Antiphospholipid Antibody Syndrome-Associated Increased Surface Expression of VLA4 Integrin on Human Monocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Mining
2.2. Study Design and Participants
2.3. Antiphospholipid Antibodies Determination
2.4. Purification of Serum aPL
2.5. Whole Blood Staining
2.6. Cell Experiments
2.6.1. Purification of Human Monocytes from Healthy Controls
2.6.2. In Vitro Stimulation of Monocytes from Healthy Controls with IgG Fractions
2.6.3. Flow Cytometry
2.6.4. Soluble TNF-α ELISA and IL-6 ELISA
2.7. Statistical Analysis
3. Results
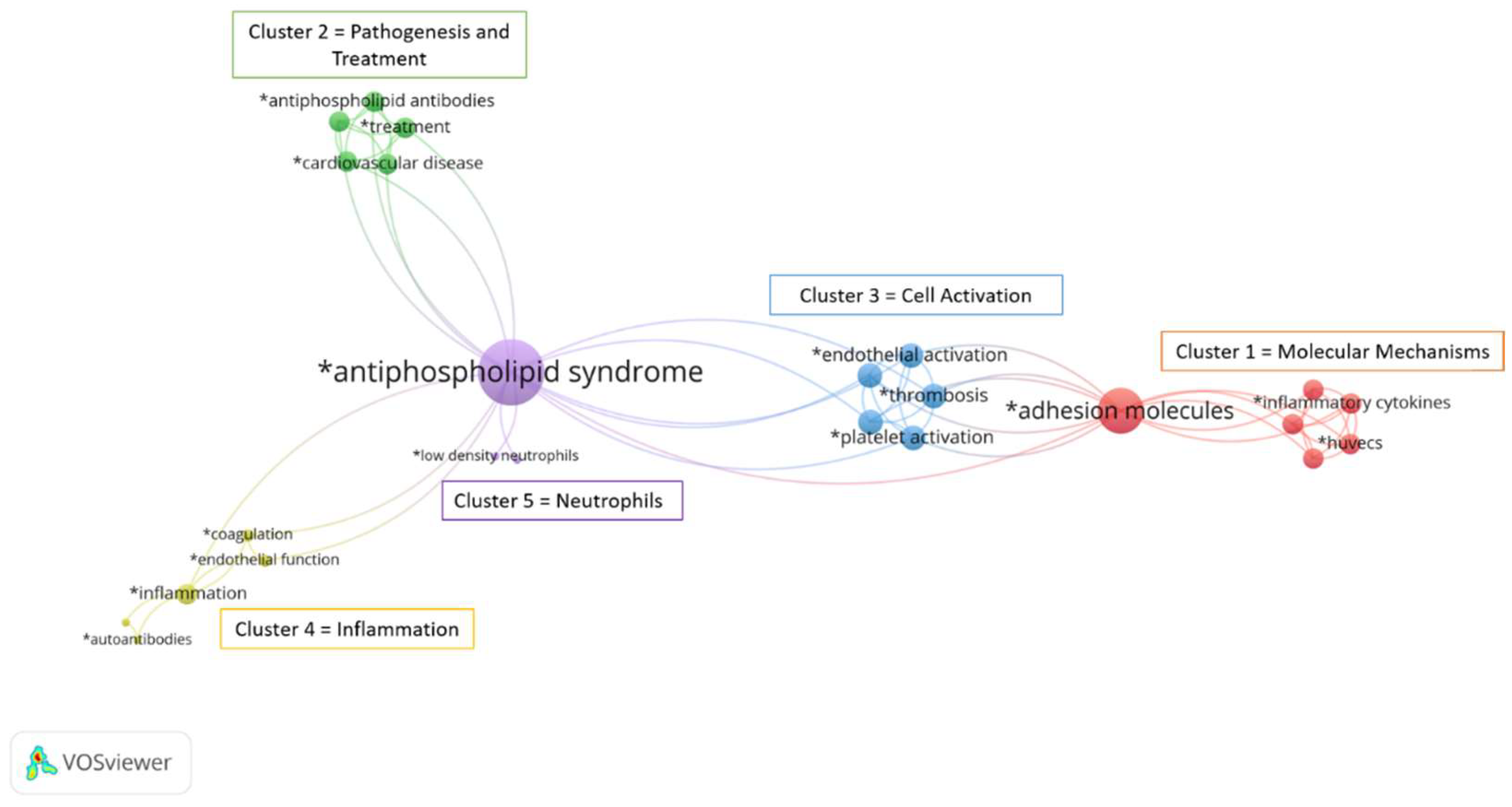
3.1. Bibliographic Analysis
3.2. Demographic and Clinical Characteristics of APS Patients and Healthy Controls
3.3. Identification of Monocyte Subsets in Whole Blood
3.4. Differential Expression of Surface Adhesion Molecules on Total Monocytes and Monocyte Subsets
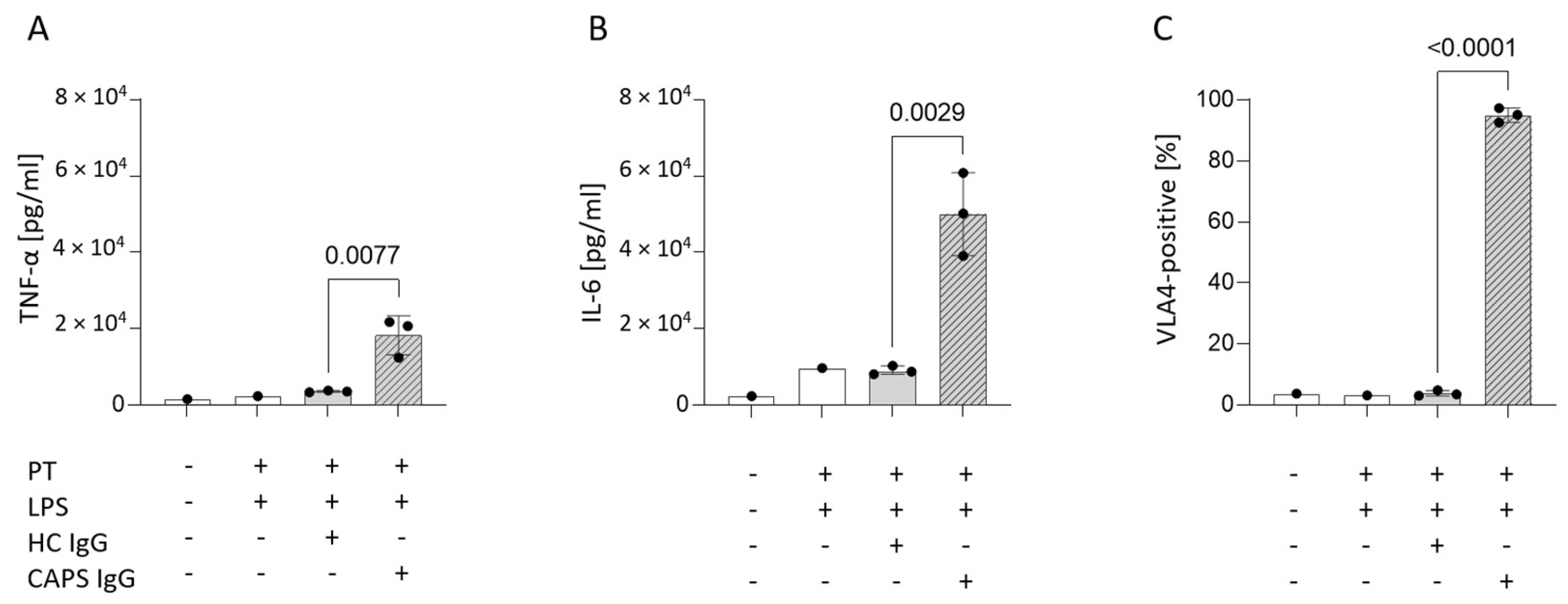
3.5. Mimicking Catastrophic APS In Vitro Significantly Increases Surface VLA4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; PG, D.E.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.; Pattanaik, D. Cellular and Molecular Mechanisms of Anti-Phospholipid Syndrome. Front. Immunol. 2018, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Žigon, P.; Čučnik, S.; Ambrožič, A.; Kveder, T.; Šemrl, S.S.; Rozman, B.; Božič, B. Detection of antiphosphatidylserine/prothrombin antibodies and their potential diagnostic value. Clin. Dev. Immunol. 2013, 2013, 724592. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, S.; Xie, Z.; You, H.; Jiang, H.; Shi, Y.; Qi, W.; Zhao, J.; Wang, Q.; Tian, X.; et al. Evaluation of the Diagnostic Value of Non-criteria Antibodies for Antiphospholipid Syndrome Patients in a Chinese Cohort. Front. Immunol. 2021, 12, 741369. [Google Scholar] [CrossRef]
- Cervera, R.; Rodríguez-Pintó, I.; Espinosa, G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: A comprehensive review. J. Autoimmun. 2018, 92, 1–11. [Google Scholar] [CrossRef]
- Knight, J.S.; Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Sorice, M.; Longo, A.; Capozzi, A.; Garofalo, T.; Misasi, R.; Alessandri, C.; Conti, F.; Buttari, B.; Rigano, R.; Ortona, E.; et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007, 56, 2687–2697. [Google Scholar] [CrossRef]
- Xie, H.; Zhou, H.; Wang, H.; Chen, D.; Xia, L.; Wang, T.; Yan, J. Anti-β(2)GPI/β(2)GPI induced TF and TNF-α expression in monocytes involving both TLR4/MyD88 and TLR4/TRIF signaling pathways. Mol. Immunol. 2013, 53, 246–254. [Google Scholar] [CrossRef]
- Müller-Calleja, N.; Köhler, A.; Siebald, B.; Canisius, A.; Orning, C.; Radsak, M.; Stein, P.; Mönnikes, R.; Lackner, K.J. Cofactor-independent antiphospholipid antibodies activate the NLRP3-inflammasome via endosomal NADPH-oxidase: Implications for the antiphospholipid syndrome. Thromb. Haemost. 2015, 113, 1071–1083. [Google Scholar] [CrossRef]
- Jajoria, P.; Murthy, V.; Papalardo, E.; Romay-Penabad, Z.; Gleason, C.; Pierangeli, S.S. Statins for the treatment of antiphospholipid syndrome? Ann. N. Y. Acad. Sci. 2009, 1173, 736–745. [Google Scholar] [CrossRef]
- Dobado-Berrios, P.M.; López-Pedrera, C.; Velasco, F.; Aguirre, M.A.; Torres, A.; Cuadrado, M.J. Increased levels of tissue factor mRNA in mononuclear blood cells of patients with primary antiphospholipid syndrome. Thromb. Haemost. 1999, 82, 1578–1582. [Google Scholar]
- López-Pedrera, C.; Buendía, P.; Cuadrado, M.J.; Siendones, E.; Aguirre, M.A.; Barbarroja, N.; Montiel-Duarte, C.; Torres, A.; Khamashta, M.; Velasco, F. Antiphospholipid antibodies from patients with the antiphospholipid syndrome induce monocyte tissue factor expression through the simultaneous activation of NF-kappaB/Rel proteins via the p38 mitogen-activated protein kinase pathway, and of the MEK-1/ERK pathway. Arthritis Rheum. 2006, 54, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.S.; Cho, M.L.; Chen, P.P.; Min, S.Y.; Hwang, S.Y.; Park, K.S.; Kim, W.U.; Min, D.J.; Min, J.K.; Park, S.H.; et al. Antiphospholipid antibodies induce monocyte chemoattractant protein-1 in endothelial cells. J. Immunol. 2002, 168, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McCrae, K.R. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2005, 105, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Clemens, N.; Frauenknecht, K.; Katzav, A.; Sommer, C.; von Landenberg, P. In vitro effects of antiphospholipid syndrome-IgG fractions and human monoclonal antiphospholipid IgG antibody on human umbilical vein endothelial cells and monocytes. Ann. N. Y. Acad. Sci. 2009, 1173, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Morrell, C.N.; Tarango, C.; Thomas, G.D.; Yuhanna, I.S.; Girardi, G.; Herz, J.; Urbanus, R.T.; de Groot, P.G.; Thorpe, P.E.; et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J. Clin. Investig. 2011, 121, 120–131. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- McEver, R.P. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 2001, 86, 746–756. [Google Scholar] [CrossRef]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Merah-Mourah, F.; Cohen, S.O.; Charron, D.; Mooney, N.; Haziot, A. Identification of Novel Human Monocyte Subsets and Evidence for Phenotypic Groups Defined by Interindividual Variations of Expression of Adhesion Molecules. Sci. Rep. 2020, 10, 4397. [Google Scholar] [CrossRef]
- Tolouei Semnani, R.; Moore, V.; Bennuru, S.; McDonald-Fleming, R.; Ganesan, S.; Cotton, R.; Anuradha, R.; Babu, S.; Nutman, T.B. Human Monocyte Subsets at Homeostasis and Their Perturbation in Numbers and Function in Filarial Infection. Infect. Immun. 2014, 82, 4438–4446. [Google Scholar] [CrossRef] [Green Version]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14++CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kanti Barman, P.; Kumar Thatoi, P.; Tripathy, R.; Kumar Das, B.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Smedman, C.; Ernemar, T.; Gudmundsdotter, L.; Gille-Johnson, P.; Somell, A.; Nihlmark, K.; Gårdlund, B.; Andersson, J.; Paulie, S. FluoroSpot Analysis of TLR-Activated Monocytes Reveals Several Distinct Cytokine-Secreting Subpopulations. Scand. J. Immunol. 2012, 75, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, L.; Patiño-Trives, A.M.; Aguirre-Zamorano, M.Á.; Luque-Tévar, M.; Ábalos-Aguilera, M.C.; Arias-De La Rosa, I.; Seguí, P.; Velasco-Gimena, F.; Barbarroja, N.; Escudero-Contreras, A.; et al. Characterization of Antiphospholipid Syndrome Atherothrombotic Risk by Unsupervised Integrated Transcriptomic Analyses. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 865–877. [Google Scholar] [CrossRef]
- Bozic, B.; Kveder, T.; Stegnar, M.; Morosini-Berus, E.; Kos-Golja, M.; Peternel, P.; Rozman, B. Influence of degraded phosphatidylserine on binding of antiphospholipid antibodies. Int. Arch. Allergy Immunol. 1997, 112, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cucnik, S. Binding of high-avidity anti- 2-glycoprotein I antibodies. Rheumatology 2004, 43, 1353–1356. [Google Scholar] [CrossRef]
- Reber, G.; Schousboe, I.; Tincani, A.; Sanmarco, M.; Kveder, T.; de Moerloose, P.; Boffa, M.C.; Arvieux, J. Inter-laboratory variability of anti-beta2-glycoprotein I measurement. A collaborative study in the frame of the European Forum on Antiphospholipid Antibodies Standardization Group. Thromb. Haemost. 2002, 88, 66–73. [Google Scholar]
- Zigon, P.; Ambrozic, A.; Cucnik, S.; Kveder, T.; Rozman, B.; Bozic, B. Modified phosphatidylserine-dependent antiprothrombin ELISA enables identification of patients negative for other antiphospholipid antibodies and also detects low avidity antibodies. Clin. Chem. Lab. Med. 2011, 49, 1573. [Google Scholar]
- Bontadi, A.; Ruffatti, A.; Falcinelli, E.; Giannini, S.; Marturano, A.; Tonello, M.; Hoxha, A.; Pengo, V.; Punzi, L.; Momi, S.; et al. Platelet and endothelial activation in catastrophic and quiescent antiphospholipid syndrome. Thromb. Haemost. 2013, 109, 901–908. [Google Scholar] [CrossRef]
- Kaplanski, G.; Cacoub, P.; Farnarier, C.; Marin, V.; Grégoire, R.; Gatel, A.; Durand, J.M.; Harlé, J.R.; Bongrand, P.; Piette, J.C. Increased soluble vascular cell adhesion molecule 1 concentrations in patients with primary or systemic lupus erythematosus-related antiphospholipid syndrome: Correlations with the severity of thrombosis. Arthritis Rheum. 2000, 43, 55–64. [Google Scholar] [CrossRef]
- Guagnozzi, D.; Caprilli, R. Natalizumab in the treatment of Crohn’s disease. Biologics 2008, 2, 275–284. [Google Scholar]
- Khoy, K.; Mariotte, D.; Defer, G.; Petit, G.; Toutirais, O.; Le Mauff, B. Natalizumab in Multiple Sclerosis Treatment: From Biological Effects to Immune Monitoring. Front. Immunol. 2020, 11, 549842. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Meng, H.; Coit, P.; Yalavarthi, S.; Sule, G.; Gandhi, A.A.; Grenn, R.C.; Mazza, L.F.; Ali, R.A.; Renauer, P.; et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight 2017, 2, e93897. [Google Scholar] [CrossRef]
- Diz-Kucukkaya, R.; Inanc, M.; Afshar-Kharghan, V.; Zhang, Q.E.; Lopez, J.A.; Pekcelen, Y. P-selectin glycoprotein ligand-1 VNTR polymorphisms and risk of thrombosis in the antiphospholipid syndrome. Ann. Rheum. Dis. 2007, 66, 1378–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| HC (n = 19) | APS (n = 20) | |
|---|---|---|
| Demographics | ||
| Age, median (IQR) | 43 (23) | 43 (18) |
| Sex, n (% female) | 10 (52%) | 11 (55%) |
| BMI, median (IQR) | 25.3 (4.6) | 25.9 (4.8) |
| Clinical data, n (%) | ||
| Arterial thrombosis | N/A | 6 (30%) |
| Venous thrombosis | N/A | 11 (55%) |
| Obstetric complications | N/A | 3 (15%) |
| Diabetes | N/A | 0 (0%) |
| Hyperlipidaemia | N/A | 9 (45%) |
| Hypertension | N/A | 5 (25%) |
| Therapy, n (%) | ||
| Anticoagulation therapy | N/A | 16 (80%) |
| Antiaggregation therapy | N/A | 2 (10%) |
| Antimalarial medication | N/A | 4 (20%) |
| Laboratory features, n (%) | ||
| aCL Ig (G/M/A), n (%) | N/A | 14 (70%) |
| IgG (mean AU ± SD), <10 AU neg | 3 | 19.9 ± 12.0 |
| IgM (AU ± SD), <10 AU neg | 3 | 15.2 ± 10.2 |
| IgA (AU ± SD), <10 AU neg | 3 | 5.9 ± 5.9 |
| anti-β2GPI Ig(G/M/A), n (%) | N/A | 13 (65%) |
| IgG (AU ± SD), <2 AU neg | 1 | 10.4 ± 7.2 |
| IgM (AU ± SD), <2 AU neg | 1 | 2.9 ± 3.0 |
| IgA (AU ± SD), <2 AU neg | 1 | 2.1 ± 1.8 |
| aPS/PT Ig(G/M/A), n (%) | N/A | 10 (50%) |
| IgG (AU ± SD), <5 AU neg | 3 | 39.5 ± 44.3 |
| IgM (AU ± SD), <5 AU neg | 3 | 22.4 ± 31.6 |
| IgA (AU ± SD), <5 AU neg | 3 | 5.1 ± 4.3 |
| Single aPL positive | N/A | 4 (20%) |
| Double aPL positive | N/A | 6 (30%) |
| Triple aPL positive | N/A | 10 (50%) |
| LA positive | N/A | 17 (85%) |
| Median % Positive (IQR) | ||||
|---|---|---|---|---|
| Molecule | HC (n = 19) | APS (n = 20) | p-Value | |
| Total monocytes | LFA1 | 100 (0.2) | 100 (0.0) | 0.149 |
| MAC1 | 98.9 (2.1) | 98.7 (2.2) | 0.647 | |
| VLA4 | 28.8 (16.4) | 40.4 (26.6) | 0.070 | |
| L-selectin | 89.9 (51.7) | 85.4 (57.9) | 0.967 | |
| PSGL1 | 99.3 (1.5) | 98.6 (1.5) | 0.184 | |
| Classical subset | LFA1 | 100 (0) | 100 (0) | 0.967 |
| MAC1 | 99.9 (0.1) | 99.9 (0.1) | 0.175 | |
| VLA4 | 23 (14) | 38.6 (31.2) | * 0.038 | |
| L-selectin | 94 (56.2) | 91.6 (61.7) | 0.647 | |
| PSGL1 | 99.9 (0.2) | 99.9 (0.4) | 0.647 | |
| Intermediate subset | LFA1 | 100 (0.0) | 100 (0.0) | 1000 |
| MAC1 | 100 (0.3) | 99.8(1.1) | 0.115 | |
| VLA4 | 64.2 (30.4) | 84.1 (28.4) | * 0.038 | |
| L-selectin | 72.8 (28.9) | 62.1 (59.5) | 0.184 | |
| PSGL1 | 100 (0.4) | 99.8 (1.1) | 0.247 | |
| Non-classical subset | LFA1 | 100 (0.9) | 100 (0.1) | 0.322 |
| MAC1 | 94.2 (18.4) | 88.8 (12.8) | 0.380 | |
| VLA4 | 39 (36.6) | 36.5 (42) | 0.923 | |
| L-selectin | 27.2 (25.5) | 32.1 (38.3) | 0.336 | |
| PSGL1 | 90.7 (13.7) | 87.5 (16.8) | 0.258 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štok, U.; Štucin, N.; Blokar, E.; Ambrožič, A.; Sodin-Šemrl, S.; Čučnik, S.; Žigon, P. Antiphospholipid Antibody Syndrome-Associated Increased Surface Expression of VLA4 Integrin on Human Monocytes. Biomedicines 2022, 10, 2341. https://doi.org/10.3390/biomedicines10102341
Štok U, Štucin N, Blokar E, Ambrožič A, Sodin-Šemrl S, Čučnik S, Žigon P. Antiphospholipid Antibody Syndrome-Associated Increased Surface Expression of VLA4 Integrin on Human Monocytes. Biomedicines. 2022; 10(10):2341. https://doi.org/10.3390/biomedicines10102341
Chicago/Turabian StyleŠtok, Ula, Neža Štucin, Elizabeta Blokar, Aleš Ambrožič, Snežna Sodin-Šemrl, Saša Čučnik, and Polona Žigon. 2022. "Antiphospholipid Antibody Syndrome-Associated Increased Surface Expression of VLA4 Integrin on Human Monocytes" Biomedicines 10, no. 10: 2341. https://doi.org/10.3390/biomedicines10102341
APA StyleŠtok, U., Štucin, N., Blokar, E., Ambrožič, A., Sodin-Šemrl, S., Čučnik, S., & Žigon, P. (2022). Antiphospholipid Antibody Syndrome-Associated Increased Surface Expression of VLA4 Integrin on Human Monocytes. Biomedicines, 10(10), 2341. https://doi.org/10.3390/biomedicines10102341






