Effect of MNCQQ Cells on Migration of Human Dermal Fibroblast in Diabetic Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fibroblast Culture
2.2. QQ Culture of PbMNCs
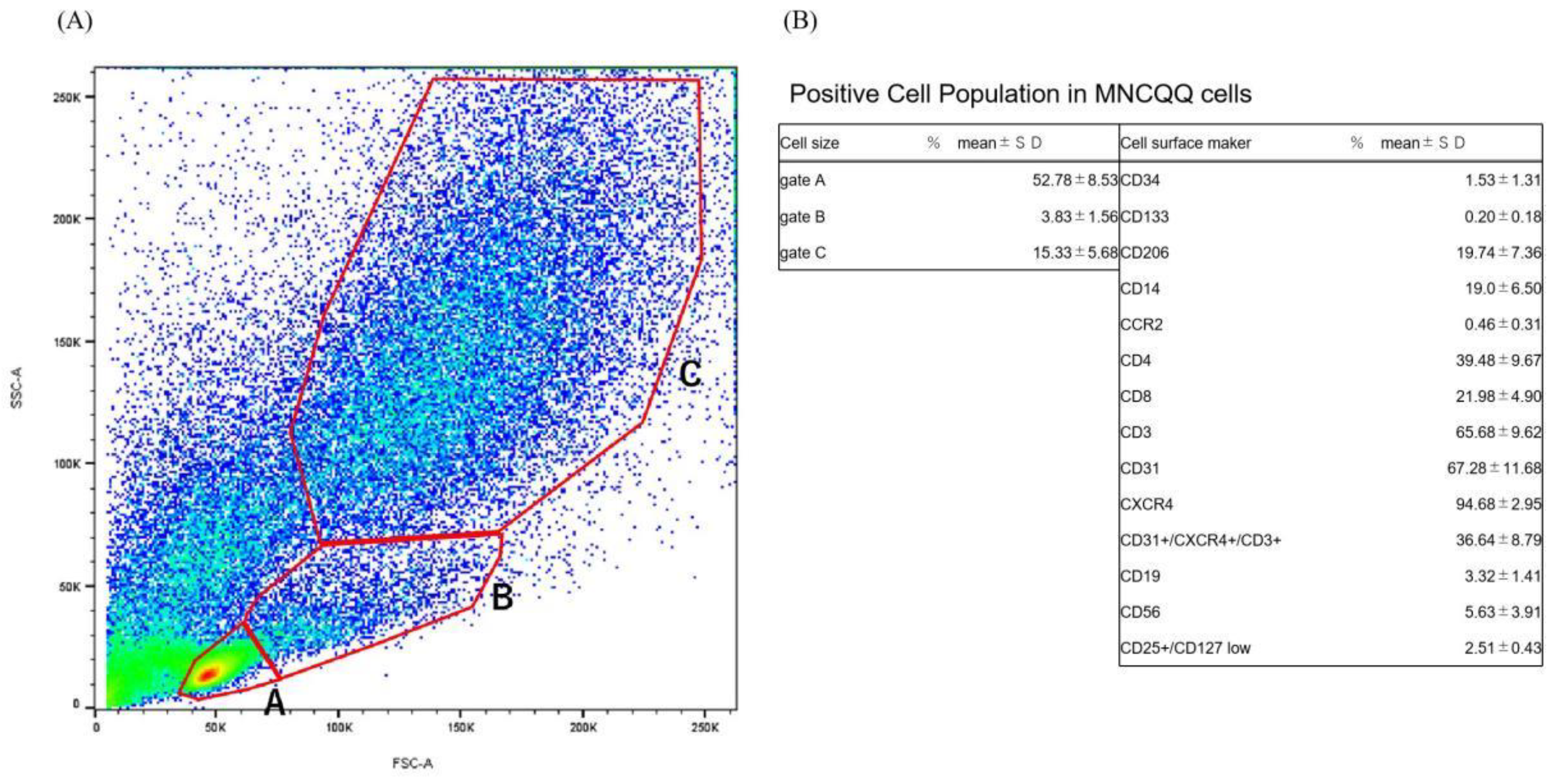
2.3. Fluorescence Activated Cell Sorting
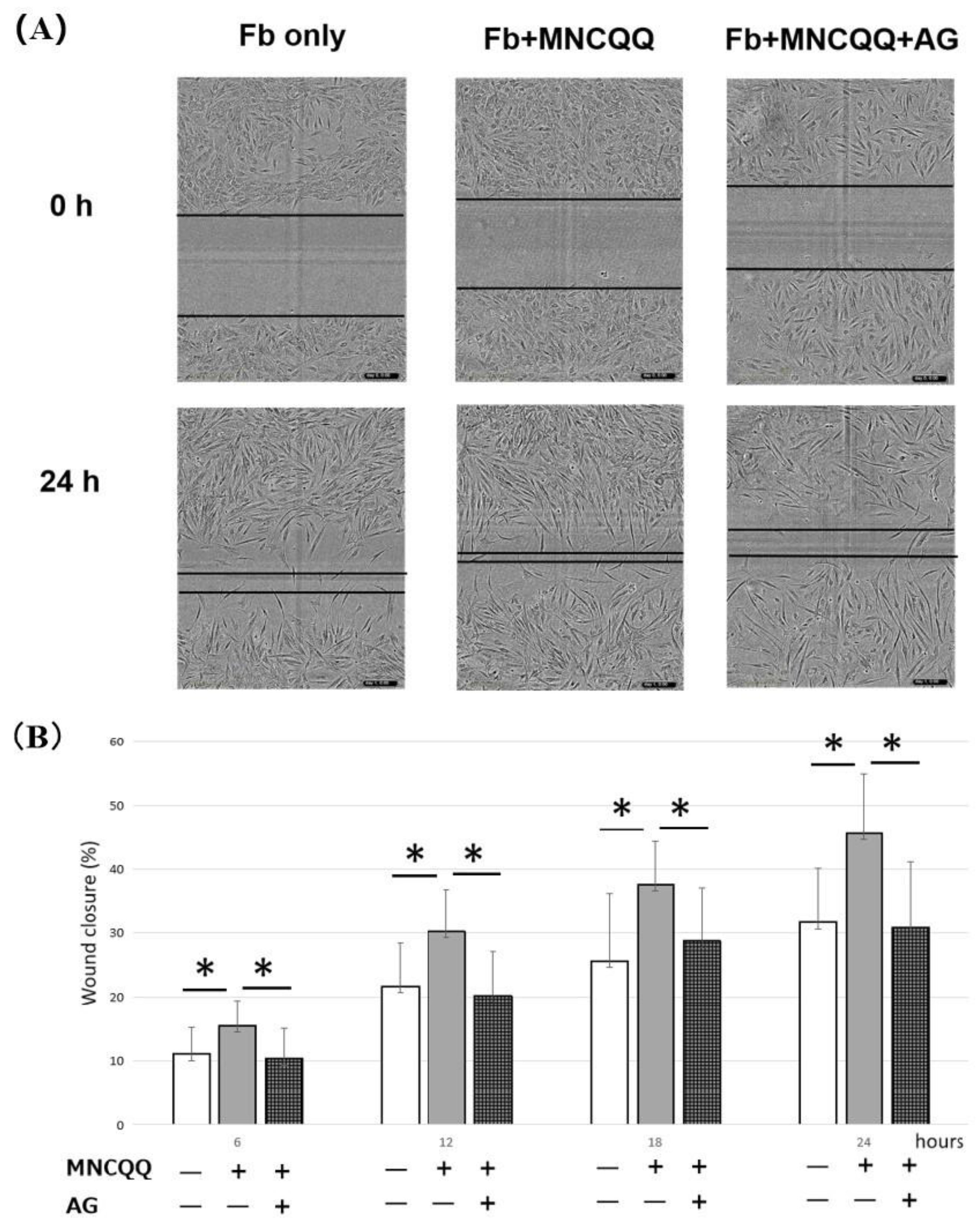
2.4. Wound-Healing Assay
2.5. qRT-PCR
2.6. Statistical Analysis
3. Results
3.1. Positive Cell Population in MNCQQ Cells
3.2. MNCQQ Promoted the Migration under Both NG and HG Conditions
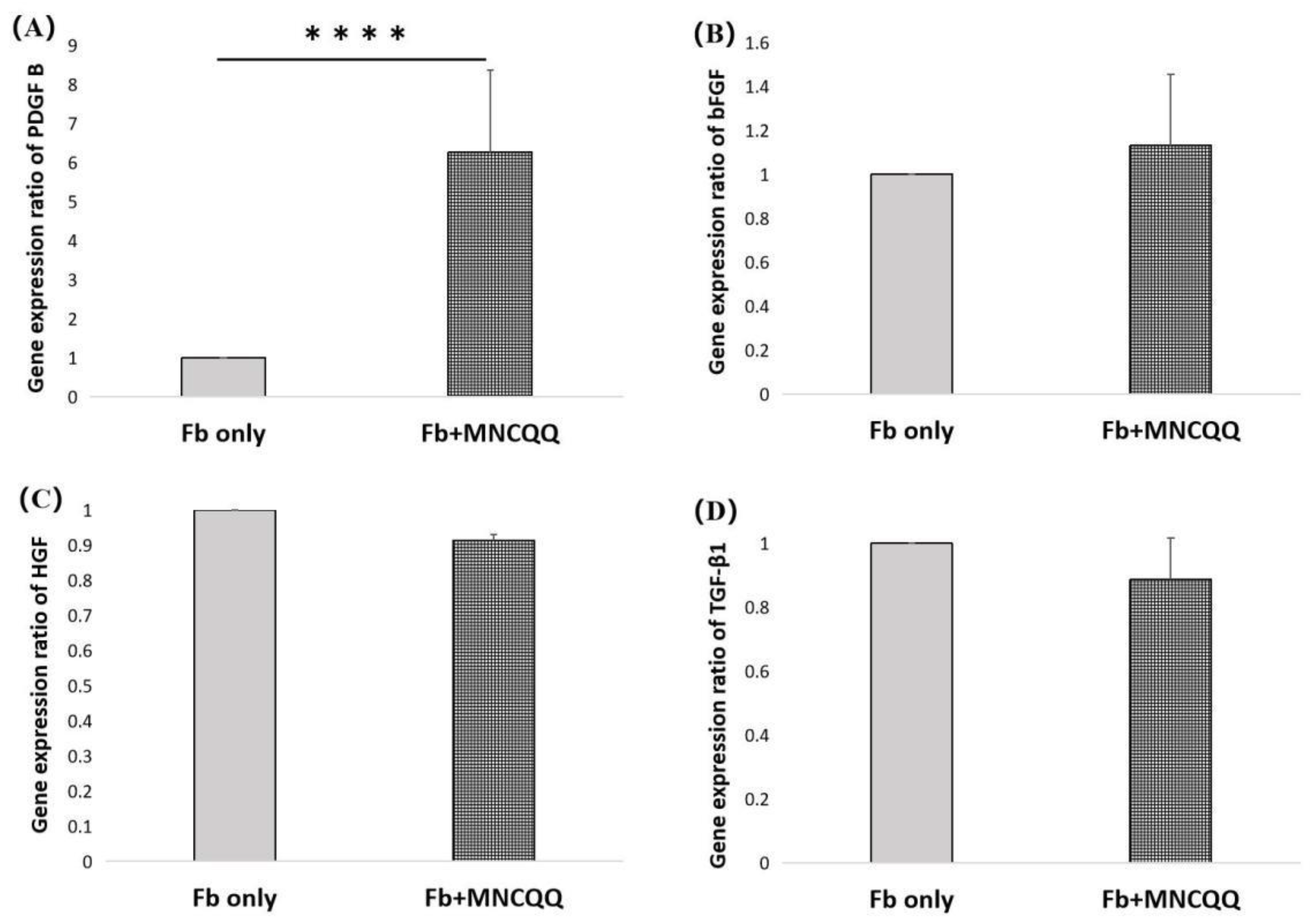
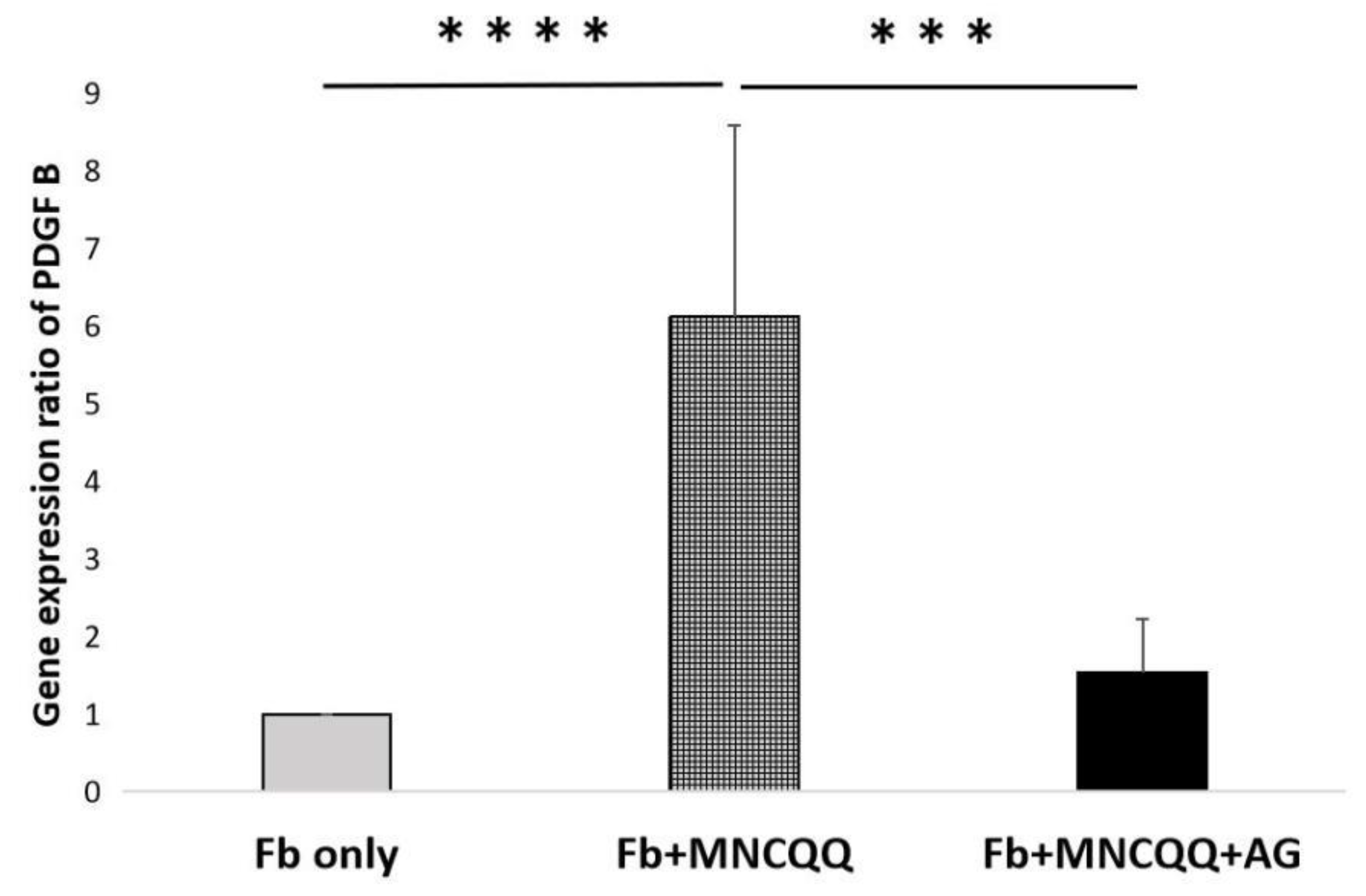
3.3. Platelet-Derived Growth Factor (PDGF) B in Fibroblasts Were Upregulated after Coculture with MNCQQ Cells
3.4. The Promoted Migration and PDGF B Expression of Fibroblasts Induced by MNCQQ Cells Was Impressed by PDGFR Inhibitor (AG)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Liu, J.; Sun, H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A meta-analysis. PLoS ONE 2020, 15, e0239236. [Google Scholar] [CrossRef]
- Pemayun, T.G.D.; Naibaho, R.M.; Novitasari, D.; Amin, N.; Minuljo, T.T. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A hospital-based case–control study. Diabet. Foot Ankle 2015, 6, 29629. [Google Scholar] [CrossRef]
- Izumi, Y.; Satterfield, K.; Lee, S.; Harkless, L.B.; Lavery, L.A. Mortality of first-time amputees in diabetics: A 10-year observation. Diabetes Res. Clin. Pr. 2009, 83, 126–131. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef]
- Shi, Q.; Rafii, S.; Wu, M.H.-D.; Wijelath, E.S.; Yu, C.; Ishida, A.; Fujita, Y.; Kothari, S.; Mohle, R.; Sauvage, L.R. Evidence for circulating bone marrow-derived endothelial cells. Blood J. Am. Soc. Hematol. 1998, 92, 362–367. [Google Scholar]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Losordo, D.W.; Schatz, R.A.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; Chaudhry, M. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115, 3165–3172. [Google Scholar] [CrossRef] [Green Version]
- Moriya, J.; Minamino, T.; Tateno, K.; Shimizu, N.; Kuwabara, Y.; Sato, Y.; Saito, Y.; Komuro, I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ. Cardiovasc. Interv. 2009, 2, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, R.; Masuda, H.; Kato, S.; Imagawa, K.; Kanabuchi, K.; Nakashioya, C.; Yoshiba, F.; Fukui, T.; Ito, R.; Kobori, M. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant. 2014, 23, 167–179. [Google Scholar] [CrossRef]
- Tanaka, R.; Wada, M.; Kwon, S.M.; Masuda, H.; Carr, J.; Ito, R.; Miyasaka, M.; Warren, S.M.; Asahara, T.; Tepper, O.M. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast. Reconstr. Surg. 2008, 121, 1929–1942. [Google Scholar] [CrossRef]
- Tepper, O.M.; Carr, J.; Allen Jr, R.J.; Chang, C.C.; Lin, C.D.; Tanaka, R.; Gupta, S.M.; Levine, J.P.; Saadeh, P.B.; Warren, S.M. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1α mediated bone marrow mobilization impair diabetic tissue repair. Diabetes 2010, 59, 1974–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Tanaka, R.; Fujimura, S.; Ishikawa, M.; Akimaru, H.; Shizuno, T.; Sato, A.; Okada, Y.; Iida, Y.; Itoh, J. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J. Am. Heart Assoc. 2014, 3, e000743. [Google Scholar] [CrossRef] [Green Version]
- Kado, M.; Tanaka, R.; Arita, K.; Okada, K.; Ito-Hirano, R.; Fujimura, S.; Mizuno, H. Human peripheral blood mononuclear cells enriched in endothelial progenitor cells via quality and quantity controlled culture accelerate vascularization and wound healing in a porcine wound model. Cell Transplant. 2018, 27, 1068–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, R.; Ito-Hirano, R.; Fujimura, S.; Arita, K.; Hagiwara, H.; Mita, T.; Itoh, M.; Kawaji, H.; Ogawa, T.; Watada, H. Ex vivo conditioning of peripheral blood mononuclear cells of diabetic patients promotes vasculogenic wound healing. Stem Cells Transl. Med. 2021, 10, 895–909. [Google Scholar] [CrossRef]
- Tanaka, R.; Fujimura, S.; Kado, M.; Fukuta, T.; Arita, K.; Hirano-Ito, R.; Mita, T.; Watada, H.; Kato, Y.; Miyauchi, K. Phase I/IIa Feasibility Trial of Autologous Quality-and Quantity-Cultured Peripheral Blood Mononuclear Cell Therapy for Non-Healing Extremity Ulcers. Stem Cells Transl. Med. 2022, 11, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.N.-Y.; Kado, M.; Hagiwara, H.; Fujimura, S.; Mizuno, H.; Tanaka, R. MMP9 secreted from mononuclear cell quality and quantity culture mediates STAT3 phosphorylation and fibroblast migration in wounds. Regen. Ther. 2021, 18, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.H.; Huang, B.B.; Tian, H.S.; Chi, L.S.; Duan, Y.M.; Wang, X.; Zhu, Z.X.; Cai, W.H.; Zhu, Y.T.; Wei, T.M. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS ONE 2014, 9, e108182. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Wehrmann, M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. J. Cell. Mol. Med. 2007, 11, 1342–1351. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, S.; Fujiwara, T.; Matsuzaki, S.; Shingaki, K.; Taniguchi, M.; Miyata, S.; Tohyama, M.; Sakai, Y.; Yano, K.; Hosokawa, K. bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes fibroblast migration in wound healing. PLoS ONE 2010, 5, e12228. [Google Scholar] [CrossRef] [Green Version]
- Lamers, M.L.; Almeida, M.E.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High glucose-mediated oxidative stress impairs cell migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef]
- Lerman, O.Z.; Galiano, R.D.; Armour, M.; Levine, J.P.; Gurtner, G.C. Cellular dysfunction in the diabetic fibroblast: Impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am. J. Pathol. 2003, 162, 303–312. [Google Scholar] [CrossRef]
- Hur, J.; Yang, H.-M.; Yoon, C.-H.; Lee, C.-S.; Park, K.-W.; Kim, J.-H.; Kim, T.-Y.; Kim, J.-Y.; Kang, H.-J.; Chae, I.-H. Identification of a novel role of T cells in postnatal vasculogenesis: Characterization of endothelial progenitor cell colonies. Circulation 2007, 116, 1671–1682. [Google Scholar] [CrossRef] [Green Version]
- Hata, S.; Okamura, K.; Hatta, M.; Ishikawa, H.; Yamazaki, J. Proteolytic and non-proteolytic activation of keratinocyte-derived latent TGF-β1 induces fibroblast differentiation in a wound-healing model using rat skin. J. Pharmacol. Sci. 2014, 124, 230–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Cheng, Y.; Ye, J.; Cai, P.; Zhang, J.; Li, R.; Yang, Y.; Wang, Z.; Zhang, H.; Lin, C. bFGF promotes the migration of human dermal fibroblasts under diabetic conditions through reactive oxygen species production via the PI3K/Akt-Rac1-JNK pathways. Int. J. Biol. Sci. 2015, 11, 845. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Pi, L.-Q.; Hwang, S.T.; Lee, W.-S. Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann. Dermatol. 2012, 24, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Calderon, T.M.; Sherman, J.; Wilkerson, H.; Hatcher, V.B.; Berman, J.W. Interleukin 6 modulates c-sis gene expression in cultured human endothelial cells. Cell. Immunol. 1992, 143, 118–126. [Google Scholar] [CrossRef]
- Kavanaugh, W.; Harsh, G., 4th; Starksen, N.F.; Rocco, C.; Williams, L. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J. Biol. Chem. 1988, 263, 8470–8472. [Google Scholar] [CrossRef]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F.; Ross, R. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet. Med. 2006, 23, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.-H.; Eriksson, U.; Östman, A. New members of the platelet-derived growth factor family of mitogens. Arch. Biochem. Biophys. 2002, 398, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Pietras, K. PDGF family function and prognostic value in tumor biology. Biochem. Biophys. Res. Commun. 2018, 503, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; You, J.; Gong, D.; Xu, Y.; Yang, B.; Jiang, C. PDGF-BB induces conversion, proliferation, migration, and collagen synthesis of oral mucosal fibroblasts through PDGFR-β/PI3K/AKT signaling pathway. Cancer Biomark. 2021, 30, 407–415. [Google Scholar] [CrossRef]
- Yamada, K.; Hamashima, T.; Ishii, Y.; Yamamoto, S.; Okuno, N.; Yoshida, N.; Yamada, M.; Huang, T.T.; Shioda, N.; Tomihara, K. Different PDGF receptor dimers drive distinct migration modes of the mouse skin fibroblast. Cell. Physiol. Biochem. 2018, 51, 1461–1479. [Google Scholar] [CrossRef]
- Robson, M.C.; Phillips, L.G.; Robson, L.; Thomason, A.; Pierce, G. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 1992, 339, 23–25. [Google Scholar] [CrossRef]
- Pierce, G.; Tarpley, J.; Tseng, J.; Bready, J.; Chang, D.; Kenney, W.; Rudolph, R.; Robson, M.; Berg, J.V.; Reid, P. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J. Clin. Investig. 1995, 96, 1336–1350. [Google Scholar] [CrossRef] [Green Version]
- Blažević, T.; Schwaiberger, A.V.; Schreiner, C.E.; Schachner, D.; Schaible, A.M.; Grojer, C.S.; Atanasov, A.G.; Werz, O.; Dirsch, V.M.; Heiss, E.H. 12/15-lipoxygenase contributes to platelet-derived growth factor-induced activation of signal transducer and activator of transcription 3. J. Biol. Chem. 2013, 288, 35592–35603. [Google Scholar] [CrossRef]

| Company, Catalog No. | Final Concentration | |
|---|---|---|
| StemlineⅡTM Hematopoietic Sten Cell Expansion Medium | Sigma-Aldrich, #S0192 | |
| rh SCF | Peprotec #300-07 | 100 ng/mL |
| rh Flt-3 ligand | Peprotec #300-19 | 100 ng/mL |
| rh TPO | Peprotec #300-18 | 20 ng/mL |
| rh VEGF | Peprotec #100-20 | 50 ng/mL |
| rh IL-6 | Peprotec #200-06 | 20 ng/mL |
| Antibody | Catalog No. | Company |
|---|---|---|
| PE/Cy 7-labeled anti-CD 31 | clone: WM59 | Biolegend, San Diego, California |
| PE-labeled anti-CD 34 | clone: 581 | Biolegend |
| APC-labeled anti-CD 184 (CXCR 4) | clone: 12G5 | Biolegend |
| APC-labeled anti-CD 133 | clone: AC133 | Miltenyi, Bergisch Gladbach, North Rhine-Westphalia, Germany |
| Alexa Fluor-700-labeled anti-CD 3+ | clone: UCHT1 | Biolegend |
| APC-Cy 7-labeled anti-CD 14 | clone: HCD14 | Biolegend |
| PE/Cy 7-labeled anti-CD 206 (MMR) | clone: 15-2 | Biolegend |
| PerCP/Cy 5.5-labeled anti-CCR 2 (CD 192) | clone: K036C2 | Biolegend |
| BV421-labeled anti-CD 127 | clone: A019D5 | Biolegend |
| BV421-labeled anti-CD 8 | clone: RPA-T8 | Biolegend |
| FITC-labeled anti-CD 4 | clone: RPA-T4 | Biolegend |
| PE-labeled anti-CD 25 | clone: M-A251 | Biolegend |
| Markers | Forward Sequences | Revers Sequences |
|---|---|---|
| GAPDH | 5′-GGCCTCCAAGGAGTAAGACC-3′ | 5′-GACTGAGTGTGGCAGGGACT-3′ |
| PDGF-B | 5′-TGAGAAAGATCGAGATTGTGCG-3′ | 5′-GGGCTTCGGGTCACAGG-3′ |
| bFGF | 5′-ACCTCACATCAAGCTAC-3′ | 5′-GTTTCAGTGCCACATACC-3′ |
| HGF | 5′-ATGCATCCAAGGTCAAGGAG-3′ | 5′-TTCCATGTTCTTGTCCCACA-3′ |
| TGF-β1 | 5′-CCCAGCATCTGCAAAGCTC-3′ | 5′-GTCAATGTACAGCTGCCCGCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Ito-Hirano, R.; Shen, T.N.-Y.; Fujimura, S.; Mizuno, H.; Tanaka, R. Effect of MNCQQ Cells on Migration of Human Dermal Fibroblast in Diabetic Condition. Biomedicines 2022, 10, 2544. https://doi.org/10.3390/biomedicines10102544
Jiang S, Ito-Hirano R, Shen TN-Y, Fujimura S, Mizuno H, Tanaka R. Effect of MNCQQ Cells on Migration of Human Dermal Fibroblast in Diabetic Condition. Biomedicines. 2022; 10(10):2544. https://doi.org/10.3390/biomedicines10102544
Chicago/Turabian StyleJiang, Sen, Rie Ito-Hirano, Tsubame Nishikai-Yan Shen, Satoshi Fujimura, Hiroshi Mizuno, and Rica Tanaka. 2022. "Effect of MNCQQ Cells on Migration of Human Dermal Fibroblast in Diabetic Condition" Biomedicines 10, no. 10: 2544. https://doi.org/10.3390/biomedicines10102544
APA StyleJiang, S., Ito-Hirano, R., Shen, T. N.-Y., Fujimura, S., Mizuno, H., & Tanaka, R. (2022). Effect of MNCQQ Cells on Migration of Human Dermal Fibroblast in Diabetic Condition. Biomedicines, 10(10), 2544. https://doi.org/10.3390/biomedicines10102544






