Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers
Abstract
1. Introduction
- Elderly 65 years of age or older: people aged 65 and over should receive a booster injection. The risk of severe COVID-19 disease increases with age.
- Long-term care facility residents aged 18 and over: Long-term care facility residents live closely together in group settings.
- People with comorbidities between the ages of 18 and 64.
- People who work or live in high-risk environments between the ages of 18 and 64.
2. Materials and Methods
2.1. General Characteristics
- -
- dental area: dental physicians, chair assistants, hygienists, and nurses: a total of 90 were evaluated. (39.13% of 230 total)
- -
- radiological area: radiology physicians, technicians, and nurses: a total of 72 were evaluated (31.30% of a total of 230)
- -
- internal medicine area: a total of 34 were evaluated (14.78% of a total of 230);
- -
- Forensic Medicine area: a total of 34 (14.78% of 230 total) were evaluated.
- Group I: subjects between 20–30 years old;
- Group II: subjects between 30–40 years old;
- Group III: subjects between 40–50 years old;
- Group IV: subjects between 50–60 years old;
- Group V: subjects between 60–70 years old;
2.2. Statistical Analysis
3. Results
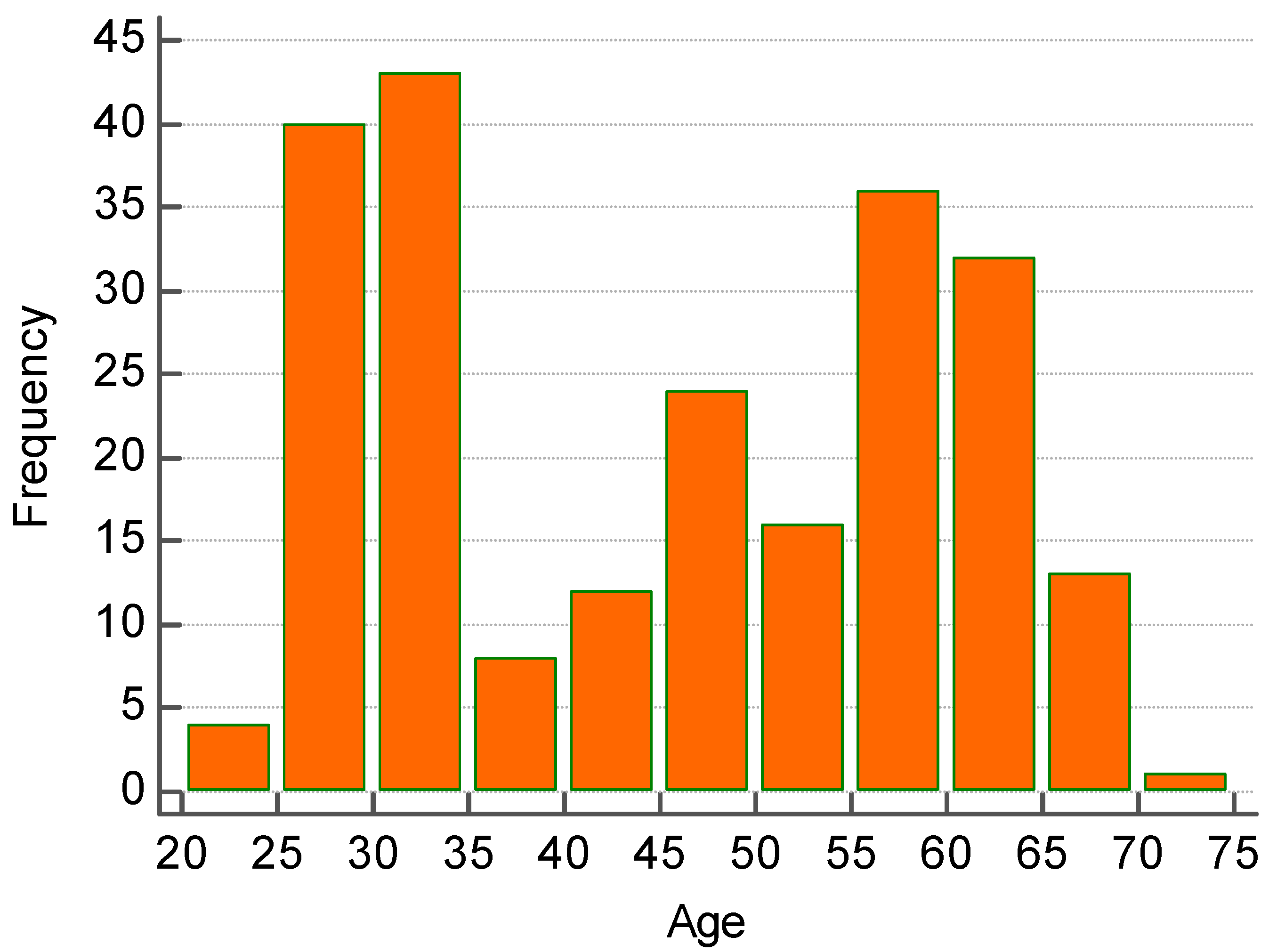
- Between the age of 20 and 30 years old there were 45 subjects (19.57% of 230 enrolled).
- Between the age of 30 and 40 years old there were 52 subjects (22.61% of 230 enrolled)
- Between the age of 40 and 50 years old there were 34 subjects (14.78% of 230 enrolled).
- Between the age 50 and 60 years old there were 53 subjects (23.04% of 230 enrolled)
- Between the age of 60 and 70 years of age there were 46 subjects (20% of 230 enrolled).
3.1. Statistical Findings
3.1.1. Age-Related Findings
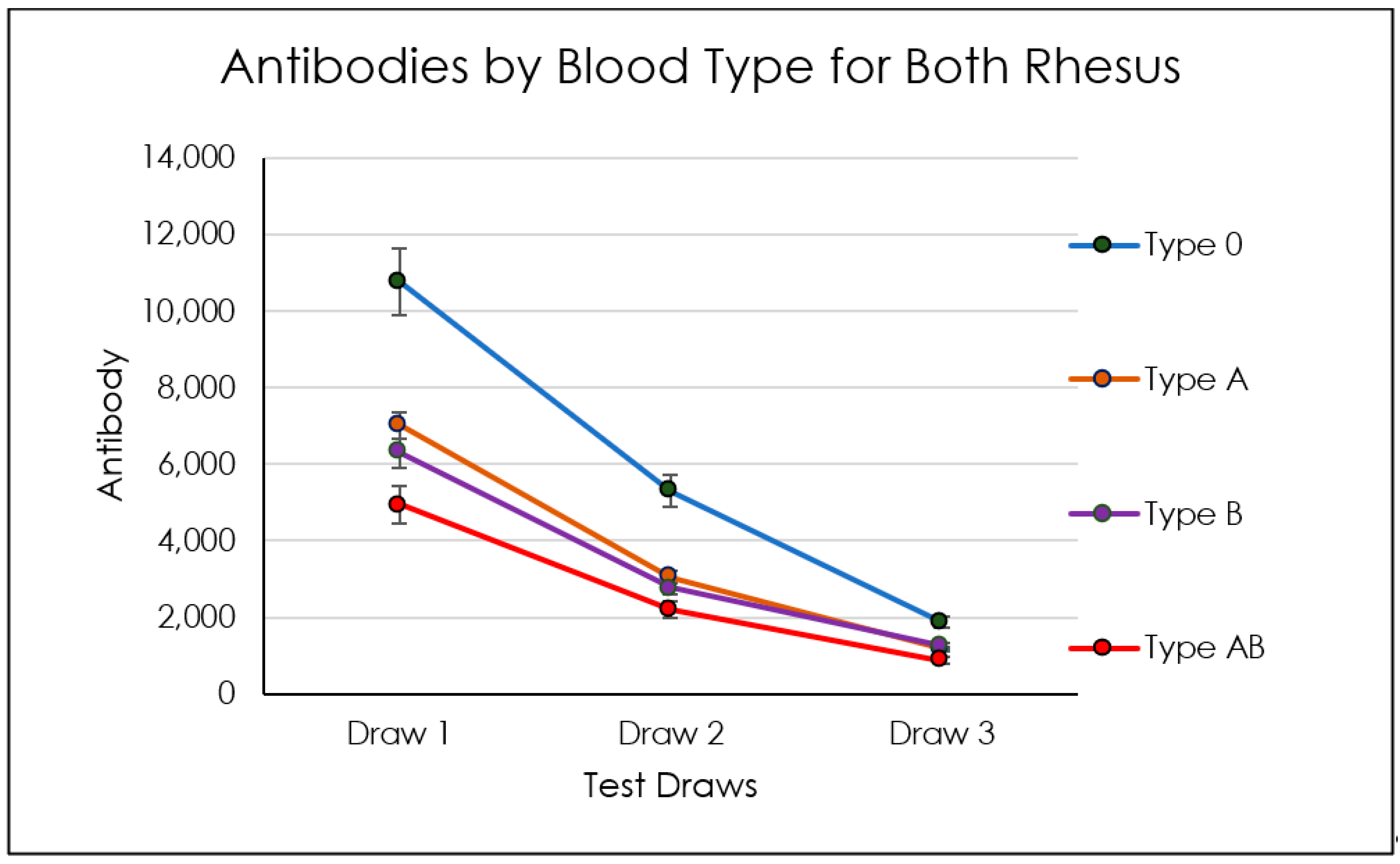
3.1.2. Blood-Type-Related Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme-2 |
| ACE | angiotensin-converting enzyme |
| ACE1 | angiotensin-converting enzyme 1 |
| AIFA | Agenzia Italiana del Farmaco |
| Alfa | English variant B.1.1.7 |
| anti-RBD IgG | Immunogloublin G anti receptor-binding domain |
| Antispike | Test IgG Antispike |
| BAU | unità arbitrarie vincolanti |
| Beta variant | (former of South Africa) |
| BMI | Body mass index |
| CI | Interval of confidence |
| CLIAs | chemiluminescence immunoassay |
| CRP | C-reactive protein |
| Delta | Indian variant B.1.617.2 |
| ELISA | enzyme-linked immunosorbent assay |
| EMA | European Medicines Agency |
| ETA | variant B.1.525; Date of designation March 2021 |
| Gamma | Brasilian variant P.1 |
| hACE2 receptor | human angiotensin I-converting enzyme 2 receptor |
| IFN | Interferon |
| IgA | Immunoglobulins A |
| IgG | Immunoglobulins G |
| IgM | Immunoglobulins M |
| IOTA | variant B.1.526; earliest documented samples USA (November 2020), Date of designation March 2021 |
| IQR | Interquartile range |
| KAPPA | Indian variant B.1.617.1 |
| LAMBA | variant C.37; earliest documented samples Peru (August 2020), Date of designation June 2021 |
| LFIAs | lateral flow immunoassays. |
| MERS | Middle East Respiratory Syndrome |
| MMF | mycophenolate mofetil |
| MPA | mycophenolic acid |
| MPPDH | inosine-5’-monophosphate dehydrogenase |
| NAAT | nucleic acid amplification test |
| NGS | Next Generation Sequencing |
| bNAbs | Broadly neutralizing antibodies |
| N-IgG | Anti-N-IgG |
| PRD | Viral Prion-like domain |
| RBD | receptor-binding domain |
| RBDs | receptor-binding domains |
| RDB-IgG | receptor-binding domain neutralizing antibodies |
| RT-PCR | real-time PCR Polymerase chain reaction |
| S | the Spike glycoprotein |
| SARS-CoV-1 | Severe Acute Respiratory Syndrome Coronavirus 1 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19) |
| SARSr-CoV Rp3 | salivar protein similar to fused 8a and 8b SARS-CoV Beta Coronavirus |
| S-IgG | Antispike IgG |
| thio-NAD | thionicotinamide-adenine dinucleotide |
| TNF | Tumor Necrosis Factor |
| VIPIT | prothrombotic immune thrombocytopenia |
| VOC | Variants of Concern |
| VOI | Variants of Interest |
| ZETA | variant P.2 |
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of Airborne Viral Emission: Quanta Emission Rate of SARS-CoV-2 for Infection Risk Assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef] [PubMed]
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Environmental Disinfection of a Dental Clinic during the COVID-19 Pandemic: A Narrative Insight. BioMed Res. Int. 2020, 2020, 8896812. [Google Scholar] [CrossRef] [PubMed]
- Isha, S.N.; Ahmad, A.; Kabir, R.; Apu, E.H. Dental Clinic Architecture Prevents COVID-19-like Infectious Diseases. HERD Health Environ. Res. Des. J. 2020, 13, 240–241. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Sun, H.; Liu, L. Exhaled Droplets Due to Talking and Coughing. J. R. Soc. Interface 2009, 6 (Suppl. 6), S703–S714. [Google Scholar] [CrossRef] [PubMed]
- Somsen, G.A.; van Rijn, C.; Kooij, S.; Bem, R.A.; Bonn, D. Small Droplet Aerosols in Poorly Ventilated Spaces and SARS-CoV-2 Transmission. Lancet Respir. Med. 2020, 8, 658–659. [Google Scholar] [CrossRef]
- Karahan, S.; Katkat, F. Impact of Serum 25(OH) Vitamin D Level on Mortality in Patients with COVID-19 in Turkey. J. Nutr. Health Aging 2021, 25, 189–196. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum—Preliminary Report. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal Profiles of Viral Load in Posterior Oropharyngeal Saliva Samples and Serum Antibody Responses during Infection by SARS-CoV-2: An Observational Cohort Study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Balzanelli, G.M.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; Nguyen, K.C.D.; et al. COVID-19 and COVID-like Patients: A Brief Analysis and Findings of Two Deceased Cases. Open Access Maced. J. Med. Sci. 2020, 8, 490–495. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Prion-Like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms 2022, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions—United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and MRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults—United States, March–July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; Li, Q.; Bagchi, S.; Dubendris, H.; Link-Gelles, R.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, 1 March–1 August 2021. MMWR Morb Mortal Wkly. Rep. 2021, 70, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. The Impact of SARS-CoV-2 Vaccination on Alpha & Delta Variant Transmission. medRxiv 2021. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- William, P.E. Fundamental Immunology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Humphrey, J. Immunology for Students of Medicine, 3rd ed.; Humphrey, J. Blackwell Scientific: Oxford, UK, 1970; pp. 65–100. [Google Scholar]
- Webster, A.D. Primary Immunodeficiency. Hum. Exp. Toxicol. 1995, 14, 99–100. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Cefalo, A.; Catucci, O.; Aityan, S.K.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Maggiore, M.E.; et al. The Vitamin D, IL-6 and the EGFR Markers a Possible Way to Elucidate the Lung–Heart–Kidney Cross-Talk in COVID-19 Disease: A Foregone Conclusion. Microorganisms 2021, 9, 1903. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.Q.; Nguyen, L.D.N.; Pham, S.T.; Nguyen, T.; Pham, P.T.T.; Nguyen, S.T.H.; Pham, D.T.; Pham, H.T.; Tran, D.K.; Le, S.H.; et al. The Distribution of Dengue Virus Serotype in Quang Nam Province (Vietnam) during the Outbreak in 2018. Int. J. Environ. Res. Public Health 2022, 19, 1285. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Bevilacqua, L.; Dotto, F.; Costantinides, F.; Lorusso, F.; Scarano, A. Observational Study on the Preparation of the Implant Site with Piezosurgery vs. Drill: Comparison between the Two Methods in Terms of Postoperative Pain, Surgical Times, and Operational Advantages. BioMed Res. Int. 2019, 2019, 8483658. [Google Scholar] [CrossRef] [PubMed]
- Interim Statement on Booster Doses for COVID-19 Vaccination. Available online: https://www.who.int/news/item/04-10-2021-interim-statement-on-booster-doses-for-covid-19-vaccination (accessed on 6 November 2021).
- CDC. CDC COVID-19 Booster Shot. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed on 6 November 2021).
- Montenegro, V.; Inchingolo, A.D.; Malcangi, G.; Limongelli, L.; Marinelli, G.; Coloccia, G.; Laudadio, C.; Patano, A.; Inchingolo, F.; Bordea, I.R.; et al. Compliance of Children with Removable Functional Appliance with Microchip Integrated during COVID-19 Pandemic: A Systematic Review. J. Biol. Regul. Homeost. Agents 2021, 35, 365–377. [Google Scholar] [PubMed]
- Booster Shots and Third Doses for COVID-19 Vaccines: What You Need to Know. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/booster-shots-and-third-doses-for-covid19-vaccines-what-you-need-to-know (accessed on 6 November 2021).
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Santacroce, L.; Marinelli, G.; Mancini, A.; Vimercati, L.; Maggiore, M.E.; D’Oria, M.T.; Hazballa, D.; et al. COVID-19 Infection in Children, Infants and Pregnant Subjects: An Overview of Recent Insights and Therapies. Microorganisms 2021, 9, 1964. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential Beneficial Role of Probiotics on the Outcome of COVID-19 Patients: An Evolving Perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 295–301. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. BNT162b2 Vaccine Booster Dose Protection: A Nationwide Study from Israel. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Shapiro Ben David, S.; Shamir-Stein, N.; Baruch Gez, S.; Lerner, U.; Rahamim-Cohen, D.; Ekka Zohar, A. Reactogenicity of a Third BNT162b2 MRNA COVID-19 Vaccine among Immunocompromised Individuals and Seniors—A Nationwide Survey. Clin. Immunol. 2021, 232, 108860. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.-F.; Hanafi, L.; Faucon, A.-L.; Housset, P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2021, 79, 185–192.e1. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Catucci, O.; Amatulli, F.; Cefalo, A.; Lazzaro, R.; Palazzo, D.; Aityan, S.K.; et al. Immunity Profiling of COVID-19 Infection, Dynamic Variations of Lymphocyte Subsets, a Comparative Analysis on Four Different Groups. Microorganisms 2021, 9, 2036. [Google Scholar] [CrossRef]
- Vomero, M.; Barbati, C.; Colasanti, T.; Celia, A.I.; Speziali, M.; Ucci, F.M.; Ciancarella, C.; Conti, F.; Alessandri, C. Autophagy Modulation in Lymphocytes From COVID-19 Patients: New Therapeutic Target in SARS-CoV-2 Infection. Front. Pharmacol. 2020, 11, 569849. [Google Scholar] [CrossRef] [PubMed]
- Bordea, I.R.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.D.; Malcangi, G.; Pham, V.H.; Inchingolo, A.M.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Bordea, I.R.; Ballini, A.; Lorusso, F.; Hazballa, D.; Isacco, C.G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Inchingolo, F.; et al. Environmental Issues and Neurological Manifestations Associated with COVID-19 Pandemic: New Aspects of the Disease? Int J. Environ. Res. Public Health 2020, 17, 8049. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 Infection Induces Long-Lived Bone Marrow Plasma Cells in Humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Algaissi, A.; Alfaleh, M.A.; Hala, S.; Abujamel, T.S.; Alamri, S.S.; Almahboub, S.A.; Alluhaybi, K.A.; Hobani, H.I.; Alsulaiman, R.M.; AlHarbi, R.H.; et al. SARS-CoV-2 S1 and N-Based Serological Assays Reveal Rapid Seroconversion and Induction of Specific Antibody Response in COVID-19 Patients. Sci Rep. 2020, 10, 16561. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 1–5. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Microorganisms 2021, 9, 525. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; D’oria, M.T.; Isacco, C.G.; Bordea, I.R.; Candrea, S.; Scarano, A.; et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Qorri, E.; Converti, I.; Lorusso, F.; Delvecchio, M.; Gnoni, A.; Scacco, S.; Scarano, A. Inflammatory Status and Glycemic Control Level of Patients with Type 2 Diabetes and Periodontitis: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2021, 18, 3018. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Sportelli, P.; Magazzino, D.; Ripa, C.; Cantatore, F.; Cagnetta, G.; De Rinaldis, C.; Montemurro, N.; De Giacomo, A. Evaluation of prosthetic quality and masticatory efficiency in patients with total removable prosthesis study of 12 cases. ORAL Implantol. 2018, 11, 230–240. [Google Scholar]
- Grassi, F.R.; Grassi, R.; Rapone, B.; Alemanno, G.; Balena, A.; Kalemaj, Z.; Gianfranco, A. Dimensional Changes of Buccal Bone Plate in Immediate Implants Inserted through Open Flap, Open Flap and Bone Grafting and Flapless Techniques: A Cone-Beam Computed Tomography Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2019, 30, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, E.; Moscufo, L.; Corsalini, M.; Coscia, D.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Rapone, B.; Carossa, M.; Carossa, S. Polyamide vs Silk Sutures in the Healing of Postextraction Sockets: A Split Mouth Study. Oral Implantol. 2018, 11, 115–120. [Google Scholar]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Cesarano, F.; Arazzi, M.; Liberato, L.D.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Periodontal Microbiological Status Influences the Occurrence of Cyclosporine-A and Tacrolimus-Induced Gingival Overgrowth. Antibiotics 2019, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Converti, I.; Santacroce, L.; Cesarano, F.; Vecchiet, F.; Cacchio, L.; Scacco, S.; Grassi, R.; Grassi, F.R.; Gnoni, A.; et al. Impact of Periodontal Inflammation on Nutrition and Inflammation Markers in Hemodialysis Patients. Antibiotics 2019, 8, 209. [Google Scholar] [CrossRef]
- Lorusso, F.; Noumbissi, S.; Francesco, I.; Rapone, B.; Khater, A.G.A.; Scarano, A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials 2020, 13, 5534. [Google Scholar] [CrossRef]
- Corsalini, M.; Di Venere, D.; Carossa, M.; Ripa, M.; Sportelli, P.; Cantatore, F.; De Rinaldis, C.; Di Santantonio, G.; Lenoci, G.; Barile, G. Comparative clinical study between zirconium-ceramic and metal-ceramic fixed rehabilitations. ORAL Implantol. 2018, 11, 150–160. [Google Scholar]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody Cocktail to SARS-CoV-2 Spike Protein Prevents Rapid Mutational Escape Seen with Individual Antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Schäfer, A.; Muecksch, F.; Lorenzi, J.C.C.; Leist, S.R.; Cipolla, M.; Bournazos, S.; Schmidt, F.; Maison, R.M.; Gazumyan, A.; Martinez, D.R.; et al. Antibody Potency, Effector Function and Combinations in Protection from SARS-CoV-2 Infection in Vivo. J. Exp. Med. 2021, 218, e20201993. [Google Scholar] [CrossRef]
- Charitos, I.A.; Del Prete, R.; Inchingolo, F.; Mosca, A.; Carretta, D.; Ballini, A.; Santacroce, L. What We Have Learned for the Future about COVID-19 and Healthcare Management of It? Acta Biomed. 2020, 91, e2020126. [Google Scholar] [PubMed]
- Patano, A.; Cirulli, N.; Beretta, M.; Plantamura, P.; Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Marinelli, G.; Scarano, A.; et al. Education Technology in Orthodontics and Paediatric Dentistry during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 6056. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Scacco, S.; Perillo, L.; Scarano, A.; Aityan, S.K.; Contaldo, M.; Cd Nguyen, K.; Santacroce, L.; Syed, J.; et al. A Comparative Study on Different Stemness Gene Expression between Dental Pulp Stem Cells vs. Dental Bud Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1626–1633. [Google Scholar] [CrossRef]
- Rapone, B.; Corsalini, M.; Converti, I.; Loverro, M.T.; Gnoni, A.; Trerotoli, P.; Ferrara, E. Does Periodontal Inflammation Affect Type 1 Diabetes in Childhood and Adolescence? A Meta-Analysis. Front. Endocrinol 2020, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Inchingolo, A.D.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; Mancini, A.; et al. COVID-19 Infection in Children and Infants: Current Status on Therapies and Vaccines. Children 2022, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Catucci, O.; Cefalo, A.; Lazzaro, R.; Inchingolo, F.; Tomassone, D.; Aityan, S.K.; Ballini, A.; Nguyen, K.C.D.; et al. Mesenchymal Stem Cells: The Secret Children’s Weapons against the SARS-CoV-2 Lethal Infection. Appl. Sci. 2021, 11, 1696. [Google Scholar] [CrossRef]
- Bemark, M.; Hazanov, H.; Strömberg, A.; Komban, R.; Holmqvist, J.; Köster, S.; Mattsson, J.; Sikora, P.; Mehr, R.; Lycke, N.Y. Limited Clonal Relatedness between Gut IgA Plasma Cells and Memory B Cells after Oral Immunization. Nat. Commun. 2016, 7, 12698. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct Antibody Responses to SARS-CoV-2 in Children and Adults across the COVID-19 Clinical Spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial Skin Temperature and Discomfort When Wearing Protective Face Masks: Thermal Infrared Imaging Evaluation and Hands Moving the Mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective Face Masks: Effect on the Oxygenation and Heart Rate Status of Oral Surgeons during Surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F.; Inchingolo, F.; Scarano, A. The Impact of The Novel COVID-19 on the Scientific Production Spread: A Five-Month Bibliometric Report of The Worldwide Research Community. Acta Med. Mediterr. 2020, 36, 3357–3360. [Google Scholar]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; D’Ettorre, E.; Nico, A.; Inchingolo, F.; Dipalma, G.; Tomassone, D.; Serlenga, E.M.; Dalagni, G.; et al. New Translational Trends in Personalized Medicine: Autologous Peripheral Blood Stem Cells and Plasma for COVID-19 Patient. J. Pers. Med. 2022, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. SARS-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte–Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Stera, G.; Pierantoni, L.; Masetti, R.; Leardini, D.; Biagi, C.; Buonsenso, D.; Pession, A.; Lanari, M. Impact of SARS-CoV-2 Pandemic on Bronchiolitis Hospitalizations: The Experience of an Italian Tertiary Center. Children 2021, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Charitos, I.A.; Ballini, A.; Bottalico, L.; Cantore, S.; Passarelli, P.C.; Inchingolo, F.; D’Addona, A.; Santacroce, L. Special Features of SARS-CoV-2 in Daily Practice. World J. Clin. Cases 2020, 8, 3920–3933. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.-H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally Enhanced Neutralizing Breadth against SARS-CoV-2 One Year after Infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 418–421; author reply 421–422. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Charrier, J.-B. Selecting a Relevant in Vitro Cell Model for Testing and Comparing the Effects of a Choukroun’s Platelet-Rich Fibrin (PRF) Membrane and a Platelet-Rich Plasma (PRP) Gel: Tricks and Traps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2010, 110, 409–411. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Qorri, E.; Dipalma, G.; Mancini, A.; Corsalini, M.; Fabbro, M.D.; Scarano, A.; Tartaglia, G.M.; Inchingolo, F. The Impact of Periodontal Inflammation on Endothelial Function Assessed by Circulating Levels of Asymmetric Dimethylarginine: A Single-Blinded Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4173. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Noumbissi, S. Infrared Thermographic Evaluation of Temperature Modifications Induced during Implant Site Preparation with Steel vs. Zirconia Implant Drill. J. Clin. Med. 2020, 9, 148. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.; Ranjan, V.; Kumar, N. Association of ABO and Rh Blood Group in Susceptibility, Severity, and Mortality of Coronavirus Disease 2019: A Hospital-Based Study from Delhi, India. Front. Cell Infect. Microbiol. 2021, 11, 767771. [Google Scholar] [CrossRef]
- Janda, A.; Engel, C.; Remppis, J.; Enkel, S.; Peter, A.; Hörber, S.; Ganzenmueller, T.; Schober, S.; Weinstock, C.; Jacobsen, E.-M.; et al. Role of ABO Blood Group in SARS-CoV-2 Infection in Households. Front. Microbiol. 2022, 13, 857965. [Google Scholar] [CrossRef] [PubMed]
- Shachor-Meyouhas, Y.; Hussein, K.; Dabaja-Younis, H.; Szwarcwort-Cohen, M.; Almog, R.; Weissman, A.; Mekel, M.; Hyams, G.; Horowitz, N.A.; Gepstein, V.; et al. Immunogenicity Trends 1 and 3 Months after Second BNT162B2 Vaccination among Healthcare Workers in Israel. Clin. Microbiol Infect. 2021, 28, 450.e1–450.e4. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection. medRxiv 2021, 10, 64. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Burnham, E.L.; Janssen, W.J.; Riches, D.W.H.; Moss, M.; Downey, G.P. The Fibroproliferative Response in Acute Respiratory Distress Syndrome: Mechanisms and Clinical Significance. Eur. Respir. J. 2014, 43, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Doykov, I.; Hällqvist, J.; Gilmour, K.C.; Grandjean, L.; Mills, K.; Heywood, W.E. ‘The Long Tail of COVID-19’—The Detection of a Prolonged Inflammatory Response after a SARS-CoV-2 Infection in Asymptomatic and Mildly Affected Patients. F1000Research 2020, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, G.; De Michele, L.; De Ceglie, M.; Pierucci, P.; Mirabile, A.; Vita, M.; Palmieri, V.O.; Carpagnano, G.E.; Scardapane, A.; D’Agostino, C. Lung Ultrasonography for Long-Term Follow-up of COVID-19 Survivors Compared to Chest CT Scan. Respir. Med. 2021, 181, 106384. [Google Scholar] [CrossRef]
- Vimercati, L.; Maria, L.D.; Quarato, M.; Caputi, A.; Gesualdo, L.; Migliore, G.; Cavone, D.; Sponselli, S.; Pipoli, A.; Inchingolo, F.; et al. Association between Long COVID and Overweight/Obesity. J. Clin. Med. 2021, 10, 4143. [Google Scholar] [CrossRef] [PubMed]
- Nath, A. Long-Haul COVID. Neurology 2020, 95, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; dos Santos Freitas, A.; Ribeiro da Silveira, P.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-Acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M. Pathogens, Commensal Symbionts, and Pathobionts: Discovery and Functional Effects on the Host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, J.; Sun, R.; Tang, X.; Liang, C.; Lin, H.; Zeng, L.; Hu, J.; Yuan, R.; Zhou, P.; et al. Prolonged Persistence of SARS-CoV-2 RNA in Body Fluids. Emerg. Infect. Dis. 2020, 26, 1834–1838. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Pede, C.D.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public Health 2022, 19, 988. [Google Scholar] [CrossRef]
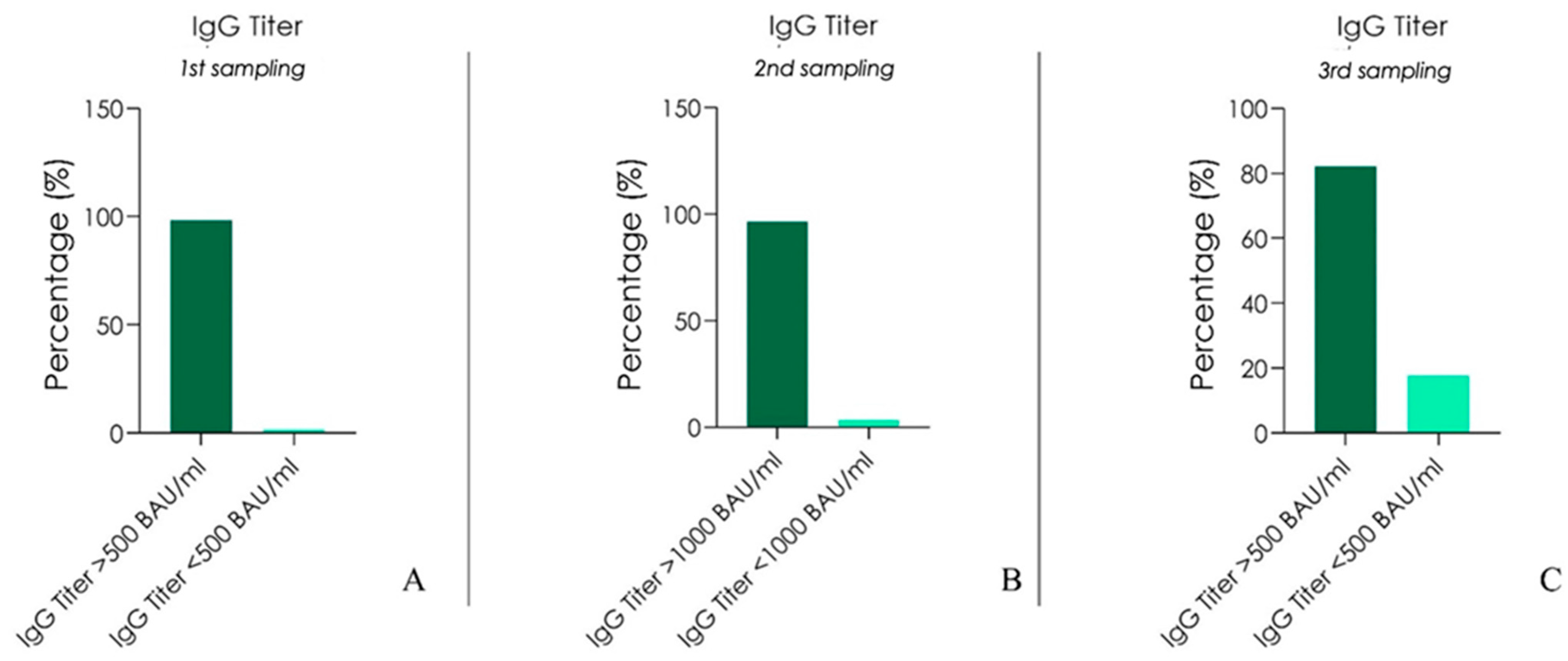



| All Blood Types | |||
|---|---|---|---|
| Titer 1 | Titer 2 | Titer 3 | |
| Average | 8.413 | 3.880 | 1.473 |
| St.Dev | 9.510 | 5.156 | 1.818 |
| Max | 64.771 | 44.352 | 15.455 |
| Min | 882 | 103 | 77 |
| Range: | 63.889 | 44.249 | 15.377 |
| # Patients | 229 | ||
| Titer 1,2 | Titer 2,3 | Titer 1–3 | |
| Correlation | 0.97 | 0.80 | 0.81 |
| Ages Related Blood Types | |||
|---|---|---|---|
| Titer 1 | Titer 2 | Titer 3 | |
| Average | 8.413 | 3.880 | 1.473 |
| St.Dev | 9.510 | 5.156 | 1.818 |
| Max | 64.771 | 44.352 | 15.455 |
| Min | 882 | 103 | 77 |
| Range: | 63.889 | 44.249 | 15.377 |
| # Patients | 229 | ||
| Titer 1,2 | Titer 2,3 | Titer 1–3 | |
| Correlation | 0.97 | 0.80 | 0.81 |
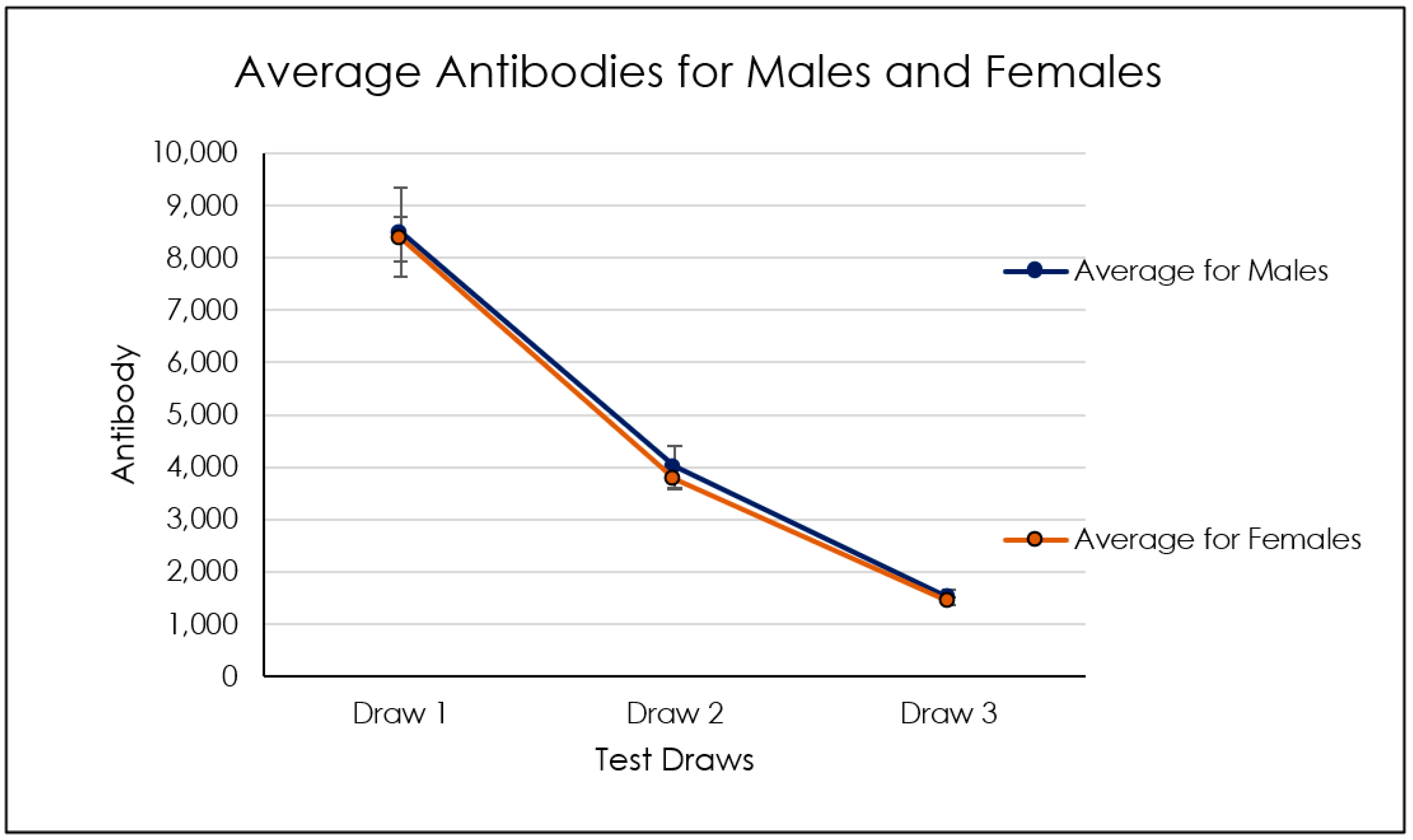
| Genders Referred Blood Types | |||
|---|---|---|---|
| Titer 1 | Titer 2 | Titer 3 | |
| Average | 8.413 | 3.880 | 1.473 |
| St.Dev | 9.510 | 5.156 | 1.818 |
| Max | 64.771 | 44.352 | 15.455 |
| Min | 882 | 103 | 77 |
| Range: | 63.889 | 44.249 | 15.377 |
| # Patients | 229 | ||
| Titer 1,2 | Titer 2,3 | Titer 1–3 | |
| Correlation | 0.97 | 0.80 | 0.81 |
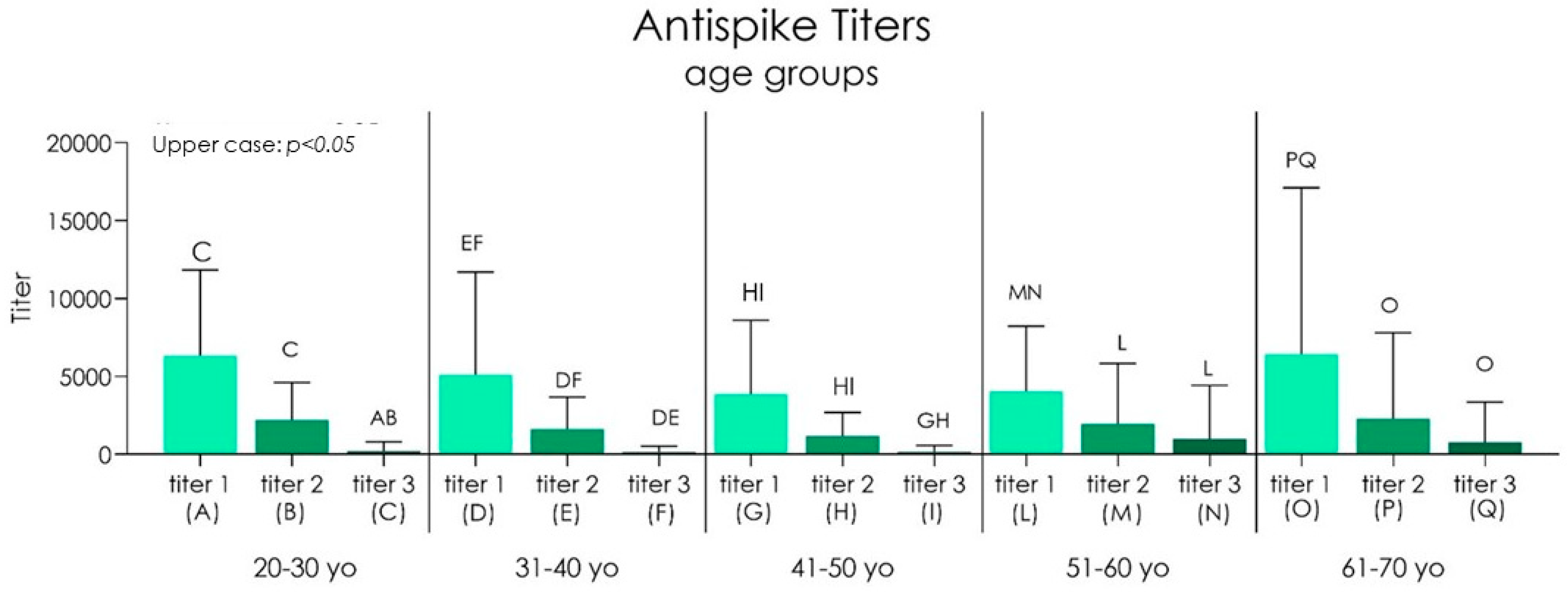
| Group I 20–30 yo | Group II 31–40 yo | Group III 41–50 yo | Group IV 51–60 yo | Group V 61–70 yo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| titer 1 | titer 2 | titer 3 | titer 1 | titer 2 | titer 3 | titer 1 | titer 2 | titer 3 | titer 1 | titer 2 | titer 3 | titer 1 | titer 2 | titer 3 | |
| Mean | 6342 | 2207 | 207.6 | 5118 | 1628 | 151.3 | 3871 | 1172 | 169.0 | 4046 | 1963 | 1010 | 6438 | 2289 | 780.6 |
| SD | 5506 | 2397 | 599.8 | 6593 | 2041 | 377.0 | 4737 | 1522 | 414.3 | 4174 | 3872 | 3413 | 10673 | 5513 | 2578 |
| Lower 95% CI | 4668 | 1479 | 25.29 | 3264 | 1054 | 45.30 | 2243 | 657.3 | 28.83 | 2896 | 895.2 | 69.02 | 3269 | 670.2 | 23.75 |
| Upper 95% CI | 8016 | 2936 | 390.0 | 6972 | 2202 | 257.4 | 5498 | 1687 | 309.2 | 5197 | 3030 | 1951 | 9608 | 3907 | 1537 |
| 0/+ | 0/− | |||||
|---|---|---|---|---|---|---|
| Titer 1 | Titer 2 | Titer 3 | Titer 1 | Titer 2 | Titer 3 | |
| Mean | 10,289 | 5025 | 1739 | 16,810 | 8710 | 3561 |
| SD | 10,013 | 6024 | 1548 | 15,992 | 9160 | 4414 |
| Lower 95% CI of mean | 8001 | 3648 | 1385 | 27.84 | 903 | −1071 |
| Upper 95% CI | 12,577 | 6401 | 2093 | 33,593 | 18,322 | 8192 |
| A/+ | A/− | |||||
| Titer 1 | Titer 1 | Titer 1 | Titer 1 | Titer 2 | Titer 3 | |
| Mean | 7327 | 5717 | 5717 | 5717 | 8710 | 3561 |
| SD | 8160 | 3095 | 3095 | 3095 | 9160 | 4414 |
| Lower 95% CI of mean | 5008 | 3638 | 3638 | 3638 | 903 | −1071 |
| Upper 95% CI | 9646 | 7797 | 7797 | 7797 | 18,322 | 8192 |
| B/+ | B/− | |||||
| Titer 1 | Titer 2 | Titer 1 | Titer 2 | Titer 1 | Titer 2 | |
| Mean | 5867 | 2574 | 5867 | 2574 | 5867 | 2574 |
| SD | 7293 | 2965 | 7293 | 2965 | 7293 | 2965 |
| Lower 95% CI of mean | 2856 | 1350 | 2856 | 1350 | 2856 | 1350 |
| Upper 95% CI | 8877 | 3798 | 8877 | 3798 | 8877 | 3798 |
| AB/+ | ||||||
| Titer 1 | Titer 1 | Titer 1 | ||||
| Mean | 4945 | 4945 | 4945 | |||
| SD | 3577 | 3577 | 3577 | |||
| Lower 95% CI of mean | 2672 | 2672 | 2672 | |||
| Upper 95% CI | 7218 | 7218 | 7218 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Malcangi, G.; Ceci, S.; Patano, A.; Corriero, A.; Azzollini, D.; Marinelli, G.; Coloccia, G.; Piras, F.; Barile, G.; et al. Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers. Biomedicines 2022, 10, 2402. https://doi.org/10.3390/biomedicines10102402
Inchingolo AD, Malcangi G, Ceci S, Patano A, Corriero A, Azzollini D, Marinelli G, Coloccia G, Piras F, Barile G, et al. Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers. Biomedicines. 2022; 10(10):2402. https://doi.org/10.3390/biomedicines10102402
Chicago/Turabian StyleInchingolo, Alessio Danilo, Giuseppina Malcangi, Sabino Ceci, Assunta Patano, Alberto Corriero, Daniela Azzollini, Grazia Marinelli, Giovanni Coloccia, Fabio Piras, Giuseppe Barile, and et al. 2022. "Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers" Biomedicines 10, no. 10: 2402. https://doi.org/10.3390/biomedicines10102402
APA StyleInchingolo, A. D., Malcangi, G., Ceci, S., Patano, A., Corriero, A., Azzollini, D., Marinelli, G., Coloccia, G., Piras, F., Barile, G., Settanni, V., Mancini, A., De Leonardis, N., Garofoli, G., Palmieri, G., Isacco, C. G., Rapone, B., Jones, M., Bordea, I. R., ... Inchingolo, F. (2022). Antispike Immunoglobulin-G (IgG) Titer Response of SARS-CoV-2 mRNA-Vaccine (BNT162b2): A Monitoring Study on Healthcare Workers. Biomedicines, 10(10), 2402. https://doi.org/10.3390/biomedicines10102402




















