GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders
Abstract
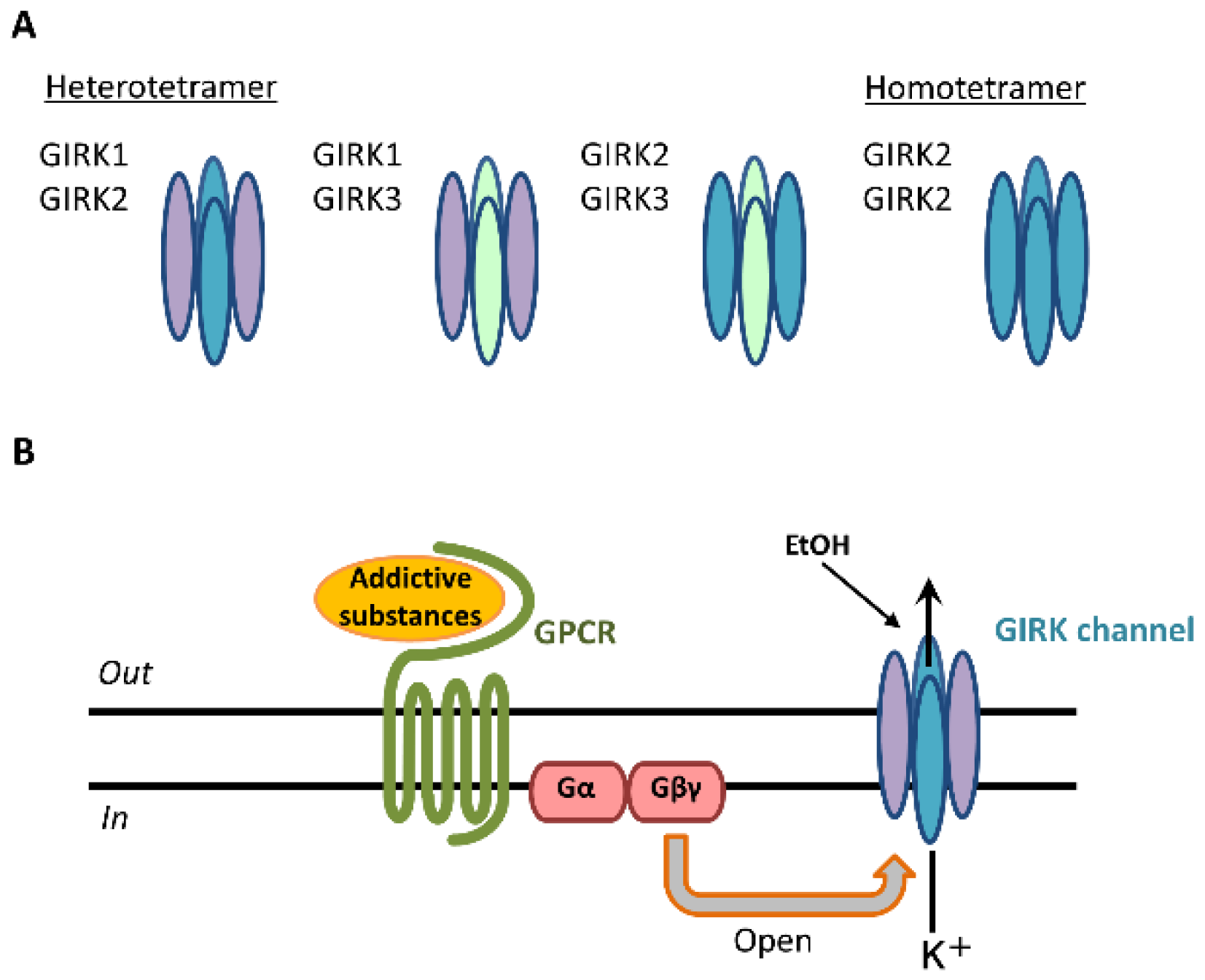
:1. Introduction
2. Fundamental Function of GIRK Channels and Response to Addictive Substances
3. Pharmacological Modulation of GIRK Channels and Therapeutic Effects
4. Future Directions
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SUD | Substance use disorder |
| GIRK | G-protein-activated inwardly rectifying potassium |
| VTA | Ventral tegmental area |
| NAc | Nucleus accumbens |
| GPCR | G-protein-coupled receptor |
| NMDA | N-methyl-D-aspartate |
| GABA | γ-aminobutyric acid |
| SNP | Single-nucleotide polymorphism |
| SRRS | Stimulant Relapse Risk Scale |
References
- Office on Drugs and Crime. World Drug Report 2021. Available online: https://www.unodc.org/unodc/en/data-and-analysis/wdr2021.html (accessed on 25 August 2022).
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, Y.; Reuveny, E.; Slesinger, P.A.; Jan, Y.N.; Jan, L.Y. Primary structure and functional expression of a rat G-proteinoupled muscarinic potassium channel. Nature 1993, 364, 802–806. [Google Scholar] [CrossRef]
- Lüscher, C.; Slesinger, P.A. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 2010, 11, 301–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karschin, C.; Dissmann, E.; Stuhmer, W.; Karschin, A. IRK(1-3) and GIRK(1-4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J. Neurosci. 1996, 16, 3559–3570. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Ikeda, K.; Ichikawa, T.; Abe, S.; Togashi, S.; Kumanishi, T. Molecular Cloning of a Mouse G-Protein-Activated K+ Channel (mGIRK1) and Distinct Distributions of 3 GIRK (GIRK1, 2 and 3) mRNAs in Mouse Brain. Biochem. Biophys. Res. Commun. 1995, 208, 1166–1173. [Google Scholar] [CrossRef]
- Kozell, L.B.; Walter, N.A.R.; Milner, L.C.; Wickman, K.; Buck, K.J. Mapping a Barbiturate Withdrawal Locus to a 0.44 Mb Interval and Analysis of a Novel Null Mutant Identify a Role for Kcnj9 (GIRK3) in Withdrawal from Pentobarbital, Zolpidem, and Ethanol. J. Neurosci. 2009, 29, 11662–11673. [Google Scholar] [CrossRef] [Green Version]
- Wickman, K.; Karschin, C.; Karschin, A.; Picciotto, M.R.; Clapham, D.E. Brain Localization and Behavioral Impact of the G-Protein-Gated K+Channel Subunit GIRK. J. Neurosci. 2000, 20, 5608–5615. [Google Scholar] [CrossRef] [Green Version]
- Poisson, C.L.; Engel, L.; Saunders, B.T. Dopamine Circuit Mechanisms of Addiction-Like Behaviors. Front. Neural Circuits 2021, 15, 752420. [Google Scholar] [CrossRef]
- Van Huijstee, A.N.; Mansvelder, H.D. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Front. Cell. Neurosci. 2014, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Sugaya, N.; Kobayashi, T.; Ikeda, K.; Nagisa, S.; Toru, K.; Kazutaka, I. Role of GIRK Channels in Addictive Substance Effects. J. Drug Alcohol Res. 2013, 2, 235823. [Google Scholar] [CrossRef]
- Andrade, R.; Malenka, R.C.; Nicoll, R.A. A G Protein Couples Serotonin and GABA B Receptors to the Same Channels in Hippocampus. Science 1986, 234, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.G.; Ivanova, T.; Lunn, M.-L.; Stoffel, M.; Slesinger, P.A.; Luescher, C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004, 7, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.M.; North, R.A. Acetylcholine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 1986, 319, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-Z.; Schonbrunn, A. Coupling Specificity between Somatostatin Receptor sst2A and G Proteins: Isolation of the Receptor-G Protein Complex with a Receptor Antibody. Mol. Endocrinol. 1997, 11, 527–537. [Google Scholar] [CrossRef]
- Inanobe, A.; Yoshimoto, Y.; Horio, Y.; Morishige, K.I.; Hibino, H.; Matsumoto, S.; Tokunaga, Y.; Maeda, T.; Hata, Y.; Takai, Y.; et al. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J. Neurosci. 1999, 19, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Christie, M.J.; North, R.A. Single potassium channels opened by opioids in rat locus ceruleus neurons. Proc. Natl. Acad. Sci. USA 1989, 86, 3419–3422. [Google Scholar] [CrossRef] [Green Version]
- Velimirovic, B.M.; Gordon, E.A.; Lim, N.F.; Navarro, B.; Clapham, D.E. The K+ channel inward rectifier subunits form a channel similar to neuronal G protein-gated K+ channel. FEBS Lett. 1996, 379, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.; North, R. Catecholamine inhibition of calcium action potentials in rat locus coeruleus neurones. Neuroscience 1985, 14, 103–109. [Google Scholar] [CrossRef]
- Kano, H.; Toyama, Y.; Imai, S.; Iwahashi, Y.; Mase, Y.; Yokogawa, M.; Osawa, M.; Shimada, I. Structural mechanism underlying G protein family-specific regulation of G protein-gated inwardly rectifying potassium channel. Nat. Commun. 2019, 10, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambright, D.G.; Sondek, J.; Bohm, A.; Skiba, N.P.; Hamm, H.E.; Sigler, P.B. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature 1996, 379, 311–319. [Google Scholar] [CrossRef]
- Clapham, D.; Neer, E.J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature 1993, 365, 403–406. [Google Scholar] [CrossRef]
- Geng, X.; Du, X.-N.; Rusinova, R.; Liu, B.-Y.; Li, F.; Zhang, X.; Chen, X.-J.; Logothetis, D.E.; Zhang, H.-L. Specificity of Gβγ Signaling Depends on Gα Subunit Coupling with G-Protein-Sensitive K+ Channels. Pharmacology 2009, 84, 82–90. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ikeda, K.; Kojima, H.; Niki, H.; Yano, R.; Yoshioka, T.; Kumanishi, T. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat. Neurosci. 1999, 2, 1091–1097. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, D.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Phencyclidine. Curr. Neuropharmacol. 2011, 9, 244–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Nishizawa, D.; Iwamura, T.; Ikeda, K. Inhibition by cocaine of G protein-activated inwardly rectifying K+ channels expressed in Xenopus oocytes. Toxicol. Vitr. 2007, 21, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Haluk, D.M.; Kourrich, S.; Pravetoni, M.; Fernández-Alacid, L.; Nicolau, J.C.; Luján, R.; Wickman, K. Altered neurotransmission in the mesolimbic reward system of Girk−/− mice. J. Neurochem. 2010, 114, 1487–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marker, C.L.; Stoffel, M.; Wickman, K. Spinal G-Protein-Gated K+ Channels Formed by GIRK1 and GIRK2 Subunits Modulate Thermal Nociception and Contribute to Morphine Analgesia. J. Neurosci. 2004, 24, 2806–2812. [Google Scholar] [CrossRef] [Green Version]
- Kotecki, L.; Hearing, M.; McCall, N.M.; de Velasco, E.M.F.; Pravetoni, M.; Arora, D.; Victoria, N.C.; Munoz, M.B.; Xia, Z.; Slesinger, P.A.; et al. GIRK Channels Modulate Opioid-Induced Motor Activity in a Cell Type- and Subunit-Dependent Manner. J. Neurosci. 2015, 35, 7131–7142. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.D.; Carroll, M.E.; Loth, A.K.; Stoffel, M.; Wickman, K. Decreased Cocaine Self-Administration in Kir3 Potassium Channel Subunit Knockout Mice. Neuropsychopharmacology 2003, 28, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, K.G.; Alva, H.; Blednov, Y.A.; Cunningham, C.L. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology 2003, 169, 108–114. [Google Scholar] [CrossRef]
- Ikeda, K.; Kobayashi, T.; Kumanishi, T.; Niki, H.; Yano, R. Involvement of G-protein-activated inwardly rectifying K+ (GIRK) channels in opioid-induced analgesia. Neurosci. Res. 2000, 38, 113–116. [Google Scholar] [CrossRef]
- Schmidt, M.; Sawyer, B.; Perry, K.; Fuller, R.; Foreman, M.; Ghetti, B. Dopamine deficiency in the weaver mutant mouse. J. Neurosci. 1982, 2, 376–380. [Google Scholar] [CrossRef]
- Ikekubo, Y.; Ide, S.; Hagino, Y.; Ikeda, K. Absence of methamphetamine-induced conditioned place preference in weaver mutant mice. Neuropsychopharmacol. Rep. 2020, 40, 324–331. [Google Scholar] [CrossRef]
- McCall, N.; Kotecki, L.; Dominguez-Lopez, S.; de Velasco, E.M.F.; Carlblom, N.; Sharpe, A.L.; Beckstead, M.J.; Wickman, K. Selective Ablation of GIRK Channels in Dopamine Neurons Alters Behavioral Effects of Cocaine in Mice. Neuropsychopharmacology 2017, 42, 707–715. [Google Scholar] [CrossRef] [Green Version]
- McCall, N.M.; De Velasco, E.M.F.; Wickman, K. GIRK Channel Activity in Dopamine Neurons of the Ventral Tegmental Area Bidirectionally Regulates Behavioral Sensitivity to Cocaine. J. Neurosci. 2019, 39, 3600–3610. [Google Scholar] [CrossRef] [Green Version]
- Tipps, M.E.; Raybuck, J.D.; Kozell, L.B.; Lattal, K.M.; Buck, K.J. G Protein-Gated Inwardly Rectifying Potassium Channel Subunit 3 Knock-Out Mice Show Enhanced Ethanol Reward. Alcohol. Clin. Exp. Res. 2016, 40, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Herman, M.A.; Sidhu, H.; Stouffer, D.G.; Kreifeldt, M.; Le, D.; Cates-Gatto, C.; Munoz, M.B.; Roberts, A.J.; Parsons, L.H.; Roberto, M.; et al. GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol. Proc. Natl. Acad. Sci. USA 2015, 112, 7091–7096. [Google Scholar] [CrossRef] [Green Version]
- Patil, N.; Cox, D.R.; Bhat, D.; Faham, M.; Myers, R.M.; Peterson, A.S. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat. Genet. 1995, 11, 126–129. [Google Scholar] [CrossRef]
- Nishizawa, D.; Fukuda, K.-I.; Kasai, S.; Ogai, Y.; Hasegawa, J.; Sato, N.; Yamada, H.; Tanioka, F.; Sugimura, H.; Hayashida, M.; et al. Association Between KCNJ6 (GIRK2) Gene Polymorphism rs2835859 and Post-operative Analgesia, Pain Sensitivity, and Nicotine Dependence. J. Pharmacol. Sci. 2014, 126, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, D.; Nagashima, M.; Katoh, R.; Satoh, Y.; Tagami, M.; Kasai, S.; Ogai, Y.; Han, W.; Hasegawa, J.; Shimoyama, N.; et al. Association between KCNJ6 (GIRK2) Gene Polymorphisms and Postoperative Analgesic Requirements after Major Abdominal Surgery. PLoS ONE 2009, 4, e7060. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ikeda, K.; Kumanishi, T. Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K+ (GIRK) channels expressed in Xenopus oocytes. J. Cereb. Blood Flow Metab. 2000, 129, 1716–1722. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). J. Cereb. Blood Flow Metab. 2003, 138, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Various Antidepressant Drugs. Neuropsychopharmacology 2004, 29, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G protein-activated inwardly rectifying K+ channels by the antidepressant paroxetine. J. Pharmacol. Sci. 2006, 102, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Ifenprodil. Neuropsychopharmacology 2006, 31, 516–524. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Different Classes of Antidepressants. PLoS ONE 2011, 6, e28208. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, Y.; Yamamoto, H.; Hagino, Y.; Markou, A.; Ikeda, K. The Selective Serotonin Reuptake Inhibitor Paroxetine, but not Fluvoxamine, Decreases Methamphetamine Conditioned Place Preference in Mice. Curr. Neuropharmacol. 2011, 9, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Takamatsu, Y.; Yamamoto, H.; Ogai, Y.; Hagino, Y.; Markou, A.; Ikeda, K. Fluoxetine as a Potential Pharmacotherapy for Methamphetamine Dependence: Studies in Mice. Ann. New York Acad. Sci. 2006, 1074, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Edinoff, A.; Akuly, H.; Hanna, T.; Ochoa, C.; Patti, S.; Ghaffar, Y.; Kaye, A.; Viswanath, O.; Urits, I.; Boyer, A.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Williams, K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: Selectivity and mechanisms at recombinant heteromeric receptors. Mol. Pharmacol. 1993, 44, 851–859. [Google Scholar]
- Mony, L.; Kew, J.N.C.; Gunthorpe, M.J.; Paoletti, P. Allosteric modulators of NR2B-containing NMDA receptors: Molecular mechanisms and therapeutic potential. J. Cereb. Blood Flow Metab. 2009, 157, 1301–1317. [Google Scholar] [CrossRef]
- Hashimoto, K.; London, E.D. Further characterization of [3H]ifenprodil binding to σ receptors in rat brain. Eur. J. Pharmacol. 1993, 236, 159–163. [Google Scholar] [CrossRef]
- Miyatake, M.; Narita, M.; Shibasaki, M.; Nakamura, A.; Suzuki, T. Glutamatergic neurotransmission and protein kinase C play a role in neuron-glia communication during the development of methamphetamine-induced psychological dependence. Eur. J. Neurosci. 2005, 22, 1476–1488. [Google Scholar] [CrossRef]
- Suzuki, T.; Kato, H.; Tsuda, M.; Suzuki, H.; Misawa, M. Effects of the non-competitive NMDA receptor antagonist ifenprodil on the morphine-induced place preference in mice. Life Sci. 1999, 64, PL151–PL156. [Google Scholar] [CrossRef]
- Li, M.-H.; Underhill, S.M.; Reed, C.; Phillips, T.J.; Amara, S.; Ingram, S.L. Amphetamine and Methamphetamine Increase NMDAR-GluN2B Synaptic Currents in Midbrain Dopamine Neurons. Neuropsychopharmacology 2017, 42, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Trovero, F.; David, S.; Bernard, P.; Puech, A.; Bizot, J.-C.; Tassin, J.-P. The Combination of Marketed Antagonists of α1b-Adrenergic and 5-HT2A Receptors Inhibits Behavioral Sensitization and Preference to Alcohol in Mice: A Promising Approach for the Treatment of Alcohol Dependence. PLoS ONE 2016, 11, e0151242. [Google Scholar] [CrossRef]
- Chen, G.; Li, T.; Xiao, J.; Wang, J.; Shang, Q.; Qian, H.; Qiao, C.; Zhang, P.; Chen, T.; Liu, X. Ifenprodil Attenuates Methamphetamine-Induced Behavioral Sensitization Through the GluN2B-PP2A-AKT Cascade in the Dorsal Striatum of Mice. Neurochem. Res. 2020, 45, 891–901. [Google Scholar] [CrossRef]
- Yomiya, K.; Matsuo, N. NMDA jyuyoutai kikkoyaku ifenprodil no chintsuuhojyoyaku toshiteno yuukousei. Kanwa Iryougaku 2003, 5, 70–78. [Google Scholar]
- Chizh, B.A.; Headley, P.; Tzschentke, T.M. NMDA receptor antagonists as analgesics: Focus on the NR2B subtype. Trends Pharmacol. Sci. 2001, 22, 636–642. [Google Scholar] [CrossRef]
- Goto, K. Arukoru izonshou no kouishougai ni taisuru ifenprodil (cerocral) no kouka. [in Japanese]. Alcohol Stud. Drug Depend. 2010, 45, 150. [Google Scholar]
- Ogai, Y.; Hori, T.; Haraguchi, A.; Asukai, N.; Senoo, E.; Ikeda, K. Influence of GIRK channel inhibition on alcohol abstinence and relapse risk in Japanese alcohol-dependent outpatients. Nihon shinkei seishin yakurigaku zasshi 2011, 31, 95–96. [Google Scholar] [PubMed]
- Sugaya, N.; Ogai, Y.; Kakibuchi, Y.; Senoo, E.; Ikeda, K. Influence of GIRK channel inhibition on relapse risk in Japanese alcohol-dependent inpatients. Nihon Shinkei Seishin Yakurigaku Zasshi 2012, 32, 165–167. [Google Scholar] [PubMed]
- Sugaya, N.; Ogai, Y.; Aikawa, Y.; Yumoto, Y.; Takahama, M.; Tanaka, M.; Haraguchi, A.; Umeno, M.; Ikeda, K. A randomized controlled study of the effect of ifenprodil on alcohol use in patients with alcohol dependence. Neuropsychopharmacol. Rep. 2018, 38, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kotajima-Murakami, H.; Takano, A.; Hirakawa, S.; Ogai, Y.; Funada, D.; Tanibuchi, Y.; Ban, E.; Kikuchi, M.; Tachimori, H.; Maruo, K.; et al. Ifenprodil for the treatment of methamphetamine use disorder: An exploratory, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacol. Rep. 2022, 42, 92–104. [Google Scholar] [CrossRef]
- Hori, T.; Komiyama, T.; Ikeda, K.; Suzuki, T. Katsubou ni taishite ifenprodil ga yuukou to kangaerareta 2 shourei [in Japanese]. Alcohol Stud. Drug Depend. 2010, 45, 151. [Google Scholar]
- Kotajima-Murakami, H.; Takano, A.; Ogai, Y.; Tsukamoto, S.; Murakami, M.; Funada, D.; Tanibuchi, Y.; Tachimori, H.; Maruo, K.; Sasaki, T.; et al. Study of effects of ifenprodil in patients with methamphetamine dependence: Protocol for an exploratory, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacol. Rep. 2019, 39, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Usami, T.; Yamamoto, T.; Funada, D.; Murakami, M.; Okita, K.; Shimane, T. Impact of COVID -19-related stress on methamphetamine users in Japan. Psychiatry Clin. Neurosci. 2021, 75, 236–238. [Google Scholar] [CrossRef]
- Weaver, C.D.; Denton, J.S. Next-generation inward rectifier potassium channel modulators: Discovery and molecular pharmacology. Am. J. Physiol. Physiol. 2021, 320, C1125–C1140. [Google Scholar] [CrossRef]
- Kaufmann, K.; Romaine, I.; Days, E.; Pascual, C.; Malik, A.; Yang, L.; Zou, B.; Du, Y.; Sliwoski, G.; Morrison, R.D.; et al. ML297 (VU0456810), the First Potent and Selective Activator of the GIRK Potassium Channel, Displays Antiepileptic Properties in Mice. ACS Chem. Neurosci. 2013, 4, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozek, K.A.; Du, Y.; Sharma, S.; Prael, F.J., 3rd; Spitznagel, B.D.; Kharade, S.V.; Denton, J.S.; Hopkins, C.R.; Weaver, C.D. Discovery and Characterization of VU0529331, a Synthetic Small-Molecule Activator of Homomeric G Protein-Gated, Inwardly Rectifying, Potassium (GIRK) Channels. ACS Chem. Neurosci. 2019, 10, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Whorton, M.R.; MacKinnon, R. X-ray structure of the mammalian GIRK2-betagamma G-protein complex. Nature 2013, 498, 190–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Abreu, N.; Levitz, J.; Kruse, A.C. Structural basis for KCTD-mediated rapid desensitization of GABAB signalling. Nature 2019, 567, 127–131. [Google Scholar] [CrossRef] [PubMed]
- SAMHSA. Medication-Assisted Treatment (MAT). Available online: https://www.samhsa.gov/medication-assisted-treatment (accessed on 11 August 2022).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; pp. 561–570. [Google Scholar]
- Blum, K.; Badgaiyan, R.D. Translational and Molecular Cytoarchitectural Genetic Guided Therapy to Induce Dopamine Homeostatic Neuro-signaling in Reward Deficiency and Associated Drug and Behavioral Addiction Seeking: A 60 Year Sojourn the Future is Now. EC Psychol. Psychiatr. 2021, 10, 1–4. [Google Scholar] [PubMed]

| Gene | Genetic Modification | Drug | Result | Reference |
|---|---|---|---|---|
| Girk1 | KO | Cocaine | ⬆ Motor activity | Arora et al., 2010 [27] |
| Morphine | ⬇ Antinociceptive effects | Marker et al., 2004 [28] | ||
| Morphine | ⬆ Motor activity | Kozell et al., 2009 [7] | ||
| Morphine | ⬆ Motor activity | Kotecki et al., 2015 [29] | ||
| Girk2 | KO | Cocaine | ⬇ Self-administration | Morgan et al., 2003 [30] |
| Cocaine | ⬆ Motor activity | Arora et al., 2010 [27] | ||
| Alcohol | ⬇ Conditioned place preference/Conditioned taste aversion | Hill et al., 2003 [31] | ||
| Morphine | ⬆ Motor activity | Kotecki et al., 2015 [29] | ||
| Morphine | ⬇ Antinociceptive effects | Marker et al., 2004 [28] | ||
| Missense mutation | Alcohol | ⬇ Antinociceptive effects | Kobayashi et al., 1999 [24] | |
| Opioids | ⬇ Antinociceptive effects | Ikeda et al., 2000 [32] | ||
| Amphetamine | ⬇ Motor activity | Schmidt et al., 1982 [33] | ||
| Methamphetamine | ⬇ Conditioned place preference | Ikekubo et al., 2020 [34] | ||
| KO in DA neurons | Cocaine | ⬆ Behavioral sensitivity | McCall et al., 2017 [35] | |
| Overexpression in DA neurons | Cocaine | ⬇ Motor activity | McCall et al., 2019 [36] | |
| Girk3 | KO | Cocaine | ⬇ Self-administration | Morgan et al., 2003 [30] |
| Morphine | ⬇ Motor activity | Kotecki et al., 2015 [29] | ||
| Alcohol | ⬇ Withdrawal | Kozell et al., 2009 [7] | ||
| Alcohol | ⬆ Conditioned place preference | Tipps et al., 2016 [37] | ||
| Alcohol | ⬆ Binge-like drinking | Herman et al., 2015 [38] | ||
| Overexpression in DA neurons | Cocaine | ⬆ Motor activity | McCall et al., 2019 [36] |
| Treatment | Study Design | Drug | Result | Reference |
|---|---|---|---|---|
| Ifenprodil | Case report * | Alcohol | ⬇ Pain in the extremities and headache ⬇ Tremors in the fingers | Goto, 2010 [61] |
| Ifenprodil, Paroxetine, and Haloperidol | Retrospective chart review ** | Alcohol | ⬇ The lack of negative expectancy for drinking on the SRRS | Ogai et al., 2011 [62] |
| Ifenprodil, Paroxetine, and Sertraline | Retrospective chart review ** | Alcohol | ⬇ The positive expectancy for alcohol on the SRRS | Sugaya et al., 2012 [63] |
| Ifenprodil | Randomized, controlled, rater-blinded study | Alcohol | ⬇ Alcohol use scores | Sugaya et al., 2018 [64] |
| Ifenprodil | Randomized, double-blind, exploratory, dose-ranging, placebo-controlled study | Methamphetamine | ⬇ The days of methamphetamine use during the follow-up period | Kotajima-Murakami et al., 2022 [65] |
| Ifenprodil | Case report * | Alcohol Bron | ⬇ Craving ⬇ Craving | Hori et al., 2010 [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotajima-Murakami, H.; Ide, S.; Ikeda, K. GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders. Biomedicines 2022, 10, 2552. https://doi.org/10.3390/biomedicines10102552
Kotajima-Murakami H, Ide S, Ikeda K. GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders. Biomedicines. 2022; 10(10):2552. https://doi.org/10.3390/biomedicines10102552
Chicago/Turabian StyleKotajima-Murakami, Hiroko, Soichiro Ide, and Kazutaka Ikeda. 2022. "GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders" Biomedicines 10, no. 10: 2552. https://doi.org/10.3390/biomedicines10102552
APA StyleKotajima-Murakami, H., Ide, S., & Ikeda, K. (2022). GIRK Channels as Candidate Targets for the Treatment of Substance Use Disorders. Biomedicines, 10(10), 2552. https://doi.org/10.3390/biomedicines10102552





