; Nackley, A.G.; Bhalang, K.; Sigurdsson, A.; Belfer, I.; Goldman, D.; Xu, K.; Shabalina, S.A.; Shagin, D.; et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum. Mol. Genet. 2005, 14, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Nackley, A.G.; Shabalina, S.A.; Tchivileva, I.E.; Satterfield, K.; Korchynskyi, O.; Makarov, S.S.; Maixner, W.; Diatchenko, L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science 2006, 314, 1930–1933. [Google Scholar] [CrossRef] [Green Version]
- Nackley, A.G.; Tan, K.S.; Fecho, K.; Flood, P.; Diatchenko, L.; Maixner, W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 2007, 128, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, D.; Wieskopf, J.S.; Rashid, N.; Sorge, R.E.; Redler, R.L.; Segall, S.K.; Mogil, J.S.; Maixner, W.; Dokholyan, N.V.; Diatchenko, L. Serotonin-induced hypersensitivity via inhibition of catechol O-methyltransferase activity. Mol. Pain 2012, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.Y.; Yang, Y.T.; She, M.P.; Tu, C.H.; Lee, T.C.; Wu, M.S.; Sun, C.H.; Hsin, L.W.; Yu, L.C. 5-HT7 receptor-dependent intestinal neurite outgrowth contributes to visceral hypersensitivity in irritable bowel syndrome. Lab. Investig. 2022, 102, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Zhang, W.; Li, T.; Yang, T.; Yuan, X.; Zhou, Y.; Zou, Q.; Yang, H.; Gao, F.; Tian, Y.; et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-κB pathway in neuropathic pain mice. Neurobiol. Learn Mem. 2021, 182, 107463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, W.; Tian, X. The pleiotropic of GLP-1/GLP-1R axis in central nervous system diseases. Int. J. Neurosci. 2021, 1–38. [Google Scholar] [CrossRef]
- Wu, H.Y.; Tang, X.Q.; Mao, X.F.; Wang, Y.X. Autocrine Interleukin-10 Mediates Glucagon-Like Peptide-1 Receptor-Induced Spinal Microglial beta-Endorphin Expression. J. Neurosci. 2017, 37, 11701–11714. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Xiao, Q.; Zhu, B.; Zhang, C.Y.; Wang, Y.C.; Fan, H.; Ma, A.N.; Wang, Y.X. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J. Neurosci. 2014, 34, 5322–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Y.; Han, Q.Q.; Deng, M.Y.; Zhao, M.J.; Apryani, E.; Shoaib, R.M.; Wei, D.Q.; Wang, Y.X. Lemairamin, isolated from the Zanthoxylum plants, alleviates pain hypersensitivity via spinal alpha7 nicotinic acetylcholine receptors. Biochem. Biophys. Res. Commun. 2020, 525, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.X. Spinal microglial beta-endorphin signaling mediates IL-10 and exenatide-induced inhibition of synaptic plasticity in neuropathic pain. CNS Neurosci. 2021, 27, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, H.; Mao, X.; Li, X.; Wang, Y. The GLP-1 receptor herbal agonist morroniside attenuates neuropathic pain via spinal microglial expression of IL-10 and beta-endorphin. Biochem. Biophys. Res. Commun. 2020, 530, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ju, P.; Wang, W.; Wei, J.; Wang, W.; Zhao, M.; Ahmad, K.A.; Wang, Y.; Chen, J. Microglial Activation of GLP-1R Signaling in Neuropathic Pain Promotes Gene Expression Adaption Involved in Inflammatory Responses. Neural Plast. 2021, 2021, 9923537. [Google Scholar] [CrossRef] [PubMed]
- Nozu, T.; Miyagishi, S.; Kumei, S.; Nozu, R.; Takakusaki, K.; Okumura, T. Glucagon-like peptide-1 analog, liraglutide, improves visceral sensation and gut permeability in rats. J. Gastroenterol. Hepatol. 2018, 33, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, R.; O’Malley, D. The Glucagon-like peptide-1 receptor agonist, exendin-4, ameliorated gastrointestinal dysfunction in the Wistar Kyoto rat model of Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2020, 32, e13738. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, P.M.; Hein, J.; Bytzer, P.; Bjornsson, E.; Kristensen, J.; Schambye, H. Clinical trial: The glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: A randomized, placebo-controlled, double-blind study. Aliment Pharm. Ther. 2009, 29, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Touny, A.A.; Kenny, E.; Mansson, M.; Webb, D.L.; Hellstrom, P.M. Pain relief and pain intensity response to GLP-1 receptor agonist ROSE-010 in irritable bowel syndrome; clinical study cross-analysis with respect to patient characteristics. Scand. J. Gastroenterol. 2022, 57, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Salhy, M.; Gundersen, D.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul. Pept. 2014, 188, 60–65. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Hausken, T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides 2015, 67, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.M.; Jain, P.; Mayerhofer, R.; Frohlich, E.E.; Farzi, A.; Reichmann, F.; Herzog, H.; Holzer, P. Visceral hyperalgesia caused by peptide YY deletion and Y2 receptor antagonism. Sci. Rep. 2017, 7, 40968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, J.R.; Alexander, T.; Covarrubias, M.; Waldman, S.A. GUCY2C-Enriched Intestinal Neuropod Cells Modulate Visceral Pain. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Bryant, A.P.; Busby, R.W.; Bartolini, W.P.; Cordero, E.A.; Hannig, G.; Kessler, M.M.; Pierce, C.M.; Solinga, R.M.; Tobin, J.V.; Mahajan-Miklos, S.; et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci. 2010, 86, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Busby, R.W.; Bryant, A.P.; Bartolini, W.P.; Cordero, E.A.; Hannig, G.; Kessler, M.M.; Mahajan-Miklos, S.; Pierce, C.M.; Solinga, R.M.; Sun, L.J.; et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur. J. Pharmacol. 2010, 649, 328–335. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef] [Green Version]
- Silos-Santiago, I.; Hannig, G.; Eutamene, H.; Ustinova, E.E.; Bernier, S.G.; Ge, P.; Graul, C.; Jacobson, S.; Jin, H.; Liong, E.; et al. Gastrointestinal pain: Unraveling a novel endogenous pathway through uroguanylin/guanylate cyclase-C/cGMP activation. Pain 2013, 154, 1820–1830. [Google Scholar] [CrossRef] [Green Version]
- Boulete, I.M.; Thadi, A.; Beaufrand, C.; Patwa, V.; Joshi, A.; Foss, J.A.; Eddy, E.P.; Eutamene, H.; Palejwala, V.A.; Theodorou, V.; et al. Oral treatment with plecanatide or dolcanatide attenuates visceral hypersensitivity via activation of guanylate cyclase-C in rat models. World J. Gastroenterol. 2018, 24, 1888–1900. [Google Scholar] [CrossRef]
- Brierley, S.M.; Grundy, L.; Castro, J.; Harrington, A.M.; Hannig, G.; Camilleri, M. Guanylate cyclase-C agonists as peripherally acting treatments of chronic visceral pain. Trends Pharm. Sci. 2022, 43, 110–122. [Google Scholar] [CrossRef]
- Gallery, M.; Zhang, J.; Bradley, D.P.; Brauer, P.; Cvet, D.; Estevam, J.; Danaee, H.; Greenfield, E.; Li, P.; Manfredi, M.; et al. A monomethyl auristatin E-conjugated antibody to guanylyl cyclase C is cytotoxic to target-expressing cells in vitro and in vivo. PLoS ONE 2018, 13, e0191046. [Google Scholar] [CrossRef] [Green Version]
- Danaee, H.; Kalebic, T.; Wyant, T.; Fassan, M.; Mescoli, C.; Gao, F.; Trepicchio, W.L.; Rugge, M. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS ONE 2017, 12, e0189953. [Google Scholar] [CrossRef] [Green Version]
- Liddle, R.A. Interactions of Gut Endocrine Cells with Epithelium and Neurons. Compr. Physiol. 2018, 8, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.T.; Poorsaadati, S.; Radkani, B.; Forootan, M. Psychological disorders in patients with chronic constipation. Gastroenterol. Hepatol. Bed Bench 2011, 4, 159–163. [Google Scholar] [PubMed]
- Dipnall, J.F.; Pasco, J.A.; Berk, M.; Williams, L.J.; Dodd, S.; Jacka, F.N.; Meyer, D. Into the Bowels of Depression: Unravelling Medical Symptoms Associated with Depression by Applying Machine-Learning Techniques to a Community Based Population Sample. PLoS ONE 2016, 11, e0167055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry 1967, 113, 1237–1264. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Smith, K.A.; Fairburn, C.G.; Cowen, P.J. Relapse of depression after rapid depletion of tryptophan. Lancet 1997, 349, 915–919. [Google Scholar] [CrossRef]
- Ruhe, H.G.; Mason, N.S.; Schene, A.H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol Psychiatry 2007, 12, 331–359. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Tian, P.; Zhu, H.; Zou, R.; Kong, Q.; Xu, M.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. An in vitro screening method for probiotics with antidepressant-like effect using the enterochromaffin cell model. Food Funct. 2021, 12, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V.; et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521.e504. [Google Scholar] [CrossRef]
- Iwai, T.; Hayashi, Y.; Narita, S.; Kasuya, Y.; Jin, K.; Tsugane, M.; Oka, J. Antidepressant-like effects of glucagon-like peptide-2 in mice occur via monoamine pathways. Behav. Brain Res. 2009, 204, 235–240. [Google Scholar] [CrossRef]
- Iwai, T.; Ohnuki, T.; Sasaki-Hamada, S.; Saitoh, A.; Sugiyama, A.; Oka, J. Glucagon-like peptide-2 but not imipramine exhibits antidepressant-like effects in ACTH-treated mice. Behav. Brain Res. 2013, 243, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Hamada, S.; Nakamura, R.; Nakao, Y.; Akimoto, T.; Sanai, E.; Nagai, M.; Horiguchi, M.; Yamashita, C.; Oka, J.I. Antidepressant-like effects exerted by the intranasal administration of a glucagon-like peptide-2 derivative containing cell-penetrating peptides and a penetration-accelerating sequence in mice. Peptides 2017, 87, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Weina, H.; Yuhu, N.; Christian, H.; Birong, L.; Feiyu, S.; Le, W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Res. 2018, 1694, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Zemdegs, J.; Martin, H.; Pintana, H.; Bullich, S.; Manta, S.; Marques, M.A.; Moro, C.; Laye, S.; Ducrocq, F.; Chattipakorn, N.; et al. Metformin Promotes Anxiolytic and Antidepressant-Like Responses in Insulin-Resistant Mice by Decreasing Circulating Branched-Chain Amino Acids. J. Neurosci. 2019, 39, 5935–5948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front Pharm. 2020, 11, 1270. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Li, Y. Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders. Biomedicines 2022, 10, 2577. https://doi.org/10.3390/biomedicines10102577
Yu L, Li Y. Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders. Biomedicines. 2022; 10(10):2577. https://doi.org/10.3390/biomedicines10102577
Chicago/Turabian StyleYu, Liangen, and Yihang Li. 2022. "Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders" Biomedicines 10, no. 10: 2577. https://doi.org/10.3390/biomedicines10102577
APA StyleYu, L., & Li, Y. (2022). Involvement of Intestinal Enteroendocrine Cells in Neurological and Psychiatric Disorders. Biomedicines, 10(10), 2577. https://doi.org/10.3390/biomedicines10102577




