Role of Tafazzin in Mitochondrial Function, Development and Disease
Abstract
1. Introduction
2. Tafazzin as the Genetic Cause of Barth Syndrome
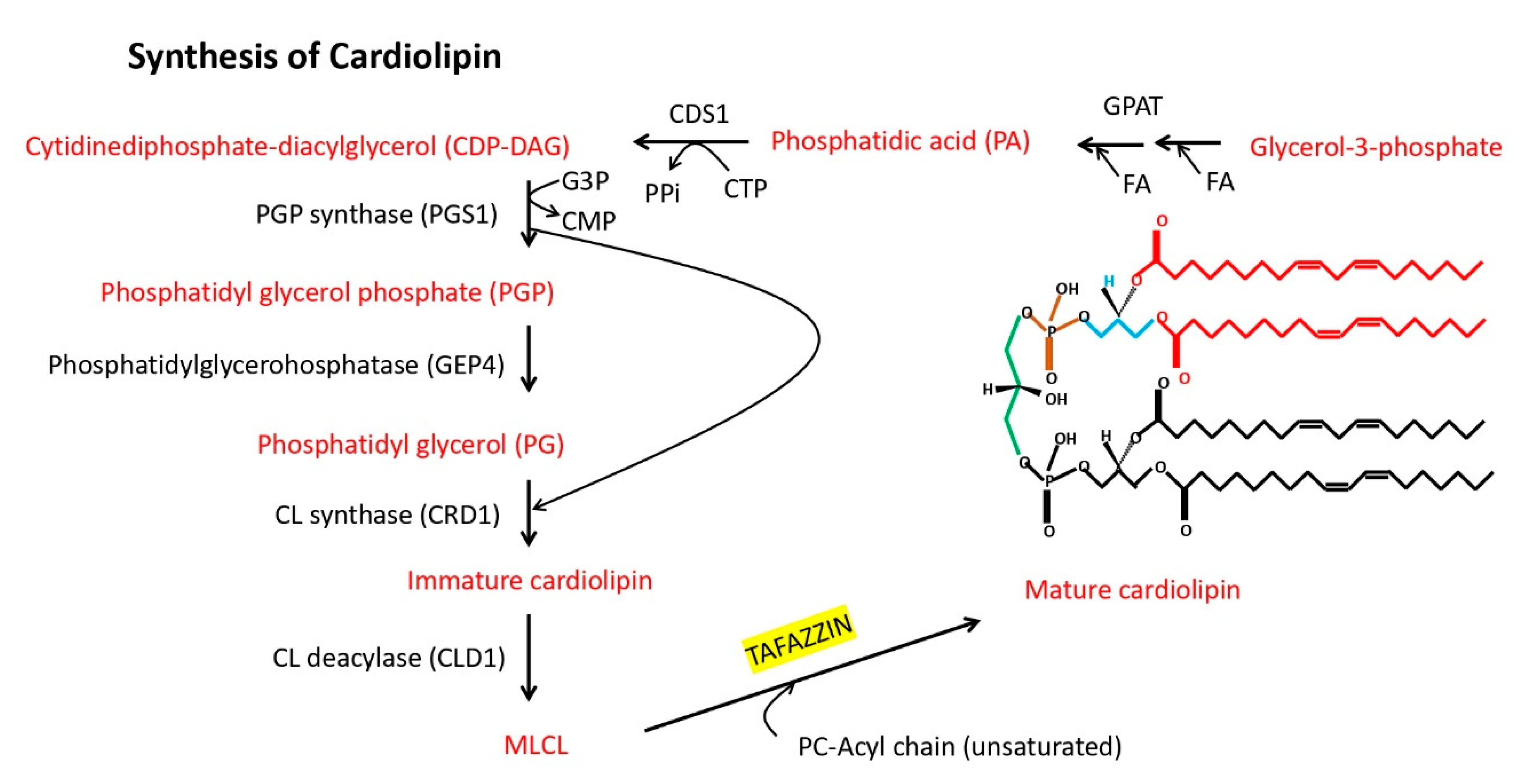
3. Tafazzin Is a Conserved, Genomically Encoded Mitochondrial Transacylase That Is Transported into the Mitochondria and Remodels Cardiolipin
4. Tafazzin Is a Regulator of Mitochondrial Structure and Function
5. The Role of Tafazzin in Cellular Differentiation and Development
6. The Role of Tafazzin in Barth Syndrome, Non-Inherited Diseases and Potential Therapies in Development
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Van der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.; Van’t Veer-Korthof, E.T.; Van der Harten, J.J.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef]
- Barth, P.G.; Van den Bogert, C.; Bolhuis, P.A.; Scholte, H.R.; van Gennip, A.H.; Schutgens, R.B.; Ketel, A.G. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): Respiratory-chain abnormalities in cultured fibroblasts. J. Inherit. Metab. Dis. 1996, 19, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Bione, S.; D’Adamo, P.; Maestrini, E.; Gedeon, A.K.; Bolhuis, P.A.; Toniolo, D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 1996, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Kelher, M.R.; Lee, D.P.; Lewin, T.M.; Coleman, R.A.; Choy, P.C.; Hatch, G.M. Complex expression pattern of the Barth syndrome gene product Tafazzin in human cell lines and murine tissues. Biochem. Cell. Biol. 2004, 82, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.L. Barth syndrome: TAZ gene mutations, mRNAs, and evolution. Am. J. Med. Genet A. 2005, 134, 409–414. [Google Scholar] [CrossRef]
- Neuwald, A.F. Barth syndrome may be due to an acyltransferase deficiency. Curr. Biol. 1997, 7, R465–R466. [Google Scholar] [CrossRef]
- Hijikata, A.; Yura, K.; Ohara, O.; Go, M. Structural and functional analyses of Barth syndrome-causing mutations and alternative splicing in the Tafazzin acyltransferase domain. Meta. Gene. 2015, 4, 92–106. [Google Scholar] [CrossRef]
- Ma, L.; Vaz, F.M.; Gu, Z.; Wanders, R.J.; Greenberg, M.L. The human TAZ gene complements mitochondrial dysfunction in the yeast taz1Delta mutant. Implications for Barth syndrome. J. Biol. Chem. 2004, 279, 44394–44399. [Google Scholar] [CrossRef]
- Xu, Y.; Condell, M.; Plesken, H.; Edelman-Novemsky, I.; Ma, J.; Ren, M.; Schlame, M. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. USA. 2006, 103, 11584–11588. [Google Scholar] [CrossRef]
- Khuchua, Z.; Yue, Z.; Batts, L.; Strauss, A.W. A zebrafish model of human Barth syndrome reveals the essential role of Tafazzin in cardiac development and function. Circ. Res. 2006, 99, 201–208. [Google Scholar] [CrossRef]
- Lou, W.; Reynolds, C.A.; Li, Y.; Liu, J.; Huttemann, M.; Schlame, M.; Stevenson, D.; Strathdee, D.; Greenberg, M.L. Loss of Tafazzin results in decreased myoblast differentiation in C2C12 cells: A myoblast model of Barth syndrome and cardiolipin deficiency. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Acehan, D.; Vaz, F.; Houtkooper, R.H.; James, J.; Moore, V.; Tokunaga, C.; Kulik, W.; Wansapura, J.; Toth, M.J.; Strauss, A.; et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 2011, 286, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Soustek, M.S.; Falk, D.J.; Mah, C.S.; Toth, M.J.; Schlame, M.; Lewin, A.S.; Byrne, B.J. Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum. Gene Ther. 2011, 22, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Phoon, C.K.L.; Acehan, D.; Schlame, M.; Stokes, D.L.; Edelman-Novemsky, I.; Yu, D.; Xu, Y.; Viswanathan, N.; Ren, M. Tafazzin Knockdown in Mice Leads to a Developmental Cardiomyopathy With Early Diastolic Dysfunction Preceding Myocardial Noncompaction. J. Am. Heart Assoc. 2012, 1, jah3-e000455. [Google Scholar] [CrossRef] [PubMed]
- Cadalbert, L.C.; Ghaffar, F.N.; Stevenson, D.; Bryson, S.; Vaz, F.M.; Gottlieb, E.; Strathdee, D. Mouse Tafazzin Is Required for Male Germ Cell Meiosis and Spermatogenesis. PLoS ONE 2015, 10, e0131066. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Xu, Y.; Erdjument-Bromage, H.; Donelian, A.; Phoon, C.K.L.; Terada, N.; Strathdee, D.; Neubert, T.A.; Schlame, M. Extramitochondrial cardiolipin suggests a novel function of mitochondria in spermatogenesis. J. Cell Biol. 2019, 218, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Xu, Y.; Ma, Q.; Lin, Z.; Schlame, M.; Bezzerides, V.J.; Strathdee, D.; Pu, W.T. AAV Gene Therapy Prevents and Reverses Heart Failure in a Murine Knockout Model of Barth Syndrome. Circ. Res. 2020, 126, 1024–1039. [Google Scholar] [CrossRef]
- Valianpour, F.; Wanders, R.J.; Overmars, H.; Vaz, F.M.; Barth, P.G.; van Gennip, A.H. Linoleic acid supplementation of Barth syndrome fibroblasts restores cardiolipin levels: Implications for treatment. J. Lipid Res. 2003, 44, 560–566. [Google Scholar] [CrossRef]
- Wang, G.; McCain, M.L.; Yang, L.; He, A.; Pasqualini, F.S.; Agarwal, A.; Yuan, H.; Jiang, D.; Zhang, D.; Zangi, L.; et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Vreken, P.; Valianpour, F.; Nijtmans, L.G.; Grivell, L.A.; Plecko, B.; Wanders, R.J.; Barth, P.G. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 2000, 279, 378–382. [Google Scholar] [CrossRef]
- Valianpour, F.; Wanders, R.J.; Overmars, H.; Vreken, P.; Van Gennip, A.H.; Baas, F.; Plecko, B.; Santer, R.; Becker, K.; Barth, P.G. Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): A study in cultured skin fibroblasts. J. Pediatr. 2002, 141, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kelley, R.I.; Blanck, T.J.; Schlame, M. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 2003, 278, 51380–51385. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M.; Towbin, J.A.; Heerdt, P.M.; Jehle, R.; DiMauro, S.; Blanck, T.J. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 2002, 51, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M.; Kelley, R.I.; Feigenbaum, A.; Towbin, J.A.; Heerdt, P.M.; Schieble, T.; Wanders, R.J.; DiMauro, S.; Blanck, T.J. Phospholipid abnormalities in children with Barth syndrome. J. Am. Coll. Cardiol. 2003, 42, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Turkenburg, M.; Poll-The, B.T.; Karall, D.; Perez-Cerda, C.; Morrone, A.; Malvagia, S.; Wanders, R.J.; Kulik, W.; Vaz, F.M. The enigmatic role of Tafazzin in cardiolipin metabolism. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 2003–2014. [Google Scholar] [CrossRef]
- Vaz, F.M.; Houtkooper, R.H.; Valianpour, F.; Barth, P.G.; Wanders, R.J. Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J. Biol. Chem. 2003, 278, 43089–43094. [Google Scholar] [CrossRef]
- Xu, Y.; Malhotra, A.; Ren, M.; Schlame, M. The enzymatic function of Tafazzin. J. Biol. Chem. 2006, 281, 39217–39224. [Google Scholar] [CrossRef]
- Schlame, M.; Acehan, D.; Berno, B.; Xu, Y.; Valvo, S.; Ren, M.; Stokes, D.L.; Epand, R.M. The physical state of lipid substrates provides transacylation specificity for Tafazzin. Nat. Chem. Biol. 2012, 8, 862–869. [Google Scholar] [CrossRef]
- Abe, M.; Hasegawa, Y.; Oku, M.; Sawada, Y.; Tanaka, E.; Sakai, Y.; Miyoshi, H. Mechanism for Remodeling of the Acyl Chain Composition of Cardiolipin Catalyzed by Saccharomyces cerevisiae Tafazzin. J. Biol. Chem. 2016, 291, 15491–15502. [Google Scholar] [CrossRef]
- Schlame, M.; Xu, Y.; Ren, M. The Basis for Acyl Specificity in the Tafazzin Reaction. J. Biol. Chem. 2017, 292, 5499–5506. [Google Scholar] [CrossRef]
- Schlame, M. Cardiolipin remodeling and the function of Tafazzin. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell. Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Esposti, M.; Cristea, I.; Gaskell, S.; Nakao, Y.; Dive, C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 2003, 10, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, S.; Malhotra, A.; Edelman-Novemsky, I.; Ma, J.; Kruppa, A.; Cernicica, C.; Blais, S.; Neubert, T.A.; Ren, M.; et al. Characterization of Tafazzin splice variants from humans and fruit flies. J. Biol. Chem. 2009, 284, 29230–29239. [Google Scholar] [CrossRef] [PubMed]
- Kirwin, S.M.; Manolakos, A.; Barnett, S.S.; Gonzalez, I.L. Tafazzin splice variants and mutations in Barth syndrome. Mol. Genet. Metab. 2014, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; McCaffery, J.M.; Koehler, C.M. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant Tafazzins. J. Cell Biol. 2006, 174, 379–390. [Google Scholar] [CrossRef]
- Claypool, S.M.; Whited, K.; Srijumnong, S.; Han, X.; Koehler, C.M. Barth syndrome mutations that cause Tafazzin complex lability. J. Cell Biol. 2011, 192, 447–462. [Google Scholar] [CrossRef]
- Lu, Y.W.; Galbraith, L.; Herndon, J.D.; Lu, Y.L.; Pras-Raves, M.; Vervaart, M.; Van Kampen, A.; Luyf, A.; Koehler, C.M.; McCaffery, J.M.; et al. Defining functional classes of Barth syndrome mutation in humans. Hum. Mol. Genet. 2016, 25, 1754–1770. [Google Scholar] [CrossRef]
- Whited, K.; Baile, M.G.; Currier, P.; Claypool, S.M. Seven functional classes of Barth syndrome mutation. Hum. Mol. Genet. 2013, 22, 483–492. [Google Scholar] [CrossRef]
- Herndon, J.D.; Claypool, S.M.; Koehler, C.M. The Taz1p transacylase is imported and sorted into the outer mitochondrial membrane via a membrane anchor domain. Eukaryot. Cell. 2013, 12, 1600–1608. [Google Scholar] [CrossRef]
- Dinca, A.A.; Chien, W.M.; Chin, M.T. Identification of novel mitochondrial localization signals in human Tafazzin, the cause of the inherited cardiomyopathic disorder Barth syndrome. J. Mol. Cell. Cardiol. 2018, 114, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sutachan, J.J.; Plesken, H.; Kelley, R.I.; Schlame, M. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Investig. 2005, 85, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Acehan, D.; Khuchua, Z.; Houtkooper, R.H.; Malhotra, A.; Kaufman, J.; Vaz, F.M.; Ren, M.; Rockman, H.A.; Stokes, D.L.; Schlame, M. Distinct effects of Tafazzin deletion in differentiated and undifferentiated mitochondria. Mitochondrion 2009, 9, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shepard, T.H.; Muffley, L.A.; Smith, L.T. Ultrastructural study of mitochondria and their cristae in embryonic rats and primate (N. nemistrina). Anat. Rec. 1998, 252, 383–392. [Google Scholar] [CrossRef]
- Brandner, K.; Mick, D.U.; Frazier, A.E.; Taylor, R.D.; Meisinger, C.; Rehling, P. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: Implications for Barth Syndrome. Mol. Biol. Cell. 2005, 16, 5202–5214. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.; Lazarou, M.; Thorburn, D.R.; Ryan, M.T. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 2006, 361, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Dudek, J.; Cheng, I.F.; Balleininger, M.; Vaz, F.M.; Streckfuss-Bomeke, K.; Hubscher, D.; Vukotic, M.; Wanders, R.J.; Rehling, P.; Guan, K. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 2013, 11, 806–819. [Google Scholar] [CrossRef]
- Chatzispyrou, I.A.; Guerrero-Castillo, S.; Held, N.M.; Ruiter, J.P.N.; Denis, S.W.; Ljlst, L.; Wanders, R.J.; van Weeghel, M.; Ferdinandusse, S.; Vaz, F.M.; et al. Barth syndrome cells display widespread remodeling of mitochondrial complexes without affecting metabolic flux distribution. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3650–3658. [Google Scholar] [CrossRef]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef]
- Kiebish, M.A.; Yang, K.; Liu, X.; Mancuso, D.J.; Guan, S.; Zhao, Z.; Sims, H.F.; Cerqua, R.; Cade, W.T.; Han, X.; et al. Dysfunctional cardiac mitochondrial bioenergetic, lipidomic, and signaling in a murine model of Barth syndrome. J. Lipid. Res. 2013, 54, 1312–1325. [Google Scholar] [CrossRef]
- Dudek, J.; Cheng, I.F.; Chowdhury, A.; Wozny, K.; Balleininger, M.; Reinhold, R.; Grunau, S.; Callegari, S.; Toischer, K.; Wanders, R.J.; et al. Cardiac-specific succinate dehydrogenase deficiency in Barth syndrome. EMBO Mol. Med. 2016, 8, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Q.; Greenberg, M.L. Loss of Tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol. Microbiol. 2008, 68, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.P.; Phoon, C.K.; Aristizábal, O.; McGrath, K.E.; Palis, J.; Turnbull, D.H. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 2003, 92, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Koushik, S.V.; Wang, J.; Rogers, R.; Moskophidis, D.; Lambert, N.A.; Creazzo, T.L.; Conway, S.J. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001, 15, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Hom, J.R.; Quintanilla, R.A.; Hoffman, D.L.; de Mesy Bentley, K.L.; Molkentin, J.D.; Sheu, S.S.; Porter, G.A., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev. Cell. 2011, 21, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.R.; Maxmillian, T.; Le, Q.; Nadtochiy, S.M.; Brookes, P.S.; Porter, G.A., Jr.; Davidson, V.L.; Ebert, S.N. Metabolomics reveals critical adrenergic regulatory checkpoints in glycolysis and pentose-phosphate pathways in embryonic heart. J. Biol. Chem. 2018, 293, 6925–6941. [Google Scholar] [CrossRef]
- Porter, G.A., Jr.; Hom, J.; Hoffman, D.; Quintanilla, R.; de Mesy Bentley, K.; Sheu, S.S. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011, 31, 75–81. [Google Scholar] [CrossRef]
- Beutner, G.; Alanzalon, R.E.; Porter, G.A., Jr. Cyclophilin D regulates the dynamic assembly of mitochondrial ATP synthase into synthasomes. Sci. Rep. 2017, 7, 14488. [Google Scholar] [CrossRef]
- Chatzispyrou, I.A.; Held, N.M.; Mouchiroud, L.; Auwerx, J.; Houtkooper, R.H. Tetracycline Antibiotics Impair Mitochondrial Function and Its Experimental Use Confounds Research. Cancer Res. 2015, 75, 4446–4449. [Google Scholar] [CrossRef]
- Vinet, L.; Rouet-Benzineb, P.; Marniquet, X.; Pellegrin, N.; Mangin, L.; Louedec, L.; Samuel, J.L.; Mercadier, J.J. Chronic doxycycline exposure accelerates left ventricular hypertrophy and progression to heart failure in mice after thoracic aorta constriction. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H352–H360. [Google Scholar] [CrossRef]
- Clarke, S.L.; Bowron, A.; Gonzalez, I.L.; Groves, S.J.; Newbury-Ecob, R.; Clayton, N.; Martin, R.P.; Tsai-Goodman, B.; Garratt, V.; Ashworth, M.; et al. Barth syndrome. Orphanet J. Rare Dis. 2013, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.L.; Forsey, J.; Dudley, D.; Steward, C.G.; Tsai-Goodman, B. Clinical Characteristics and Outcomes of Cardiomyopathy in Barth Syndrome: The UK Experience. Pediatric Cardiol. 2016, 37, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Steward, C.G.; Newbury-Ecob, R.A.; Hastings, R.; Smithson, S.F.; Tsai-Goodman, B.; Quarrell, O.W.; Kulik, W.; Wanders, R.; Pennock, M.; Williams, M.; et al. Barth syndrome: An X-linked cause of fetal cardiomyopathy and stillbirth. Prenat. Diagn. 2010, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Makaryan, V.; Kulik, W.; Vaz, F.M.; Allen, C.; Dror, Y.; Dale, D.C.; Aprikyan, A.A. The cellular and molecular mechanisms for neutropenia in Barth syndrome. Eur. J. Haematol. 2012, 88, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Lax, N.Z.; Gorman, G.S.; Turnbull, D.M. Review: Central nervous system involvement in mitochondrial disease. Neuropathol. Appl. Neurobiol. 2017, 43, 102–118. [Google Scholar] [CrossRef]
- Orstavik, K.H.; Orstavik, R.E.; Naumova, A.K.; D’Adamo, P.; Gedeon, A.; Bolhuis, P.A.; Barth, P.G.; Toniolo, D. X chromosome inactivation in carriers of Barth syndrome. Am. J. Hum. Genet. 1998, 63, 1457–1463. [Google Scholar] [CrossRef]
- Cosson, L.; Toutain, A.; Simard, G.; Kulik, W.; Matyas, G.; Guichet, A.; Blasco, H.; Maakaroun-Vermesse, Z.; Vaillant, M.C.; Le Caignec, C.; et al. Barth syndrome in a female patient. Mol. Genet. Metab. 2012, 106, 115–120. [Google Scholar] [CrossRef]
- Avdjieva-Tzavella, D.M.; Todorova, A.P.; Kathom, H.M.; Ivanova, M.B.; Yordanova, I.T.; Todorov, T.P.; Litvinenko, I.O.; Dasheva-Dimitrova, A.T.; Tincheva, R.S. Barth syndrome in male and female siblings caused by a novel mutation in the TAZ gene. Genet. Couns. 2016, 27, 495–501. [Google Scholar]
- Belmont, J.W. Genetic control of X inactivation and processes leading to X-inactivation skewing. Am. J. Hum. Genet. 1996, 58, 1101–1108. [Google Scholar]
- Sparagna, G.C.; Chicco, A.J.; Murphy, R.C.; Bristow, M.R.; Johnson, C.A.; Rees, M.L.; Maxey, M.L.; McCune, S.A.; Moore, R.L. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid. Res. 2007, 48, 1559–1570. [Google Scholar] [CrossRef]
- Saini-Chohan, H.K.; Holmes, M.G.; Chicco, A.J.; Taylor, W.A.; Moore, R.L.; McCune, S.A.; Hickson-Bick, D.L.; Hatch, G.M.; Sparagna, G.C. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid. Res. 2009, 50, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, K.C.; Sparagna, G.C.; Sucharov, C.C.; Miyamoto, S.D.; Grudis, J.E.; Sobus, R.D.; Hijmans, J.; Stauffer, B.L. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J. Mol. Cell Cardiol. 2014, 74, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Zheng, P.S. Tafazzin (TAZ) promotes the tumorigenicity of cervical cancer cells and inhibits apoptosis. PLoS ONE 2017, 12, e0177171. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Meng, W.J.; Zhang, H.; Gnosa, S.; Nandy, S.K.; Adell, G.; Holmlund, B.; Sun, X.F. Tafazzin protein expression is associated with tumorigenesis and radiation response in rectal cancer: A study of Swedish clinical trial on preoperative radiotherapy. PLoS ONE 2014, 9, e98317. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, A.K.; Xu, M.; Aristizabal Henao, J.J.; Fajardo, V.A.; Hao, Z.; Voisin, V.; Xu, G.W.; Hurren, R.; Kim, S.; MacLean, N.; et al. The Mitochondrial Transacylase, Tafazzin, Regulates AML Stemness by Modulating Intracellular Levels of Phospholipids. Cell Stem Cell 2019, 24, 1007. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Ferrara, P.J.; Verkerke, A.R.P.; Coleman, C.B.; Wentzler, E.J.; Neufer, P.D.; Kew, K.A.; de Castro Bras, L.E.; Funai, K. Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J. Mol. Cell. Cardiol. 2018, 121, 94–102. [Google Scholar] [CrossRef]
- Suzuki-Hatano, S.; Saha, M.; Rizzo, S.A.; Witko, R.L.; Gosiker, B.J.; Ramanathan, M.; Soustek, M.S.; Jones, M.D.; Kang, P.B.; Byrne, B.J.; et al. AAV-Mediated TAZ Gene Replacement Restores Mitochondrial and Cardioskeletal Function in Barth Syndrome. Hum. Gene Ther. 2019, 30, 139–154. [Google Scholar] [CrossRef]
- Suzuki-Hatano, S.; Saha, M.; Soustek, M.S.; Kang, P.B.; Byrne, B.J.; Cade, W.T.; Pacak, C.A. AAV9-TAZ Gene Replacement Ameliorates Cardiac TMT Proteomic Profiles in a Mouse Model of Barth Syndrome. Mol. Ther. Methods Clin. Dev. 2019, 13, 167–179. [Google Scholar] [CrossRef]
- Suzuki-Hatano, S.; Sriramvenugopal, M.; Ramanathan, M.; Soustek, M.; Byrne, B.J.; Cade, W.T.; Kang, P.B.; Pacak, C.A. Increased mtDNA Abundance and Improved Function in Human Barth Syndrome Patient Fibroblasts Following AAV-TAZ Gene Delivery. Int. J. Mol. Sci. 2019, 20, 3416. [Google Scholar] [CrossRef]
- Schlame, M.; Xu, Y. The Function of Tafazzin, a Mitochondrial Phospholipid-Lysophospholipid Acyltransferase. J. Mol. Biol. 2020. pii: S0022-2836(20)30259-X. [Google Scholar] [CrossRef]
| Model Species | Genetic Manipulation | Major Phenotypes |
|---|---|---|
| Saccharomyces cerevisiae (yeast) | Taz1∆ null mutation (no Taz) | temperature-sensitive growth, mitochondrial abnormalities, abnormal cardiolipin remodeling [8] |
| Drosophila melanogaster (fruit fly) | excision of upstream P element in Taz coding region (no Taz) | reduced locomotor activity, mitochondrial abnormalities, cardiolipin deficiency, defective spermatogenesis [9] |
| Danio rerio (zebrafish) | morpholino knockdown of Taz (reduced wildtype Taz) | dose-dependent embryonic lethality, growth retardation, abnormal heart formation and function [10] |
| Mus musculus (mouse) | CRISPR-generated Taz exon 3 knockout in immortalized C2C12 myoblast line (no Taz) | impaired myocyte differentiation, mitochondrial abnormalities, cardiolipin deficiency, increased mitochondrial ROS production [11] |
| Taz short hairpin RNA knockdown (reduced wildtype Taz) | variable male embryonic lethality, developmental growth retardation, mitochondrial abnormalities, cardiolipin deficiency, abnormal heart formation, adult heart failure [12,13,14] | |
| high % Tazwt/TazKO chimeras selected by coat color (reduced wildtype Taz) | male growth retardation, abnormal cardiolipin remodeling, defective spermatogenesis [15,16] | |
| Taz exons 5–10 loxP flanked global knockout (no Taz) | extensive male embryonic and neonatal lethality, growth retardation mitochondrial abnormalities, abnormal cardiolipin remodeling, poor adult cardiac function, cardiac and skeletal muscle defects [9,17] | |
| Taz exons 5–10 loxP flanked cardiomyocyte specfic knockout (no Taz in cardiac myocytes) | normal survival of mutant males, abnormal cardiolipin remodeling, mitochondrial abnormalities, reduced cardiac function, myocardial fibrosis and cardiomyocyte apoptosis [16,17] | |
| Homo sapiens (human) | BTHS male patient skin fibroblasts (mutant Taz present) | abnormal cardiolipin remodeling, mitochondrial abnormalities [18] |
| BTHS male patient induced pluripotent stem cells (iPSCs) (mutant Taz present) | iPSCs-cardiomyocytes exhibit abnormal cardiolipin remodeling, mitochondrial abnormalities, increased ROS production, sarcomere assembly and myocardial contraction abnormalities [19] |
| Common BTHS Patient Phenotypes * | Potential Developmental Origin/s of Phenotypes |
|---|---|
| male miscarriage and stillbirths | zebrafish and male mouse knockdown/knockout studies indicate that Tafazzin deficiency leads to profound cardiac developmental defects incompatible with survival |
| cardiolipin abnormalities and mitochondrial morphological and functional defects | defective CL remodeling results in abnormal embryonic mitochondrial morphogenesis, maturation, numbers, biogenesis and/or function with consequences for organ development |
| growth retardation/short stature | functioning mitochondria are essential for successful fetal development and intrauterine growth. |
| increased levels of 3-methylglutaconic acid in blood/urine | reduced mitochondrial energy production results in 3-methylglutaconic acid accumulation that can lead to metabotoxic effects in developing organs |
| neutropenia (absent to severe; persistent or cyclical) | reduced mitochondrial function affects the myeloid precursors leading to reduced production of mature neutrophils |
| dilated cardiomyopathy (often with left ventricular noncompaction) and/or endocardial fibroelastosis | reduced mitochondrial function leads to poor cardiac function, maturation and remodeling, with subsequent susceptibility to injury, manifest as ectopic lipid deposits, cardiac fibrosis, ventricular arrhythmia, prolonged QTc intervals, and ventricular dilation |
| skeletal myopathy | reduced mitochondrial function affects skeletal muscle maturation leading to smaller myocyte and muscle fiber sizes and reduced muscle strength |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, M.T.; Conway, S.J. Role of Tafazzin in Mitochondrial Function, Development and Disease. J. Dev. Biol. 2020, 8, 10. https://doi.org/10.3390/jdb8020010
Chin MT, Conway SJ. Role of Tafazzin in Mitochondrial Function, Development and Disease. Journal of Developmental Biology. 2020; 8(2):10. https://doi.org/10.3390/jdb8020010
Chicago/Turabian StyleChin, Michael T., and Simon J. Conway. 2020. "Role of Tafazzin in Mitochondrial Function, Development and Disease" Journal of Developmental Biology 8, no. 2: 10. https://doi.org/10.3390/jdb8020010
APA StyleChin, M. T., & Conway, S. J. (2020). Role of Tafazzin in Mitochondrial Function, Development and Disease. Journal of Developmental Biology, 8(2), 10. https://doi.org/10.3390/jdb8020010






