Correlation between Retinal Vascularization and Disease Aggressiveness in Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
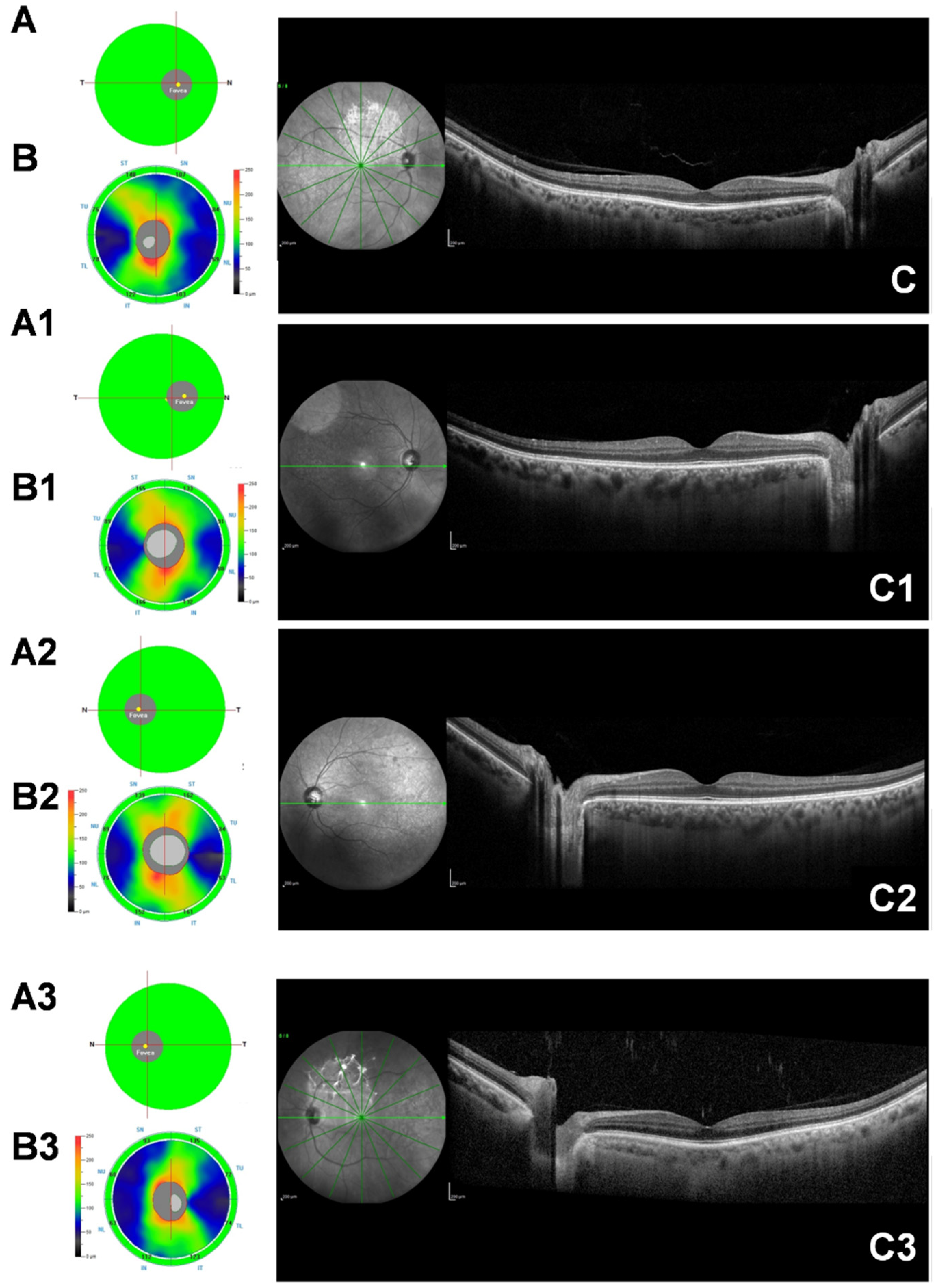
2.2. Spectral-Domain Optical Coherence Tomography (SD-OCT)
2.3. Subfoveal Choroidal Thickness Measurement (SFCT)
2.4. Optical Coherence Tomography Angiography (OCT-A)
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features
3.2. SD-OCT and OCT-A Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef]
- Bensimon, G.; Lacomblez, L.; Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef]
- Lacomblez, L.; Bensimon, G.; Leigh, P.N.; Guillet, P.; Meininger, V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis/riluzole study group II. Lancet 1996, 347, 1425–1431. [Google Scholar] [CrossRef]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef]
- Spiller, K.J.; Restrepo, C.R.; Khan, T.; Dominique, M.A.; Fang, T.C.; Canter, R.C.; Roberts, C.J.; Miller, K.R.; Ransohoff, R.M.; Trojanowski, J.Q.; et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat. Neurosci. 2018, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Li, Y.; Fukaya, M.; Lorenzini, I.; Cleveland, D.W.; Ostrow, L.W.; Rothstein, J.D.; Bergles, D.E. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat. Neurosci. 2013, 16, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; O’Banion, M.K.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef]
- Månberg, A.; Skene, N.; Sanders, F.; Trusohamn, M.; Remnestål, J.; Szczepińska, A.; Aksoylu, I.S.; Lönnerberg, P.; Ebarasi, L.; Wouters, S.; et al. Altered perivascular fibroblast activity precedes ALS disease onset. Nat. Med. 2021, 27, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Patel, V.; Xiao, J.; Khan, M.M. The role of neurovascular system in neurodegenerative diseases. Mol. Neurobiol. 2020, 57, 4373–4393. [Google Scholar] [CrossRef]
- Tsokolas, G.; Tsaousis, K.T.; Diakonis, V.F.; Matsou, A.; Tyradellis, S. Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review. Eye Brain 2020, 12, 73–87. [Google Scholar] [CrossRef]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Roisman, L.; Goldhardt, R. OCT angiography: An upcoming non-invasive tool for diagnosis of age-related macular degeneration. Curr. Ophthalmol. Rep. 2017, 5, 136–140. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot. Neuron Disord 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Shefner, J.M.; Al-Chalabi, A.; Baker, M.R.; Cui, L.Y.; de Carvalho, M.; Eisen, A.; Grosskreutz, J.; Hardiman, O.; Henderson, R.; Matamala, J.M.; et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 2020, 131, 1975–1978. [Google Scholar] [CrossRef]
- Kimura, F.; Fujimura, C.; Ishida, S.; Nakajima, H.; Furutama, D.; Uehara, H.; Shinoda, K.; Sugino, M.; Hanafusa, T. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006, 66, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Poesen, K.; De Schaepdryver, M.; Stubendorff, B.; Gille, B.; Muckova, P.; Wendler, S.; Prell, T.; Ringer, T.M.; Rhode, H.; Stevens, O.; et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 2017, 88, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, T.; Hangai, M.; Nukada, M.; Nakano, N.; Morooka, S.; Akagi, T.; Nonaka, A.; Yoshimura, N. Frequency doubling technology, and retinal measurements with spectral-domain optical coherence tomography in preperimetric glaucoma. Graefes. Arch. Clin. Exp. Ophthalmol. 2013, 251, 129–137. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express. 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. Dev. Ophthalmol. 2016, 56, 6–12. [Google Scholar] [PubMed]
- Rao, H.L.; Pradhan, Z.S.; Weinreb, R.N.; Reddy, H.B.; Riyazuddin, M.; Dasari, S.; Palakurthy, M.; Puttaiah, N.K.; Rao, D.A.; Webers, C.A. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2016, 171, 75–83. [Google Scholar] [CrossRef]
- Cerveró, A.; Casado, A.; Riancho, J. Retinal changes in amyotrophic lateral sclerosis: Looking at the disease through a new window. J. Neurol. 2021, 268, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Ringelstein, M.; Albrecht, P.; Südmeyer, M.; Harmel, J.; Müller, A.K.; Keser, N.; Finis, D.; Ferrea, S.; Guthoff, R.; Schnitzler, A.; et al. Subtle retinal pathology in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2014, 1, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Simonett, J.M.; Huang, R.; Siddique, N.; Farsiu, S.; Siddique, T.; Volpe, N.J.; Fawzi, A.A. Macular sub-layer thinning and association with pulmonary function tests in Amyotrophic Lateral Sclerosis. Sci. Rep. 2016, 6, 29187. [Google Scholar] [CrossRef]
- Mukherjee, N.; McBurney-Lin, S.; Kuo, A.; Bedlack, R.; Tseng, H. Retinal thinning in amyotrophic lateral sclerosis patients without ophthalmic disease. PLoS ONE 2017, 12, e0185242. [Google Scholar] [CrossRef]
- Rohani, M.; Meysamie, A.; Zamani, B.; Sowlat, M.M.; Akhoundi, F.H. Reduced retinal nerve fiber layer (RNFL) thickness in ALS patients: A window to disease progression. J. Neurol. 2018, 265, 1557–1562. [Google Scholar] [CrossRef]
- Roth, N.M.; Saidha, S.; Zimmermann, H.; Brandt, A.U.; Oberwahrenbrock, T.; Maragakis, N.J.; Tumani, H.; Ludolph, A.C.; Meyer, T.; Calabresi, P.A.; et al. Optical coherence tomography does not support optic nerve involvement in amyotrophic lateral sclerosis. Eur. J. Neurol. 2013, 20, 1170–1176. [Google Scholar] [CrossRef]
- Abdelhak, A.; Hübers, A.; Böhm, K.; Ludolph, A.C.; Kassubek, J.; Pinkhardt, E.H. In vivo assessment of retinal vessel pathology in amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 949–953. [Google Scholar] [CrossRef]
- Hübers, A.; Müller, H.P.; Dreyhaupt, J.; Böhm, K.; Lauda, F.; Tumani, H.; Kassubek, J.; Ludolph, A.C.; Pinkhardt, E.H. Retinal involvement in amyotrophic lateral sclerosis: A study with optical coherence tomography and diffusion tensor imaging. J. Neural. Transm. 2016, 123, 281–287. [Google Scholar] [CrossRef]
- Buckley, A.F.; Bossen, E.H. Skeletal muscle microvasculature in the diagnosis of neuromuscular disease. J Neuropathol Exp Neurol. 2013, 72, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Kolde, G.; Bachus, R.; Ludolph, A.C. Skin involvement in amyotrophic lateral sclerosis. Lancet 1996, 347, 1226–1227. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Manganelli, F.; Iodice, R.; Caporaso, G.; Stancanelli, A.; Marinou, K.; Lanzillo, B.; Santoro, L.; Mora, G. Non-motor involvement in amyotrophic lateral sclerosis: New insight from nerve and vessel analysis in skin biopsy. Neuropathol. Appl. Neurobiol. 2017, 43, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K. The Role of Mitochondria, Oxidative Stress and Altered Calcium Homeostasis in Amyotrophic Lateral Sclerosis: From Current Developments in the Laboratory to Clinical Treatments, 1st ed.; Frontiers in Cellular Neuroscience: Lausanne, Switzerland, 2017. [Google Scholar]
- Béland, L.C.; Markovinovic, A.; Jakovac, H.; De Marchi, F.; Bilic, E.; Mazzini, L.; Kriz, J.; Munitic, I. Immunity in amyotrophic lateral sclerosis: Blurred lines between excessive inflammation and inefficient immune responses. Brain Commun. 2020, 2, fcaa124. [Google Scholar] [CrossRef] [PubMed]
- Kunis, G.; Baruch, K.; Miller, O.; Schwartz, M. Immunization with a Myelin-Derived Antigen Activates the Brain’s Choroid Plexus for Recruitment of Immunoregulatory Cells to the CNS and Attenuates Disease Progression in a Mouse Model of ALS. J. Neurosci. 2015, 35, 6381–6393. [Google Scholar] [CrossRef]
- Baruch, K.; Deczkowska, A.; David, E.; Castellano, J.M.; Miller, O.; Kertser, A.; Berkutzki, T.; Barnett-Itzhaki, Z.; Bezalel, D.; Tony, W.-C.; et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 2014, 346, 89–93. [Google Scholar] [CrossRef]
- Esen, E.; Sizmaz, S.; Demir, T.; Demirkiran, M.; Unal, I.; Demircan, N. Evaluation of Choroidal Vascular Changes in Patients with Multiple Sclerosis Using Enhanced Depth Imaging Optical Coherence Tomography. Ophthalmologica 2016, 235, 65–71. [Google Scholar] [CrossRef]
- Agarwal, A.; Invernizzi, A.; Singh, R.B.; Foulsham, W.; Aggarwal, K.; Handa, S.; Agrawal, R.; Pavesio, C.; Gupta, V. An update on inflammatory choroidal neovascularization: Epidemiology, multimodal imaging, and management. J. Ophthalmic. Inflamm. Infect. 2018, 8, 13. [Google Scholar] [CrossRef]
- Thakur, K.; Tiwari, A.; Sharma, K.; Modgil, S.; Khosla, R.; Anand, A. Angiogenesis-Centered Molecular Cross-Talk in Amyotrophic Lateral Sclerosis Survival: Mechanistic Insights. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 137–151. [Google Scholar] [CrossRef]
- Saul, J.; Hutchins, E.; Reiman, R.; Saul, M.; Ostrow, L.W.; Harris, B.T.; Van Keuren-Jensen, K.; Bowser, R.; Bakkar, N. Global alterations to the choroid plexus blood-CSF barrier in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2020, 8, 92. [Google Scholar] [CrossRef]
- Lewandowski, D.; Sander, C.L.; Tworak, A.; Gao, F.; Xu, Q.; Skowronska-Krawczyk, D. Dynamic lipid turnover in photoreceptors and retinal pigment epithelium throughout life. Prog. Retin. Eye Res. 2021, 89, 101037. [Google Scholar] [CrossRef]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Baruch, K. The resolution of neuroinflammation in neurodegeneration: Leukocyte recruitment via the choroid plexus. EMBO J. 2014, 33, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Trojano, M.; Cutter, G.; Reingold, S.; Sorensen, P.S. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult. Scler. 2015, 21, 318–331. [Google Scholar] [CrossRef] [PubMed]

| Controls | ALS Patients | p Value | |
|---|---|---|---|
| Eyes (N.) | 90 | 96 | - |
| Age (years) | 58.9 ± 10 | 59.7 ± 11 | |
| Sex (female/male) | 20/25 | 15/33 | |
| ALSFRS-r | - | 36 ± 3.6 | - |
| ΔFS score | - | 0.64 ± 0.63 | - |
| Disease duration (months) | - | 40.2 ± 40 | - |
| Diagnostic delay (months) | 26.5 ± 29.1 | ||
| OCT-A parameters (%) | |||
| SCP Whole | 48.81 ± 3.44 | 48.26 ± 4.34 | 0.875 |
| DCP Whole | 49.45 ± 4.92 | 50.10 ± 5.47 | 0.764 |
| CC Whole | 71.59 ± 4.83 | 70.27 ± 4.55 | 0.768 |
| RPC Whole | 48.57 ± 2.99 | 47.70 ± 3.12 | 0.896 |
| FAZ area (mm2) | 0.278 ± 0.08 | 0.281 ± 0.07 | 0.922 |
| SD-OCT parameters (µm) | |||
| GCC average | 98.45 ± 7.01 | 98.37 ± 6.80 | 0.914 |
| RNFL average | 100.9 ± 9.63 | 101.74 ± 8.32 | 0.862 |
| SFCT average | 301.3 ± 55.80 | 357.94 ± 55.15 | <0.001 |
| BCVA (logMAR) | 0.06 ± 0.05 | 0.07 ± 0.03 | 0.789 |
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Eyes (N.) | 20 | 48 | 28 |
| Age (years) | 60.2 ± 5 | 61.6 ± 10 | 56 ± 15 |
| Sex (female/male) | -/10 | 11/13 | 4/10 |
| ALSFRS-r | 36.5 ± 4.2 | 32.1 ± 5 | 33.7 ± 4.3 |
| ΔFS score | 0.13 ± 0.04 | 0.48 ± 0.18 | 1.43 ± 0.73 |
| Disease duration (months) | 96.3 ± 65 | 37 ± 15 | 11.6 ± 5 |
| Diagnostic delay (months) | 54.15 ± 44.66 | 21.4 ± 10.3 | 8.3 ± 4.0 |
| OCT-A parameters (%) | |||
| SCP Whole | 48.80 ± 4.26 | 48.26 ± 4.15 | 46.8 ± 6.55 |
| DCP Whole | 50.58 ± 6.60 | 48.93 ± 7.08 | 48.7 ± 7.44 |
| CC Whole | 70.95 ± 6.94 | 70.03 ± 3.16 | 68.7 ± 6.44 |
| RPC Whole | 47.53 ± 2.63 | 46.11 ± 3.44 | 47.5 ± 5.14 |
| FAZ area (mm2) | 0.279 ± 0.08 | 0.280 ± 0.12 | 0.281 ± 0.11 |
| OCT parameters (µm) | |||
| GCC average | 98.45 ± 6.93 | 97.25 ± 11.54 | 95.1 ± 10.33 |
| RNFL average | 101.07 ± 8.44 | 99.51 ± 10.38 | 98.5 ± 12.25 |
| SFCT average | 394.45 ± 53.73 | 393.09 ± 42.17 | 267.71 ± 56.24 |
| BCVA (logMAR) | 0.07 ± 0.04 | 0.01 ± 0.03 | 0.03 ± 0.05 |
| Group 1 vs. Group 2 | ||
|---|---|---|
| SD-OCT | β | p-Value |
| GCC average | 1.445 | 0.622 |
| RNFL average | 2.371 | 0.694 |
| SFCT average | 1.321 | 0.824 |
| OCT-A | ||
| SCP Whole | 0.130 | 0.784 |
| DCP Whole | 2.142 | 0.344 |
| CC Whole | 0.240 | 0.633 |
| RPC Whole | 1.342 | 0.405 |
| FAZ area | −0.011 | 0.626 |
| Group 1 vs. Group 3 | ||
| SD-OCT | β | p-Value |
| GCC average | 3.621 | 0.514 |
| RNFL average | 3.469 | 0.531 |
| SFCT average | 126.32 | <0.001 |
| OCT-A | ||
| SCP Whole | 2.417 | 0.534 |
| DCP Whole | 2.342 | 0.426 |
| CC Whole | 2.602 | 0.524 |
| RPC Whole | −0.047 | 0.726 |
| FAZ area | −0.019 | 0.844 |
| Group 2 vs. Group 3 | ||
| SD-OCT | β | p-Value |
| GCC average | 2.134 | 0.663 |
| RNFL average | 1.247 | 0.752 |
| SFCT average | 125.41 | <0.001 |
| OCT-A | ||
| SCP Whole | 2.345 | 0.431 |
| DCP Whole | 0.034 | 0.741 |
| CC Whole | 2.311 | 0.524 |
| RPC Whole | −1.674 | 0.423 |
| FAZ area | −0.015 | 0.647 |
| Disease Duration | ALSFRS-r | SCP | DCP | CC | RPC | FAZ | GCC | RNFL | SFCT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Disease Duration | 1 | |||||||||
| ALSFRS-r | −0.1149 | 1 | ||||||||
| SCP | 0.0707 | 0.1413 | 1 | |||||||
| DCP | −0.1326 | 0.2561 | 0.3858 | 1 | ||||||
| CC | −0.1048 | 0.0976 | 0.2002 | 0.1941 | 1 | |||||
| RPC | −0.0103 | 0.0760 | 0.4693 | 0.0301 | 0.2016 | 1 | ||||
| FAZ | 0.2218 | −0.2739 | −0.1753 | 0.0719 | −0.0130 | −0.0080 | 1 | |||
| GCC | 0.0843 | 0.2179 | 0.4119 | 0.1434 | 0.1148 | 0.3980 | −0.1272 | 1 | ||
| RNFL | 0.0701 | 0.2469 | 0.3771 | 0.3122 | 0.1507 | 0.4082 | 0.1271 | 0.6505 | 1 | |
| SFCT | 0.2443 | 0.7526 | 0.0728 | 0.0827 | 0.0472 | −0.1976 | −0.0603 | 0.0754 | 0.0009 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, G.; Montorio, D.; Ausiello, F.P.; Magno, L.; Iodice, R.; Mazzucco, A.; Iuzzolino, V.V.; Senerchia, G.; Brescia Morra, V.; Nolano, M.; et al. Correlation between Retinal Vascularization and Disease Aggressiveness in Amyotrophic Lateral Sclerosis. Biomedicines 2022, 10, 2390. https://doi.org/10.3390/biomedicines10102390
Cennamo G, Montorio D, Ausiello FP, Magno L, Iodice R, Mazzucco A, Iuzzolino VV, Senerchia G, Brescia Morra V, Nolano M, et al. Correlation between Retinal Vascularization and Disease Aggressiveness in Amyotrophic Lateral Sclerosis. Biomedicines. 2022; 10(10):2390. https://doi.org/10.3390/biomedicines10102390
Chicago/Turabian StyleCennamo, Gilda, Daniela Montorio, Francesco Pio Ausiello, Luigifilippo Magno, Rosa Iodice, Alberto Mazzucco, Valentina Virginia Iuzzolino, Gianmaria Senerchia, Vincenzo Brescia Morra, Maria Nolano, and et al. 2022. "Correlation between Retinal Vascularization and Disease Aggressiveness in Amyotrophic Lateral Sclerosis" Biomedicines 10, no. 10: 2390. https://doi.org/10.3390/biomedicines10102390
APA StyleCennamo, G., Montorio, D., Ausiello, F. P., Magno, L., Iodice, R., Mazzucco, A., Iuzzolino, V. V., Senerchia, G., Brescia Morra, V., Nolano, M., Costagliola, C., & Dubbioso, R. (2022). Correlation between Retinal Vascularization and Disease Aggressiveness in Amyotrophic Lateral Sclerosis. Biomedicines, 10(10), 2390. https://doi.org/10.3390/biomedicines10102390









