Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes
Abstract
:1. Introduction
2. Materials and Methods
- ls—signal in the lesion;
- bs—signal in the background (parenchyma);
- σ—standard deviation of the signal in the parenchyma.
2.1. Histopathological Examination
2.2. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Funke, M. Diagnostic Imaging of Breast Cancer: An Update. Radiologe 2016, 56, 921–938. [Google Scholar] [CrossRef] [PubMed]
- Lalji, U.; Lobbes, M. Contrast-Enhanced Dual-Energy Mammography: A Promising New Imaging Tool in Breast Cancer Detection. Women’s. Health 2014, 10, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Smetherman, D.H. Screening, Imaging, and Image-Guided Biopsy Techniques for Breast Cancer. Surg. Clin. N. Am. 2013, 93, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Porzio, M.; Lorenzon, L.; Milan, L.; Sghedoni, R.; Russo, G.; Massafra, R.; Fanizzi, A.; Barucci, A.; Ardu, V.; et al. Artificial Intelligence Applications in Medical Imaging: A Review of the Medical Physics Research in Italy. Phys. Medica 2021, 83, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, R.; Bagnasco, S.; Bottigli, U.; Castellano, M.; Cataldo, R.; Catanzariti, E.; Cerello, P.; Cheran, S.C.; De Carlo, F.; Delogu, P.; et al. The MAGIC-5 Project: Medical Applications on a Grid Infrastructure Connection. In Proceedings of the IEEE Symposium Conference Record Nuclear Science 2004, Rome, Italy, 16–22 October 2004; IEEE: Piscataway Township, NJ, USA, 2004; Volume 3, pp. 1902–1906. [Google Scholar]
- Milosevic, M.; Jankovic, D.; Milenkovic, A.; Stojanov, D. Early Diagnosis and Detection of Breast Cancer. Technol. Health Care 2018, 26, 729–759. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.J.; Pinker-Domenig, K. Diagnosis and Staging of Breast Cancer: When and How to Use Mammography, Tomosynthesis, Ultrasound, Contrast-Enhanced Mammography, and Magnetic Resonance Imaging; Springer: Berlin, Germany, 2019; ISBN 9783030111489. [Google Scholar]
- Lobbes, M.B.I.; Heuts, E.M.; Moossdorff, M.; van Nijnatten, T.J.A. Contrast Enhanced Mammography (CEM) versus Magnetic Resonance Imaging (MRI) for Staging of Breast Cancer: The pro CEM Perspective. Eur. J. Radiol. 2021, 142, 109883. [Google Scholar] [CrossRef]
- Sogani, J.; Mango, V.L.; Keating, D.; Sung, J.S.; Jochelson, M.S. Contrast-Enhanced Mammography: Past, Present, and Future. Clin. Imaging 2021, 69, 269–279. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-Enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion on the Optimal Primary Breast Cancer Treatment. Breast Care 2019, 14, 103–110. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the Benefits of Therapy for Early-Stage Breast Cancer: The St. Gallen International Consensus Guidelines for the Primary Therapy of Early Breast Cancer 2019. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Lee, M.; Lee, C.S.; Tan, P.H. Hormone Receptor Expression in Breast Cancer: Postanalytical Issues. J. Clin. Pathol. 2013, 66, 478–484. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Nagini, S. Breast Cancer: Current Molecular Therapeutic Targets and New Players. Anticancer. Agents Med. Chem. 2017, 17, 152–163. [Google Scholar] [CrossRef]
- de Gregorio, A.; Friedl, T.W.P.; Hering, E.; Widschwendter, P.; de Gregorio, N.; Bekes, I.; Janni, W.; Dayan, D.; Huober, J.B. Ki67 as Proliferative Marker in Patients with Early Breast Cancer and Its Association with Clinicopathological Factors. Oncology 2021, 99, 780–789. [Google Scholar] [CrossRef]
- Erić, I.; Petek Erić, A.; Kristek, J.; Koprivčić, I.; Babić, M. Breast cancer in young women: Pathologic and immunohistochemical features. Acta Clin. Croat. 2018, 57, 497–502. [Google Scholar] [CrossRef]
- Boughey, J.C.; Hoskin, T.L.; Goetz, M.P. Neoadjuvant Chemotherapy and Nodal Response Rates in Luminal Breast Cancer: Effects of Age and Tumor Ki67. Ann. Surg. Oncol. 2022, 1–10. [Google Scholar] [CrossRef]
- Bauer, E.; Levy, M.S.; Domachevsky, L.; Anaby, D.; Nissan, N. Background Parenchymal Enhancement and Uptake as Breast Cancer Imaging Biomarkers: A State-of-the-Art Review. Clin. Imaging 2022, 83, 41–50. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, W.K.; Shin, H.J.; Cha, J.H.; Chae, E.Y.; Kim, H.H. Evaluation of the Tumor Response After Neoadjuvant Chemotherapy in Breast Cancer Patients: Correlation Between Dynamic Contrast-Enhanced Magnetic Resonance Imaging and Pathologic Tumor Cellularity. Clin. Breast Cancer 2018, 18, e115–e121. [Google Scholar] [CrossRef]
- Barra, F.R.; Sobrinho, A.B.; Barra, R.R.; Magalhães, M.T.; Aguiar, L.R.; de Albuquerque, G.F.L.; Costa, R.P.; Farage, L.; Pratesi, R. Contrast-Enhanced Mammography (CEM) for Detecting Residual Disease after Neoadjuvant Chemotherapy: A Comparison with Breast Magnetic Resonance Imaging (MRI). Biomed Res. Int. 2018, 2018, 8531916. [Google Scholar] [CrossRef]
- Hartley, R.L.; Stone, J.P.; Temple-Oberle, C. Breast Cancer in Transgender Patients: A Systematic Review. Part 1: Male to Female. Eur. J. Surg. Oncol. 2018, 44, 1455–1462. [Google Scholar] [CrossRef]
- Fanizzi, A.; Basile, T.M.A.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Fausto, A.; Massafra, R.; Moschetta, M.; et al. A Machine Learning Approach on Multiscale Texture Analysis for Breast Microcalcification Diagnosis. BMC Bioinform. 2020, 21, 91. [Google Scholar] [CrossRef]
- Bove, S.; Comes, M.C.; Lorusso, V.; Cristofaro, C.; Didonna, V.; Gatta, G.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.I.; et al. A Ultrasound-Based Radiomic Approach to Predict the Nodal Status in Clinically Negative Breast Cancer Patients. Sci. Rep. 2022, 12, 7914. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Newstead, G. Controversies in Breast MRI. Radiologia 2010, 10, 26–29. [Google Scholar]
- La Forgia, D.; Fanizzi, A.; Campobasso, F.; Bellotti, R.; Didonna, V.; Lorusso, V.; Moschetta, M.; Massafra, R.; Tamborra, P.; Tangaro, S.; et al. Radiomic Analysis in Contrast-Enhanced Spectral Mammography for Predicting Breast Cancer Histological Outcome. Diagnostics 2020, 10, 708. [Google Scholar] [CrossRef]
- Massafra, R.; Bove, S.; Lorusso, V.; Biafora, A.; Comes, M.C.; Didonna, V.; Diotaiuti, S.; Fanizzi, A.; Nardone, A.; Nolasco, A.; et al. Radiomic Feature Reduction Approach to Predict Breast Cancer by Contrast-Enhanced Spectral Mammography Images. Diagnostics 2021, 11, 684. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of Radiomics in Breast Cancer Diagnosis and Prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef]
- Li, H.; Mendel, K.R.; Lan, L.; Sheth, D.; Giger, M.L. Digital Mammography in Breast Cancer: Additive Value of Radiomics of Breast Parenchyma. Radiology 2019, 291, 15–20. [Google Scholar] [CrossRef]
- Comes, M.C.; Fanizzi, A.; Bove, S.; Didonna, V.; Diotaiuti, S.; La Forgia, D.; Latorre, A.; Martinelli, E.; Mencattini, A.; Nardone, A.; et al. Early Prediction of Neoadjuvant Chemotherapy Response by Exploiting a Transfer Learning Approach on Breast DCE-MRIs. Sci. Rep. 2021, 11, 14123. [Google Scholar] [CrossRef]
| Characteristic | Description | Proportion (%) |
|---|---|---|
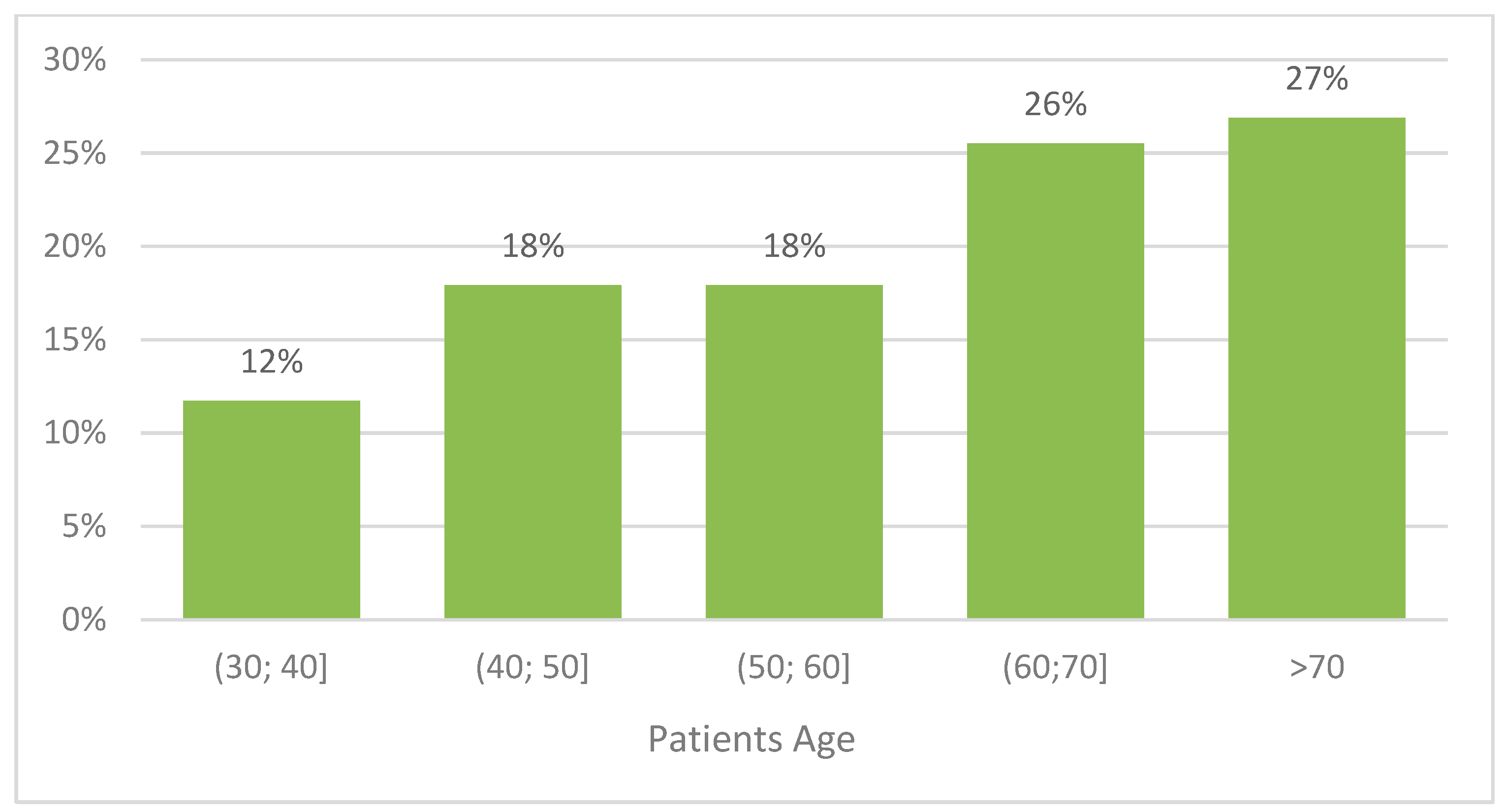
| Age | <45 years | 34/145 23% |
| ≥45 years | 111/145 77% | |
| Histological type | DCIS | 15/145 10% |
| Ca lobular | 21/145 14% | |
| Ca NST | 109/145 75% | |
| Malignancy | No | 15/145 10% |
| Yes | 130/145 90% | |
| Molecular subtypes | Luminal | 118/145 81% |
| -Luminal A | 73/145 50% | |
| -Luminal B | 45/145 31% | |
| HER2, TNBC | 27/145 18% | |
| -HER2 | 9/145 6% | |
| -TNBC | 18/145 12% | |
| ER | Negative | 28/145 19% |
| Positive | 117/145 81% | |
| PR | Negative | 33/145 23% |
| Positive | 112/145 77% | |
| HER2 | Negative | 98/130 75% |
| Positive | 32/130 25% | |
| Ki67 | Low | 63/109 58% |
| High (>20%) | 46/109 42% | |
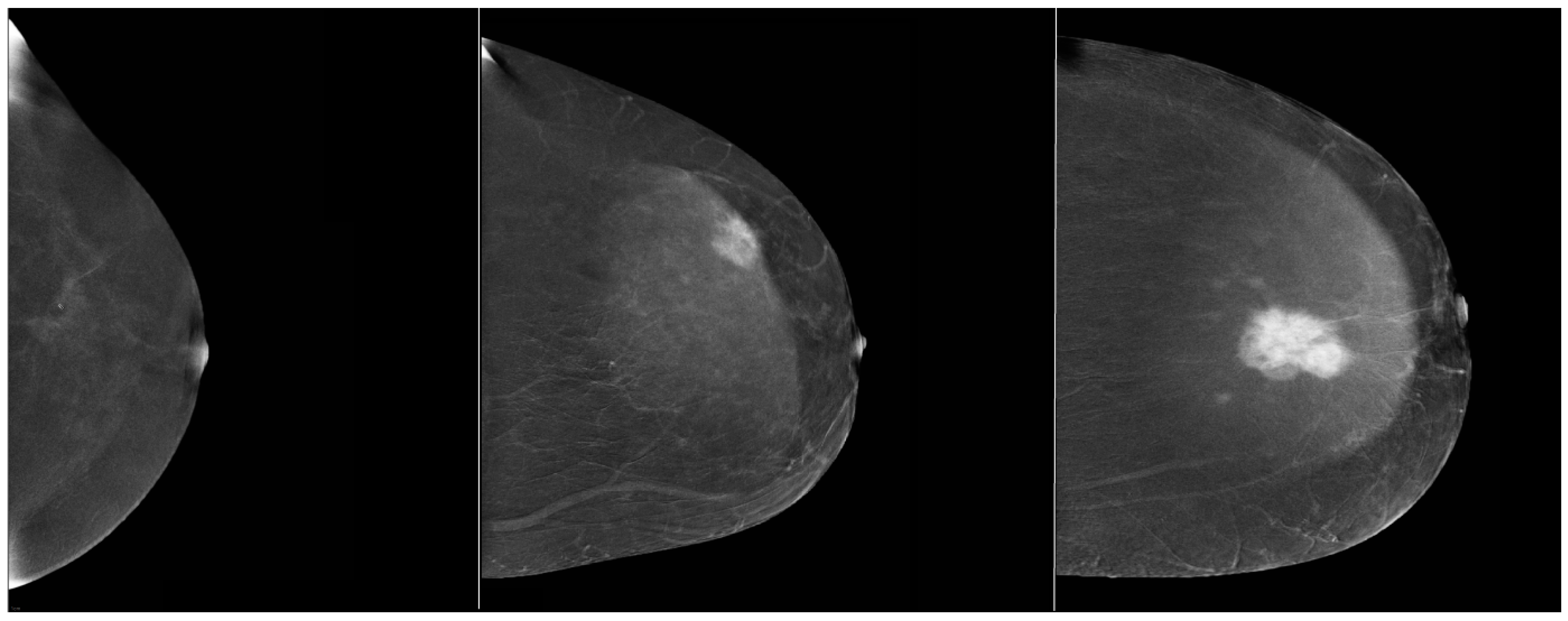
| Enhancement degree | Weak | 19/145 13% |
| Medium | 46/145 32% | |
| Strong | 80/145 55% |
| Continuous Variables | ER+ (N = 117) | ER- (N = 28) | p Value | PR+ (N = 112) | PR- (N = 33) | p Value |
|---|---|---|---|---|---|---|
| Age | 63 (48, 72) | 57.5 (42, 69) | 0.144 | 63 (48, 71.8) | 59 (42, 70) | 0.230 |
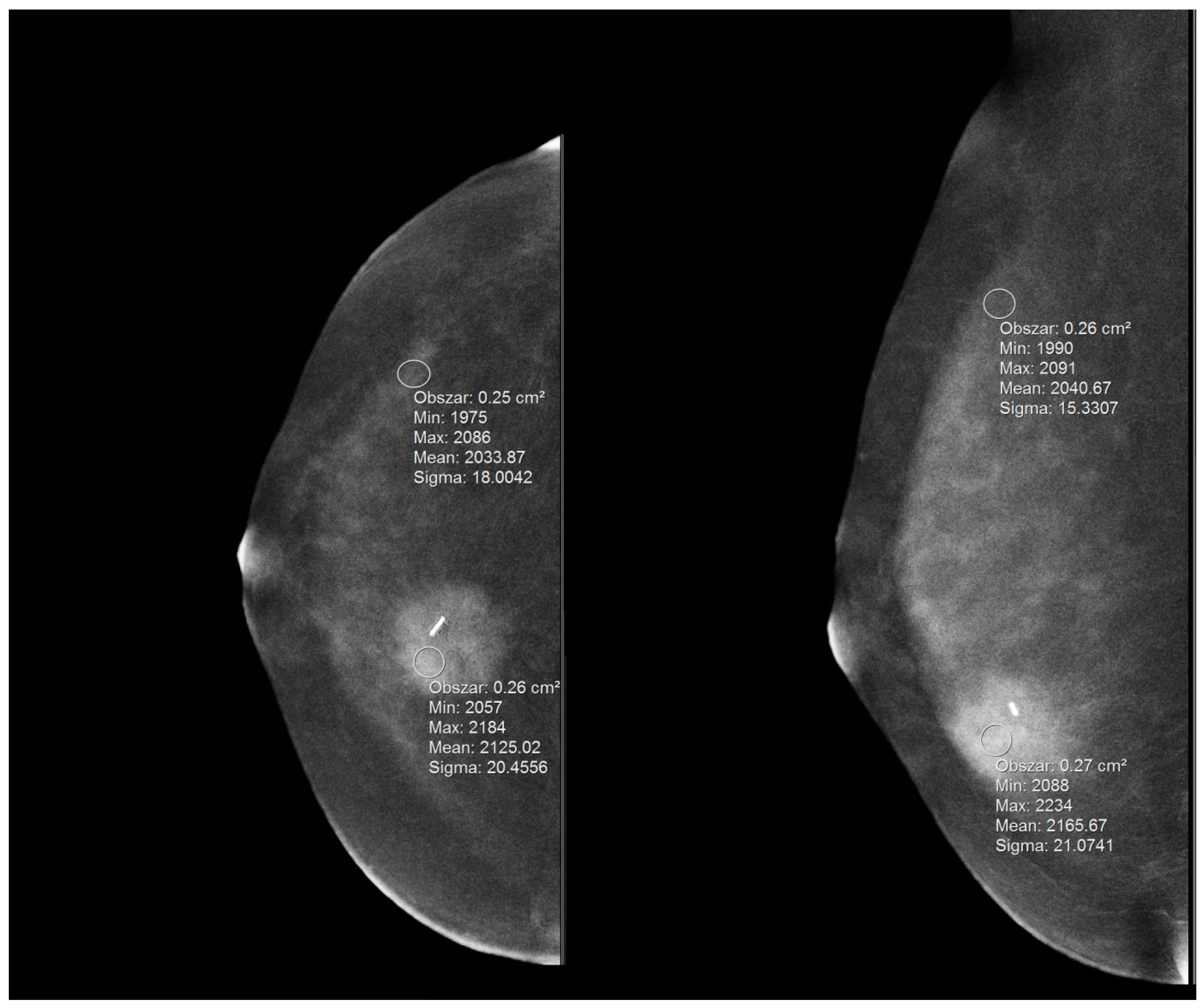
| Mean Enhancement | 2089.5 (2055.8, 2129.5) | 2091.3 (2071.8, 2115.8) | 0.397 | 2092.3 (2056, 2130.8) | 2090.5 (2060.5, 2111.5) | 0.354 |
| Mean BPE | 2020.9 (2014, 2035.6) | 2029.3 (2019.5, 2039.6) | 0.014 | 2020.7 (2014, 2034.2) | 2032.2 (2020.5, 2039.2) | 0.004 |
| SDNR_CC | 4.4 (1.8, 6.9) | 3.1 (1.2, 4.8) | 0.016 | 4.6 (1.8, 6.9) | 2.9 (1.2, 4.5) | 0.005 |
| SDNR_MLO | 4.3 (1.9, 6.8) | 5.2 (2.5, 6.9) | 0.221 | 4.5 (1.8, 7.1) | 4.1 (2.5, 6.5) | 0.368 |
| SDNR | 4.5 (2.2, 6.9) | 3.8 (2.3, 5.4) | 0.228 | 4.5 (2.2, 7) | 3.1 (2.2, 5.3) | 0.095 |
| %RS_CC | 3.2 (1.3, 5.2) | 2.4 (0.8, 3.7) | 0.026 | 3.4 (1.3, 5.2) | 2.1 (0.8, 3.4) | 0.005 |
| %RS_MLO | 3.3 (1.5, 5.1) | 3.9 (1.9, 4.9) | 0.179 | 3.4 (1.4, 5.3) | 3.4 (1.7, 4.8) | 0.390 |
| %RS | 3.4 (1.6, 5) | 2.8 (1.8, 3.8) | 0.265 | 3.5 (1.7, 5) | 2.6 (1.6, 3.8) | 0.085 |
| Quantitative Variable | HER+ (N = 32) | HER- (N = 98) | ϕ | p Value | Ki-67 (High) (N = 46) | Ki-67 (Low) (N = 63) | ϕ | p Value |
|---|---|---|---|---|---|---|---|---|
| Age | 0.07 | 0.59 | 0.28 | 0.006 | ||||
| <45 ≥45 | 9/32 28% 23/32 72% | 21/98 21% 77/98 79% | 18/46 39% 28/46 61% | 9/63 14% 54/63 86% |
| Continuous Variables | Luminal Subtype (N = 118) | Non-Luminal Subtype (N = 27) | p Value |
|---|---|---|---|
| Age | 63 (48, 72) | 56 (40, 69) | 0.107 |
| Mean Enhancement | 2090.5 (2056, 2130.3) | 2091 (2057.5, 2112) | 0.47 |
| Mean BPE | 2020.9 (2014, 2035.5) | 2032 (2018.5, 2039.6) | 0.012 |
| SDNR_CC | 4.5 (1.8, 6.8) | 2.9 (1, 4.8) | 0.011 |
| SDNR_MLO | 4.5 (1.9, 6.9) | 4.1 (2.4, 6.4) | 0.457 |
| SDNR | 4.5 (2.2, 6.9) | 3.1 (2.2, 5.4) | 0.107 |
| %RS_CC | 3.3 (1.3, 5.2) | 2.3 (0.7, 3.6) | 0.013 |
| %RS_MLO | 3.4 (1.5, 5.3) | 3.4 (1.8, 4.9) | 0.37 |
| %RS | 3.4 (1.7, 5) | 2.7 (1.7, 3.8) | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luczynska, E.; Piegza, T.; Szpor, J.; Heinze, S.; Popiela, T.; Kargol, J.; Rudnicki, W. Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes. Biomedicines 2022, 10, 2384. https://doi.org/10.3390/biomedicines10102384
Luczynska E, Piegza T, Szpor J, Heinze S, Popiela T, Kargol J, Rudnicki W. Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes. Biomedicines. 2022; 10(10):2384. https://doi.org/10.3390/biomedicines10102384
Chicago/Turabian StyleLuczynska, Elzbieta, Tomasz Piegza, Joanna Szpor, Sylwia Heinze, Tadeusz Popiela, Jaromir Kargol, and Wojciech Rudnicki. 2022. "Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes" Biomedicines 10, no. 10: 2384. https://doi.org/10.3390/biomedicines10102384
APA StyleLuczynska, E., Piegza, T., Szpor, J., Heinze, S., Popiela, T., Kargol, J., & Rudnicki, W. (2022). Contrast-Enhanced Mammography (CEM) Capability to Distinguish Molecular Breast Cancer Subtypes. Biomedicines, 10(10), 2384. https://doi.org/10.3390/biomedicines10102384







