Determinants of Six-Minute Walk Distance in Idiopathic Pulmonary Fibrosis and Idiopathic Pleuroparenchymal Fibroelastosis
Abstract
:1. Introduction
2. Materials and Methods
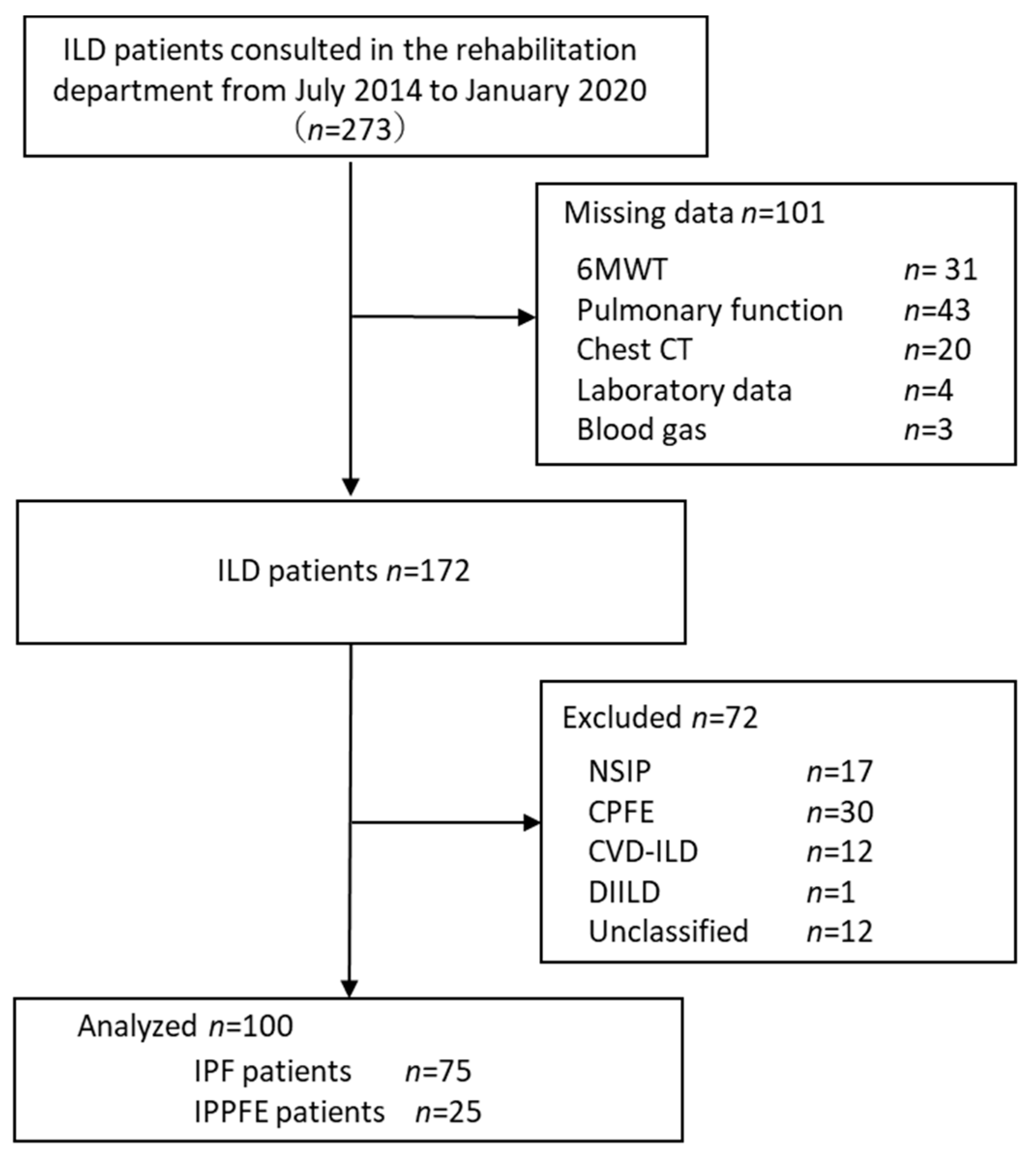
2.1. Study Population and Selection Criteria
2.2. Clinical Endpoints of the Original Study
2.3. Grouping
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [Green Version]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Hallstrand, T.S.; Boitano, L.J.; Johnson, W.C.; Spada, C.A.; Hayes, J.G.; Raghu, G.; Allstrand, T.S. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur. Respir. J. 2005, 25, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caminati, A.; Bianchi, A.; Cassandro, R.; Mirenda, M.R.; Harari, S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir. Med. 2009, 103, 117–123. [Google Scholar] [CrossRef] [Green Version]
- du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; Szwarcberg, J.; et al. Six-minute-walk test in idiopathic pulmonary fibrosis: Test validation and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 183, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Enright, P.L.; Sherrill, D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amitani, R.; Niimi, A.; Kuse, F. Idiopathic pulmonary upper lobe fibrosis. Kokyu 1992, 11, 693–699. (In Japanese) [Google Scholar]
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004, 126, 2007–2013. [Google Scholar] [CrossRef]
- Harada, T.; Yoshida, Y.; Kitasato, Y.; Tsuruta, N.; Wakamatsu, K.; Hirota, T.; Tanaka, M.; Tashiro, N.; Ishii, H.; Shiraishi, M.; et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur. Respir. Rev. 2014, 23, 263–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, H.; Watanabe, K.; Kushima, H.; Baba, T.; Watanabe, S.; Yamada, Y.; Arai, T.; Tsushima, K.; Kondoh, Y.; Nakamura, Y.; et al. Diffuse Lung Disease Study Group Pleuroparenchymal fibroelastosis diagnosed by multidisciplinary discussions in Japan. Respir. Med. 2018, 141, 190–197. [Google Scholar] [CrossRef]
- Hayashi, H.; Nei, T.; Abe, S.; Saito, Y.; Kokuho, N.; Atsumi, K.; Fujita, K.; Saito, T.; Tanaka, T.; Gemma, A.; et al. Body Mass Index and arterial blood oxygenation as prognostic factors in patients with idiopathic pleuroparenchymal fibroelastosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 35–40. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fujisawa, T.; Sumikawa, H.; Tanaka, T.; Sugimoto, C.; Kono, M.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; et al. Disease course and prognosis of pleuroparenchymal fibroelastosis compared with idiopathic pulmonary fibrosis. Respir. Med. 2020, 171, 106078. Available online: https://www.sciencedirect.com/science/article/pii/S0954611120302183 (accessed on 10 September 2022). [CrossRef]
- Tanizawa, K.; Handa, T.; Kubo, T.; Chen-Yoshikawa, T.F.; Aoyama, A.; Motoyama, H.; Hijiya, K.; Yoshizawa, A.; Oshima, Y.; Ikezoe, K.; et al. Clinical significance of radiological pleuroparenchymal fibroelastosis pattern in interstitial lung disease patients registered for lung transplantation: A retrospective cohort study. Respir. Res. 2018, 19, 162. [Google Scholar] [CrossRef] [Green Version]
- Shiiya, H.; Tian, D.; Sato, M.; Karasaki, T.; Kitano, K.; Nagayama, K.; Anraku, M.; Kaga, K.; Matsui, Y.; Nakajima, J. Differences Between Patients with Idiopathic Pleuroparenchymal Fibroelastosis and Those with Other Types of Idiopathic Interstitial Pneumonia in Candidates for Lung Transplants. Transplant. Proc. 2019, 51, 2014–2021. [Google Scholar] [CrossRef]
- du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; King, T.E., Jr. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, A.; Iwanami, Y.; Sugino, K.; Gocho, K.; Homma, S.; Ebihara, S. Using 6-Min Walk Distance Expressed as a Percentage of Reference to Evaluate the Effect of Pulmonary Rehabilitation in Elderly Patients with Interstitial Lung Disease. J. Cardiopulm. Rehabil. Prev. 2018, 38, 342–347. Available online: https://journals.lww.com/jcrjournal/Fulltext/2018/09000/Using_6_Min_Walk_Distance_Expressed_as_a.12.aspx (accessed on 10 September 2022). [CrossRef]
- Watanabe, K.; Ishii, H.; Kiyomi, F.; Terasaki, Y.; Hebisawa, A.; Kawabata, Y.; Johkoh, T.; Sakai, F.; Kondoh, Y.; Inoue, Y.; et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: A proposal. Respir. Investig. 2019, 57, 312–320. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Ley, B.; Ryerso, C.J.; Vittinghoff, E.; Ryu, J.H.; Tomassetti, S.; Lee, J.S.; Poletti, V.; Buccioli, M.; Elicker, B.M.; Jones, K.D.; et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012, 156, 684–691. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [Green Version]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Ebihara, K.; Iwanami, Y.; Yamasaki, K.; Takemura, A.; Sato, N.; Usui, Y.; Nakamura, Y.; Kishi, K.; Homma, S.; Ebihara, S. Appendicular Skeletal Muscle Mass Correlates with Patient-Reported Outcomes and Physical Performance in Patients with Idiopathic Pulmonary Fibrosis. Tohoku J. Exp. Med. 2021, 253, 61–68. [Google Scholar] [CrossRef]
- Troosters, T.; Gosselink, R.; Decramer, M. Six minute walking distance in healthy elderly subjects. Eur. Respir. J. 1999, 14, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Morakami, F.K.; Morita, A.A.; Bisca, G.W.; Felcar, J.M.; Ribeiro, M.; Furlanetto, K.C.; Hernandes, N.A.; Pitta, F. Can the six-minute walk distance predict the occurrence of acute exacerbations of COPD in patients in Brazil? J. Bras. Pneumol. 2017, 43, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, O.; Yamazaki, R.; Sano, H.; Iwanaga, T.; Higashimoto, Y.; Kume, H.; Tohda, Y. Fat-free mass index predicts survival in patients with idiopathic pulmonary fibrosis. Respirology 2017, 22, 480–485. [Google Scholar] [CrossRef]
- Guler, S.A. Body composition, muscle function, and physical performance in fibrotic interstitial lung disease: A prospective cohort study. Respir. Res. 2019, 20, 56. [Google Scholar] [CrossRef] [Green Version]
- Bonifazi, M.; Montero, M.A.; Renzoni, E.A. Idiopathic Pleuroparenchymal Fibroelastosis. Curr. Pulmonol. Rep. 2017, 6, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Ischaki, E.; Papatheodorou, G.; Gaki, E.; Papa, I.; Koulouris, N.; Loukides, S. Body mass and fat-free mass indices in COPD: Relation with variables expressing disease severity. Chest 2007, 132, 164–169. [Google Scholar] [CrossRef]
- Rambod, M.; Porszasz, J.; Make, B.J.; Crapo, J.D.; Casaburi, R. Six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest 2012, 141, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Schols, A.M.; Mostert, R.; Soeters, P.B.; Wouters, E.F. Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 1991, 46, 695–699. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.N.; Diaz, A.A.; Ross, J.C.; San Jose Estepar, R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef]
- Joppa, P.; Tkacova, R.; Franssen, F.M.; Hanson, C.; Rennard, S.I.; Silverman, E.K.; McDonald, M.L.; Calverley, P.M.; Tal-Singer, R.; Spruit, M.A.; et al. Sarcopenic Obesity, Functional Outcomes, and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2016, 17, 712–718. [Google Scholar] [CrossRef]
- Moreira, G.L.; Donária, L.; Furlanetto, K.C.; Paes, T.; Sant’Anna, T.; Hernandes, N.A.; Pitta, F. GOLD B-C-D groups or GOLD II-III-IV grades: Which one better reflects the functionality of patients with chronic obstructive pulmonary disease? Chronic Respir. Dis. 2015, 12, 102–110. [Google Scholar] [CrossRef]
- Nathan, S.D.; du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; et al. Validation of test performance characteristics and minimal clinically important difference of the 6-minute walk test in patients with idiopathic pulmonary fibrosis. Respir. Med. 2015, 109, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Oda, T.; Ogura, T.; Kitamura, H.; Hagiwara, E.; Baba, T.; Enomoto, Y.; Iwasawa, T.; Okudela, K.; Takemura, T.; Sakai, F.; et al. Distinct characteristics of pleuroparenchymal fibroelastosis with usual interstitial pneumonia compared with idiopathic pulmonary fibrosis. Chest 2014, 146, 1248–1255. [Google Scholar] [CrossRef]
- Nakatani, T.; Arai, T.; Kitaichi, M.; Akira, M.; Tachibana, K.; Sugimoto, C.; Hirooka, A.; Tsuji, T.; Minomo, S.; Hayashi, S.; et al. Pleuroparenchymal fibroelastosis from a consecutive database: A rare disease entity? Eur. Respir. J. 2015, 45, 1183–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, H.; Kinoshita, Y.; Kushima, H.; Nagata, N.; Watanabe, K. The similarities and differences between pleuroparenchymal fibroelastosis and idiopathic pulmonary fibrosis. Chron. Respir. Dis. 2019, 16, 1479973119867945. [Google Scholar] [CrossRef]
| IPF (n = 75) | IPPFE (n = 25) | p Value | |
|---|---|---|---|
| Age (years) | 73.3 ± 7.6 | 73.4 ± 6.1 | 0.511 † |
| Gender (male/female) | 58/17 | 15/10 | 0.093 ‡ |
| BMI (kg/m2) | 23.5 ± 3.1 | 17.6 ± 2.3 | <0.001 † |
| mMRC (0/1/2/3/4) | 19/29/16/8/3 | 3/5/10/4/3 | 0.010 † |
| Severity | |||
| GAP Index (Ⅰ/Ⅱ/Ⅲ) | 35/30/10 | 8/10/7 | 0.097 † |
| Pulmonary function | |||
| FVC (L) | 2.3 ± 0.7 | 1.6 ± 0.5 | <0.001 † |
| FVC (%predicted) | 76.6 ± 19.1 | 58.2 ± 15.6 | <0.001 † |
| FEV1 (L) | 1.9 ± 0.5 | 1.4 ± 0.5 | 0.001 † |
| FEV1 (%predicted) | 95.0 ± 24.2 | 78.0 ± 19.9 | 0.006 † |
| FEV1/FVC (%) | 89.9 ± 16.4 | 96.6 ± 17.5 | 0.005 † |
| TLC (L) | 3.6 ± 1.0 | 3.2 ± 0.9 | 0.044 † |
| TLC (%predicted) | 74.7 ± 16.5 | 70.0 ± 15.8 | 0.209 † |
| RV (L) | 1.3 ± 0.4 | 1.6 ± 0.7 | 0.430 † |
| RV (%predicted) | 79.7 ± 22.9 | 98.6 ± 33.4 | 0.020 † |
| RV/TLC (%) | 37.6 ± 7.5 | 48.9 ± 8.9 | <0.001 † |
| RV/TLC (%predicted) | 100.6 ± 22.3 | 131.2 ± 28.3 | <0.001 † |
| DLco (%predicted) | 60.6 ± 16.4 | 61.7 ± 17.0 | 0.786 † |
| pH | 7.4 ± 4.2 | 7.4 ± 0.0 | 0.693 † |
| PaO2 (mmHg) | 86.5.7 ± 13.9 | 86.5 ± 13.6 | 0.705 † |
| PaCO2 (mmHg) | 40.7 ± 4.2 | 45.5 ± 6.4 | <0.001 † |
| Serum IP marker | |||
| KL-6 (U/mL) | 978.8 ± 663.0 | 964.5 ± 299.9 | 0.159 † |
| SP-A (ng/mL) | 65.5 ± 31.8 | 59.1 ± 37.5 | 0.128 † |
| SP-D (ng/mL) | 261.7 ± 210.8 | 267.1 ± 201.2 | 0.982 † |
| Physical assessment | |||
| 6MWD (m) | 395.1 ± 106.9 | 373.4 ± 103.2 | 0.627 † |
| ≥250 m {No. (%)} | 6 (8) | 2 (8) | 0.682 ‡ |
| %6MWD (%predicted) | 84.9 ± 22.5 | 72.1 ± 23.0 | 0.013 † |
| Quadriceps force (Nm/kg) | 1.3 ± 0.4 | 1.2 ± 0.5 | 0.471 † |
| Handgrip strength (kg) | 27.3 ± 7.5 | 22.4 ± 7.1 | 0.007 † |
| Skeletal muscle assessment | |||
| PMCSA (cm2) | 34.8 ± 8.4 | 24.9 ± 7.2 | <0.001 † |
| ESMCSA (cm2) | 29.6 ± 6.8 | 22.6 ± 6.4 | <0.001 † |
| Normal Group (n = 54) | Decline Group (n = 46) | p Value | |
|---|---|---|---|
| IPF/IPPFE (n) | 46/8 | 29/17 | 0.011 ‡ |
| Age (years) | 72.7 ± 7.5 | 74.5 ± 6.7 | 0.163 † |
| Sex (male/female) | 45/9 | 28/18 | 0.008 ‡ |
| BMI (kg/m2) | 23.4 ± 3.7 | 20.5 ± 3.6 | 0.002 † |
| mMRC (0/1/2/3/4) | 16/26/11/1/0 | 6/8/15/11/6 | <0.001 † |
| Severity | |||
| GAP Index (I/II/III) | 33/19/2 | 10/21/15 | <0.001 † |
| Pulmonary function | |||
| FVC (L) | 2.4 ± 0.7 | 1.8 ± 0.6 | <0.001 † |
| FVC (%predicted) | 79.1 ± 17.7 | 63.7 ± 19.6 | <0.001 † |
| FEV1 (L) | 2.0 ± 0.5 | 1.6 ± 0.5 | <0.001 † |
| FEV1 (%predicted) | 96.5 ± 20.1 | 84.0 ± 27.2 | 0.002 † |
| FEV1/FVC (%) | 88.9 ± 16.2 | 94.7 ± 17.5 | 0.011 † |
| TLC (L) | 3.9 ± 0.9 | 3.2 ± 0.9 | p < 0.001 † |
| TLC (%predicted) | 77.5 ± 15.3 | 68.8 ± 16.5 | 0.005 † |
| RV (L) | 1.4 ± 0.4 | 1.4 ± 0.6 | 0.181 † |
| RV (%predicted) | 86.3 ± 25.9 | 82.5 ± 28.5 | 0.209 † |
| RV/TLC (%) | 37.5 ± 15.3 | 44.2 ± 9.2 | 0.001 † |
| %RV/TLC (%predicted) | 99.8 ± 24.3 | 118.5 ± 27.5 | 0.001 † |
| DLco (%predicted) | 65.9 ± 14.9 | 54.5 ± 16.8 | 0.001 † |
| pH | 7.4 ± 0.0 | 7.4 ± 0.0 | 0.157 † |
| PaO2 (mmHg) | 89.0 ± 12.9 | 82.4 ± 14.4 | 0.036 † |
| PaCO2 (mmHg) | 41.3 ± 3.9 | 42.6 ± 6.6 | 0.352 † |
| Serum IP marker | |||
| KL-6 (U/mL) | 888.0 ± 651.4 | 931.6 ± 453.7 | 0.201 † |
| SP-A (ng/mL) | 60.2 ± 31.6 | 68.8.1 ± 36.3 | 0.170 † |
| SP-D (ng/mL) | 247.0 ± 192.6 | 286.1 ± 233.4 | 0.197 † |
| Physical assessment | |||
| Quadriceps force (Nm/kg) | 1.3 ± 0.4 | 1.2 ± 0.4 | 0.054 † |
| Handgrip strength (kg) | 29.1 ± 6.9 | 22.4 ± 6.9 | <0.001 † |
| Skeletal muscle assessment | |||
| PMCSA (cm2) | 36.1 ± 8.8 | 27.9 ± 7.7 | <0.001 † |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| Disease type (IPF/IPPFE) | 0.383 | 0.650–2.444 | 0.288 |
| Sex (male/female) | 2.103 | 0.238–18.535 | 0.503 |
| BMI (kg/m2) | 0.745 | 0.570–0.974 | 0.032 |
| mMRC (0/1/2/3/4) | 2.131 | 1.097–4.139 | 0.026 |
| Severity | |||
| GAP Index (I/II/III) | 3.483 | 1.038–11.684 | 0.043 |
| Pulmonary function | |||
| FEV1 (%predicted) | 1.009 | 0.961–1.060 | 0.708 |
| TLC (%predicted) | 1.002 | 0.941–1.060 | 0.962 |
| RV/TLC (%predicted) | 0.992 | 0.951–1.034 | 0.700 |
| PaO2 (mmHg) | 0.988 | 0.942–1.036 | 0.619 |
| Physical assessment | |||
| Handgrip strength (kg) | 0.911 | 0.803–1.034 | 0.149 |
| Skeletal muscle assessment | |||
| PMCSA (cm2) | 0.951 | 0.846–1.070 | 0.407 |
| ESMCSA (cm2) | 1.065 | 0.937–1.210 | 0.338 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, N.; Iwanami, Y.; Ebihara, K.; Nakao, K.; Miyagi, M.; Nakamura, Y.; Kishi, K.; Homma, S.; Ebihara, S. Determinants of Six-Minute Walk Distance in Idiopathic Pulmonary Fibrosis and Idiopathic Pleuroparenchymal Fibroelastosis. Biomedicines 2022, 10, 2556. https://doi.org/10.3390/biomedicines10102556
Sato N, Iwanami Y, Ebihara K, Nakao K, Miyagi M, Nakamura Y, Kishi K, Homma S, Ebihara S. Determinants of Six-Minute Walk Distance in Idiopathic Pulmonary Fibrosis and Idiopathic Pleuroparenchymal Fibroelastosis. Biomedicines. 2022; 10(10):2556. https://doi.org/10.3390/biomedicines10102556
Chicago/Turabian StyleSato, Naofumi, Yuji Iwanami, Kento Ebihara, Keiko Nakao, Midori Miyagi, Yasuhiko Nakamura, Kazuma Kishi, Sakae Homma, and Satoru Ebihara. 2022. "Determinants of Six-Minute Walk Distance in Idiopathic Pulmonary Fibrosis and Idiopathic Pleuroparenchymal Fibroelastosis" Biomedicines 10, no. 10: 2556. https://doi.org/10.3390/biomedicines10102556
APA StyleSato, N., Iwanami, Y., Ebihara, K., Nakao, K., Miyagi, M., Nakamura, Y., Kishi, K., Homma, S., & Ebihara, S. (2022). Determinants of Six-Minute Walk Distance in Idiopathic Pulmonary Fibrosis and Idiopathic Pleuroparenchymal Fibroelastosis. Biomedicines, 10(10), 2556. https://doi.org/10.3390/biomedicines10102556





