How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins
Abstract
1. Peculiarities of Mammalian Prophase I Oocytes
- Oocytes are very large cells, even in mammals. For example, the mouse oocyte is 70–80 µm in diameter and the human oocyte is 100–120 µm.
- The nucleus occupies an extremely large territory: its absolute diameter is about 25–30 µm in the mouse and up to 40 µm in the human.
- The centrosomes have been destroyed during prophase I, so the formation of microtubules depends on acentriolar microtubule organizing centers (MTOCs). Chromosomes themselves direct the formation of multiple active MTOCs that later assemble the meiotic spindle.
- Because the distance of action of microtubules is limited (they can effectively bind to chromosomes only in cells with a diameter not exceeding 30 µm), the positioning of chromosomes is helped by other types of cytoskeletal elements, namely, microfilaments and intermediate filaments.
- Long before nuclear envelope breakdown (about 4 h), nuclear pores lose their function to be checkpoints for nucleocytoplasmic transport. Large openings in the envelope (up to 800 nm in diameter, reflecting the large size of the nucleus itself) appear and provide access for cytoplasmic cytoskeletal fibers to the chromatin [3].
- At that period, the shape of the nucleus remains visibly unchanged, but the free penetration of cytoplasmic components means that it has effectively stopped being a separate compartment of the cell.
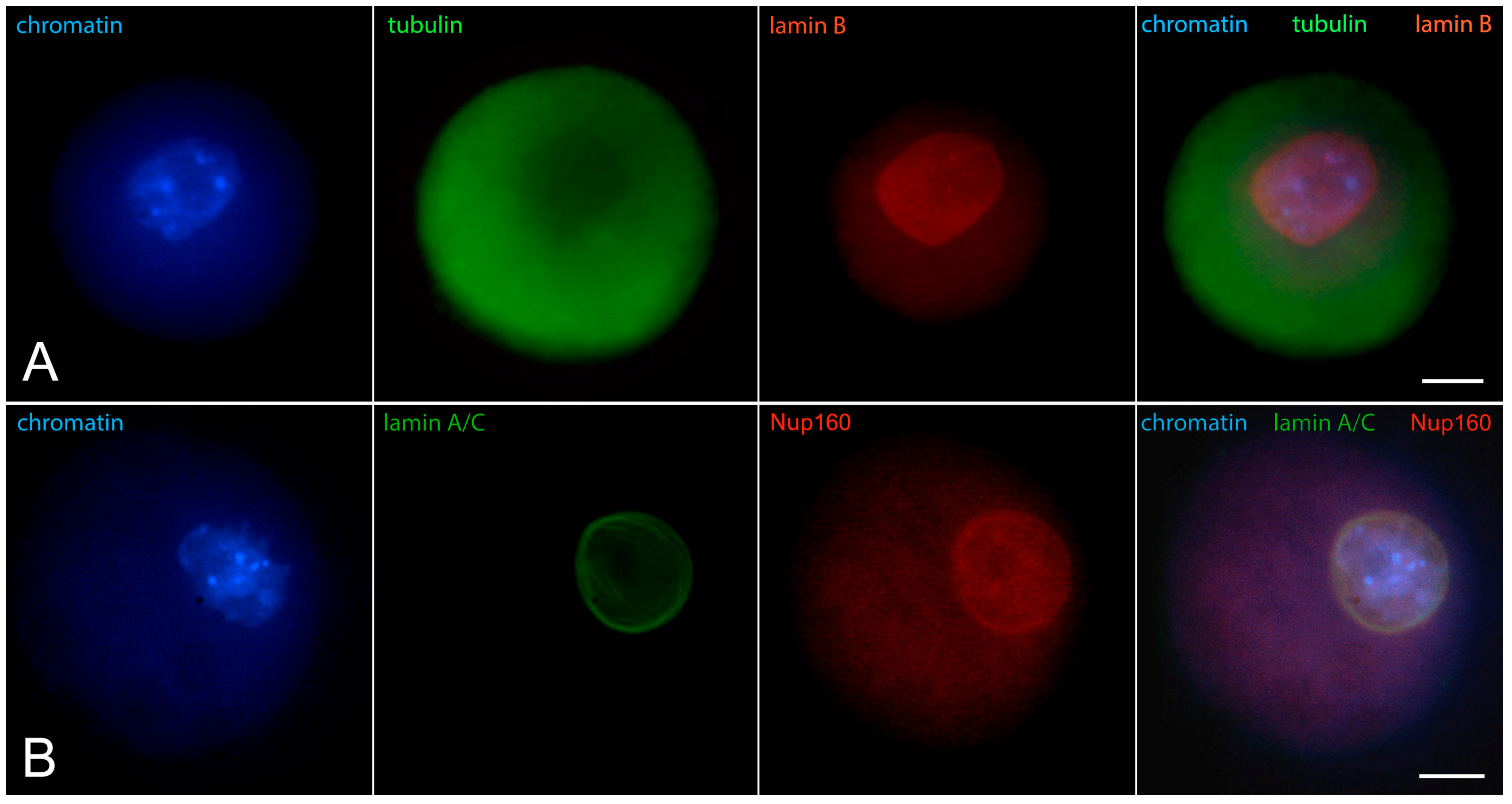
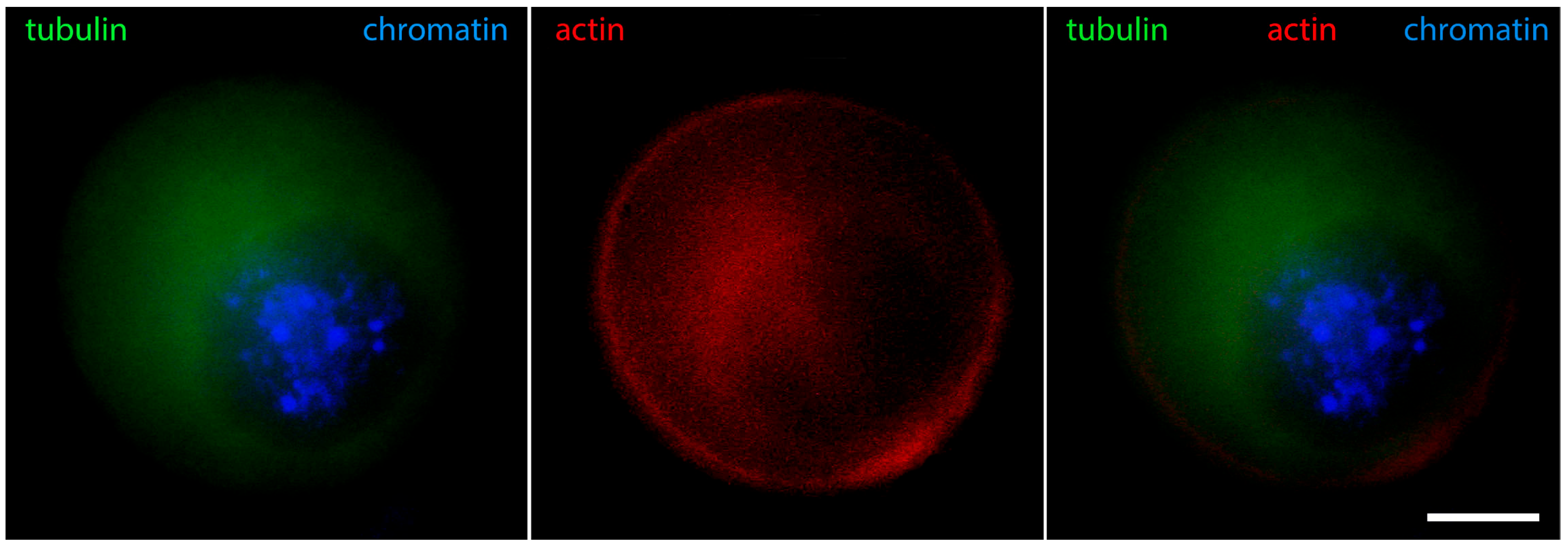
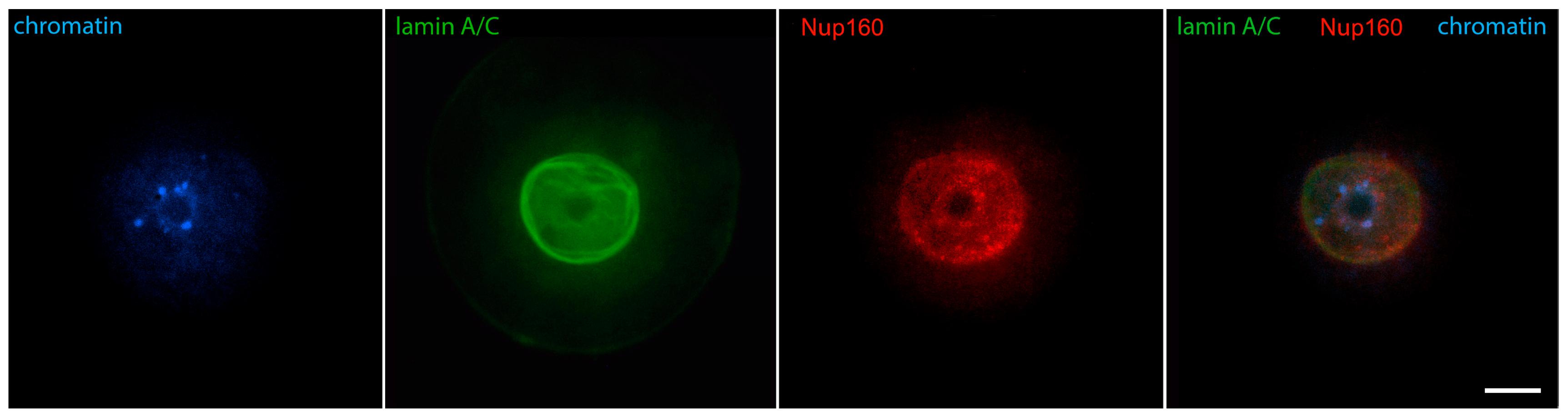
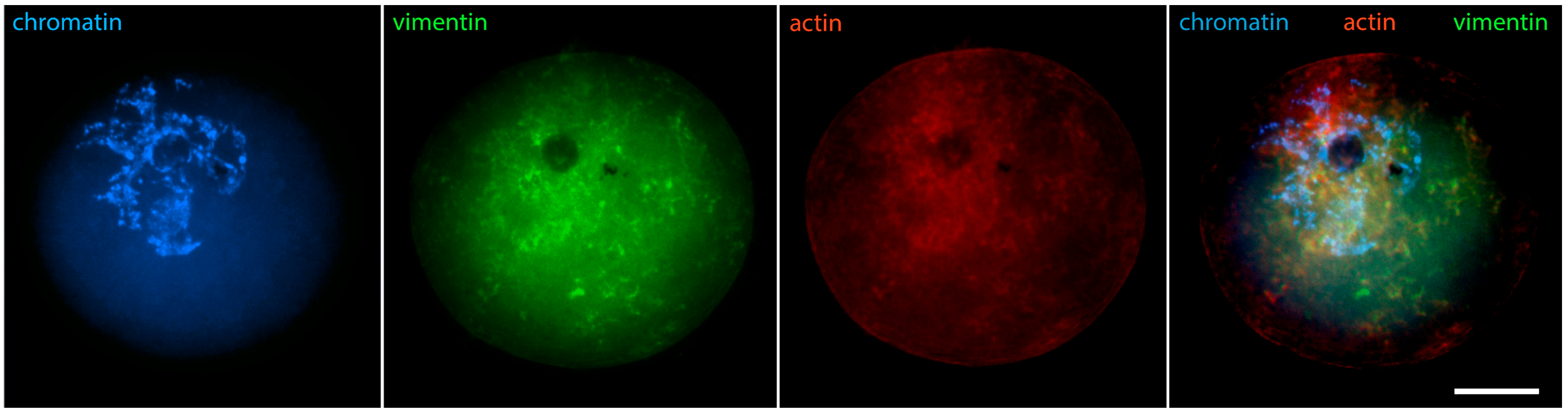
2. The “Surrounded Nucleolus” (Karyosphere) of GV Stage Oocytes
3. Early GV Oocytes Without Karyosphere (Non-Surrounded Nucleolus)
4. GV Oocytes in the Process of Karyosphere Formation (Partially Non-Surrounded and Partially Surrounded Nucleolus)
5. GV Oocytes with Fully Formed Karyosphere (Surrounded Nucleolus)
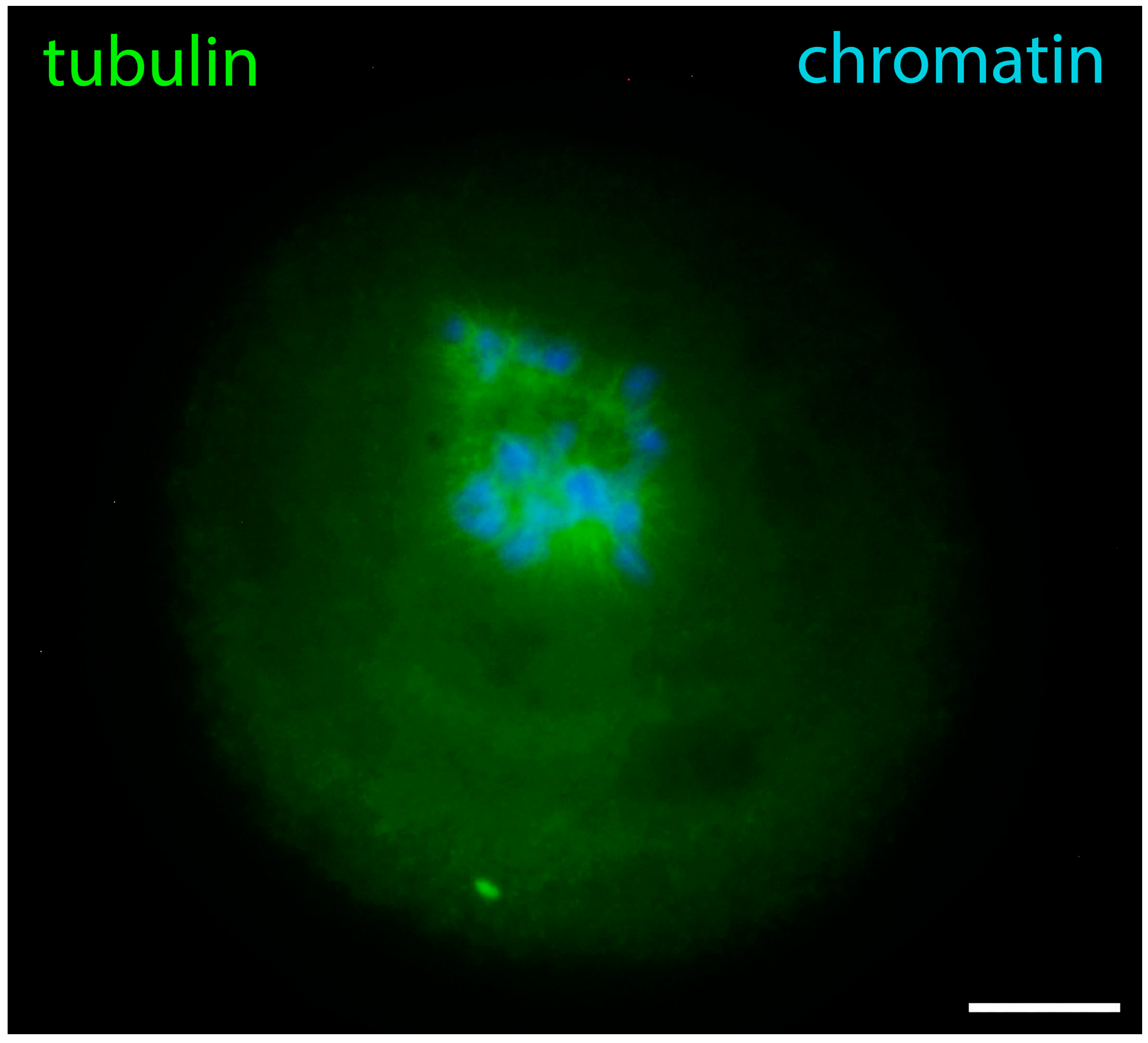
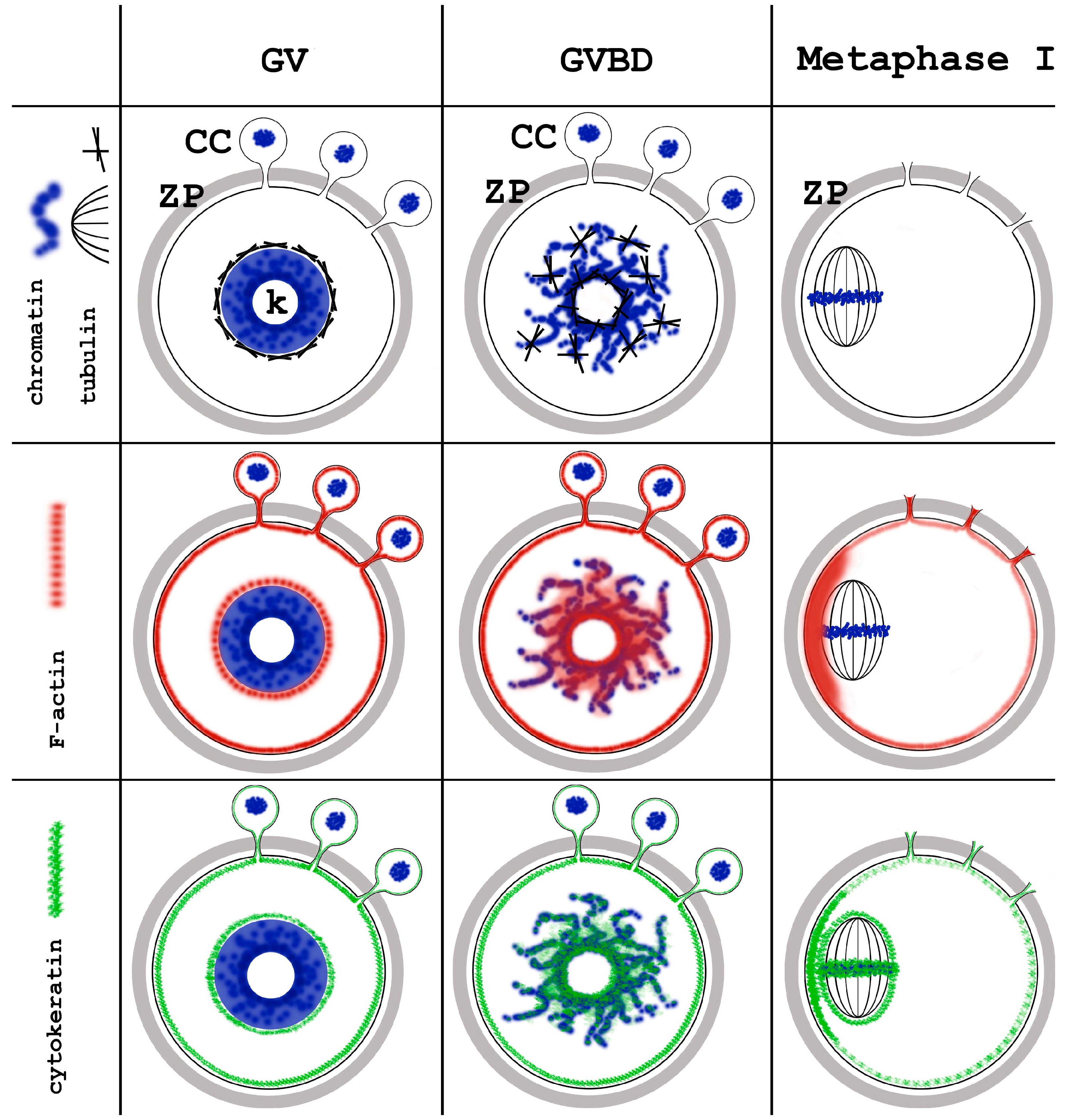
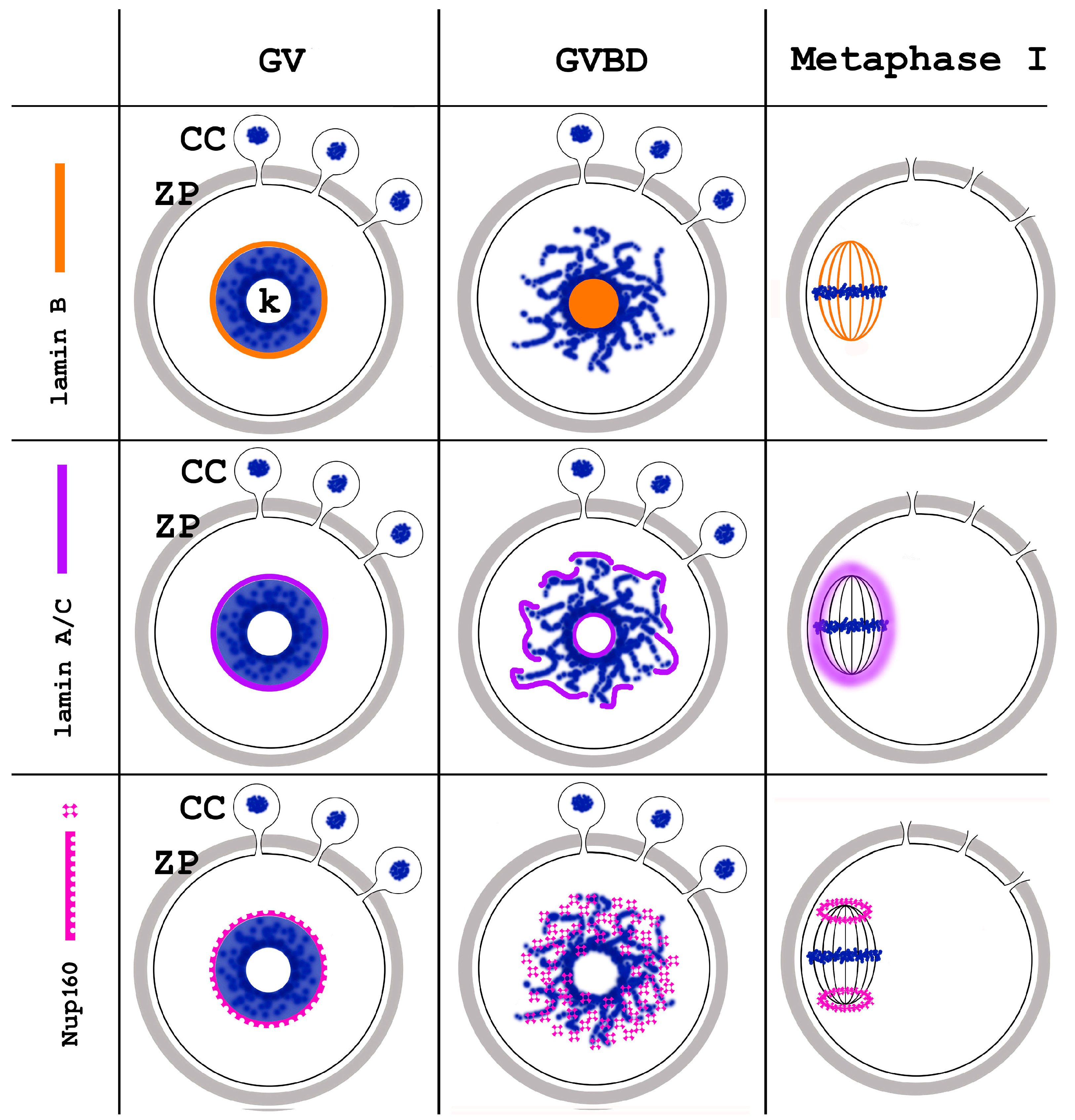
6. GVBD and Prometaphase I Oocytes
7. Metaphase Oocytes
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffin, J.; Emery, B.R.; Huang, I.; Peterson, C.M.; Carrell, D.T. Comparative analysis of follicle morphology and oocyte diameter in four mammalian species (mouse, hamster, pig, and human). J. Exp. Clin. Assist. Reprod. 2006, 3, 2. [Google Scholar] [CrossRef]
- Lénárt, P.; Bacher, C.P.; Daigle, N.; Hand, A.R.; Eils, R.; Terasaki, M.; Ellenberg, J. A contractile nuclear actin network drives chromosome congression in oocytes. Nature 2005, 436, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.; Rulong, S.; Resau, J.; Fukasawa, K.; Matten, W.; Kuriyama, R.; Mansour, S.; Ahn, N.; Vande Woude, G.F. Mos/mitogen-activated protein kinase can induce early meiotic phenotypes in the absence of maturation-promoting factor: A novel system for analyzing spindle formation during meiosis I. Proc. Natl. Acad. Sci. USA 1996, 93, 4730–4735. [Google Scholar] [CrossRef]
- Radford, S.J.; Nguyen, A.L.; Schindler, K.; McKim, K.S. The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes. Chromosoma 2017, 126, 351–364. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev. Biol. 2006, 292, 1–12. [Google Scholar] [CrossRef]
- Harris, H. (Ed.) The discovery of the cell nucleus. In The Birth of the Cell; Yale University Press: New Haven, CT, USA, 1999; pp. 76–93. [Google Scholar]
- Shishova, K.V.; Lavrentyeva, E.A.; Dobrucki, J.W.; Zatsepina, O.V. Nucleolus-like bodies of fully-grown mouse oocytes contain key nucleolar proteins but are impoverished for rRNA. Dev. Biol. 2015, 397, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Salimov, D.; Lisovskaya, T.; Otsuki, J.; Gzgzyan, A.; Bogolyubova, I.; Bogolyubov, D. Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles. Cells 2023, 12, 1976. [Google Scholar] [CrossRef]
- Robinson, R. Embryos need Mom’s nucleolus. J. Cell Biol. 2008, 180, 650. [Google Scholar] [CrossRef]
- Ma, J.Y.; Li, M.; Luo, Y.B.; Song, S.; Tian, D.; Yang, J.; Zhang, B.; Hou, Y.; Schatten, H.; Liu, Z.; et al. Maternal factors required for oocyte developmental competence in mice: Transcriptome analysis of non-surrounded nucleolus (NSN) and surrounded nucleolus (SN) oocytes. Cell Cycle 2013, 12, 1928–1938. [Google Scholar] [CrossRef]
- Can, A.; Semiz, O.; Cinar, O. Centrosome and microtubule dynamics during early stages of meiosis in mouse oocytes. Mol. Hum. Reprod. 2003, 9, 749–756. [Google Scholar] [CrossRef]
- De La Fuente, R.; Viveiros, M.M.; Burns, K.H.; Adashi, E.Y.; Matzuk, M.M.; Eppig, J.J. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev. Biol. 2004, 275, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Bogolyubova, I.; Bogolyubov, D. Heterochromatin Morphodynamics in Late Oogenesis and Early Embryogenesis of Mammals. Cells 2020, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Ponce, R.H.; Boiani, M.; Guizzardi, S.; Govoni, P.; Scandroglio, R.; Garagna, S.; Redi, C.A. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote 2002, 10, 73–78. [Google Scholar] [CrossRef]
- Zuccotti, M.; Merico, V.; Bellone, M.; Mulas, F.; Sacchi, L.; Rebuzzini, P.; Prigione, A.; Redi, C.A.; Bellazzi, R.; Adjaye, J.; et al. Gatekeeper of pluripotency: A common Oct4 transcriptional network operates in mouse eggs and embryonic stem cells. BMC Genom. 2011, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Lodde, V.; Modina, S.; Galbusera, C.; Franciosi, F.; Luciano, A.M. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol. Reprod. Dev. 2007, 74, 740–749. [Google Scholar] [CrossRef]
- Bonnet-Garnier, A.; Feuerstein, P.; Chebrout, M.; Fleurot, R.; Jan, H.U.; Debey, P.; Beaujean, N. Genome organization and epigenetic marks in mouse germinal vesicle oocytes. Int. J. Dev. Biol. 2012, 56, 877–887. [Google Scholar] [CrossRef]
- Diao, Y.F.; Lin, T.; Li, X.; Oqani, R.K.; Lee, J.E.; Kim, S.Y.; Jin, D.I. Dynamic changes of SETD2, a histone H3K36 methyltransferase, in porcine oocytes, IVF and SCNT embryos. PLoS ONE 2018, 13, e0191816. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Yin, Y.; Guo, S.M.; Wang, Y.F.; Zhao, G.N.; Ji, D.M.; Zhou, L.Q. The landscape of transcriptional profiles in human oocytes with different chromatin configurations. J. Ovarian Res. 2024, 17, 99. [Google Scholar] [CrossRef]
- Bogolyubov, D.S. Karyosphere (Karyosome): A Peculiar Structure of the Oocyte Nucleus. Int. Rev. Cell Mol. Biol. 2018, 337, 1–48. [Google Scholar] [CrossRef]
- Longo, F.; Garagna, S.; Merico, V.; Orlandini, G.; Gatti, R.; Scandroglio, R.; Redi, C.A.; Zuccotti, M. Nuclear localization of NORs and centromeres in mouse oocytes during folliculogenesis. Mol. Reprod. Dev. 2003, 66, 279–290. [Google Scholar] [CrossRef]
- Tan, J.H.; Wang, H.L.; Sun, X.S.; Liu, Y.; Sui, H.S.; Zhang, J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol. Hum. Reprod. 2009, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Garagna, S.; Merico, V.; Sebastiano, V.; Monti, M.; Orlandini, G.; Gatti, R.; Scandroglio, R.; Redi, C.A.; Zuccotti, M. Three-dimensional localization and dynamics of centromeres in mouse oocytes during folliculogenesis. J. Mol. Histol. 2004, 35, 631–638. [Google Scholar] [CrossRef] [PubMed]
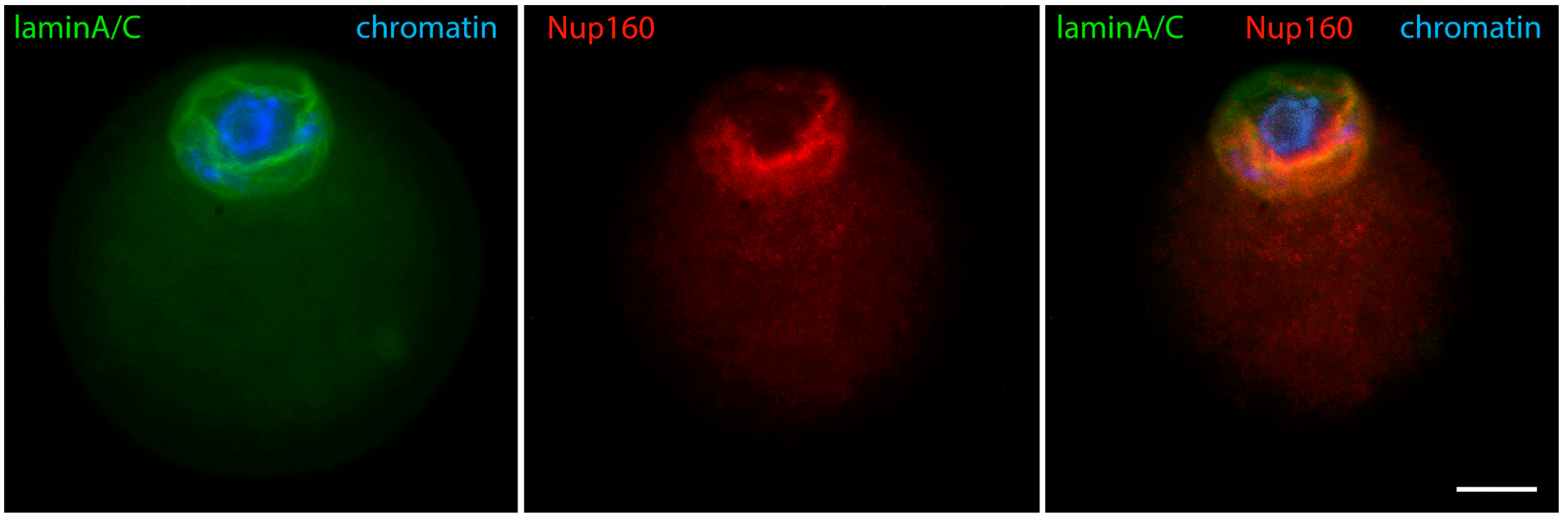
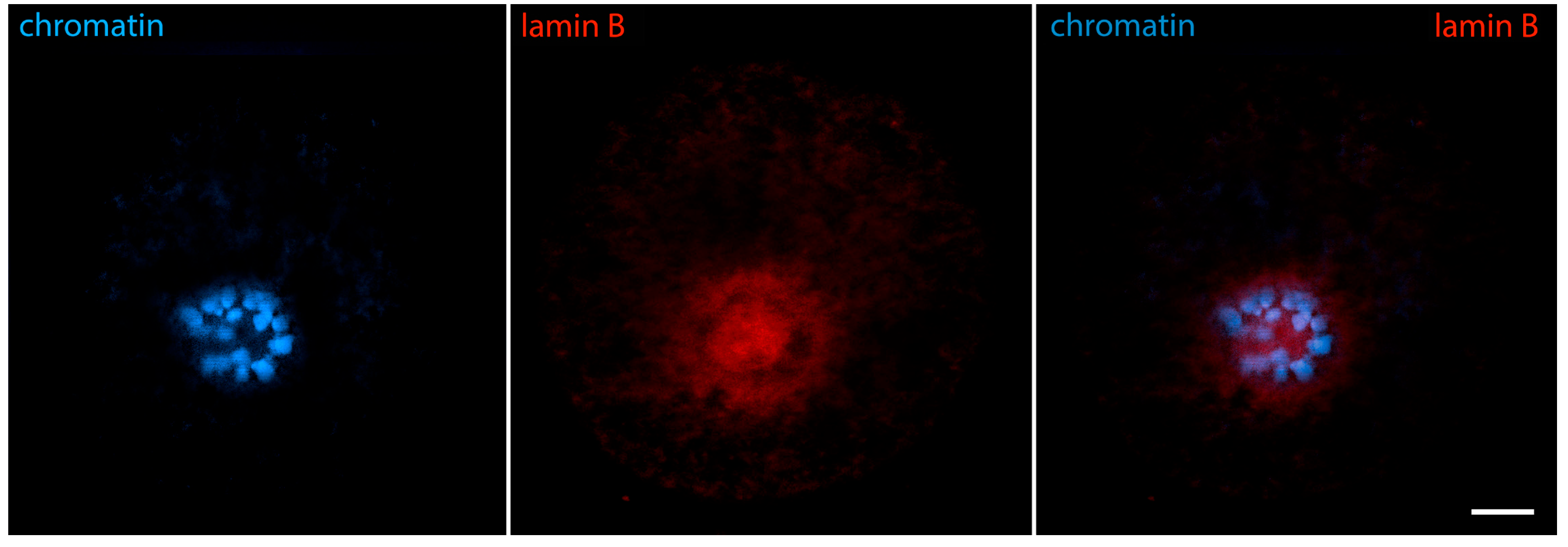
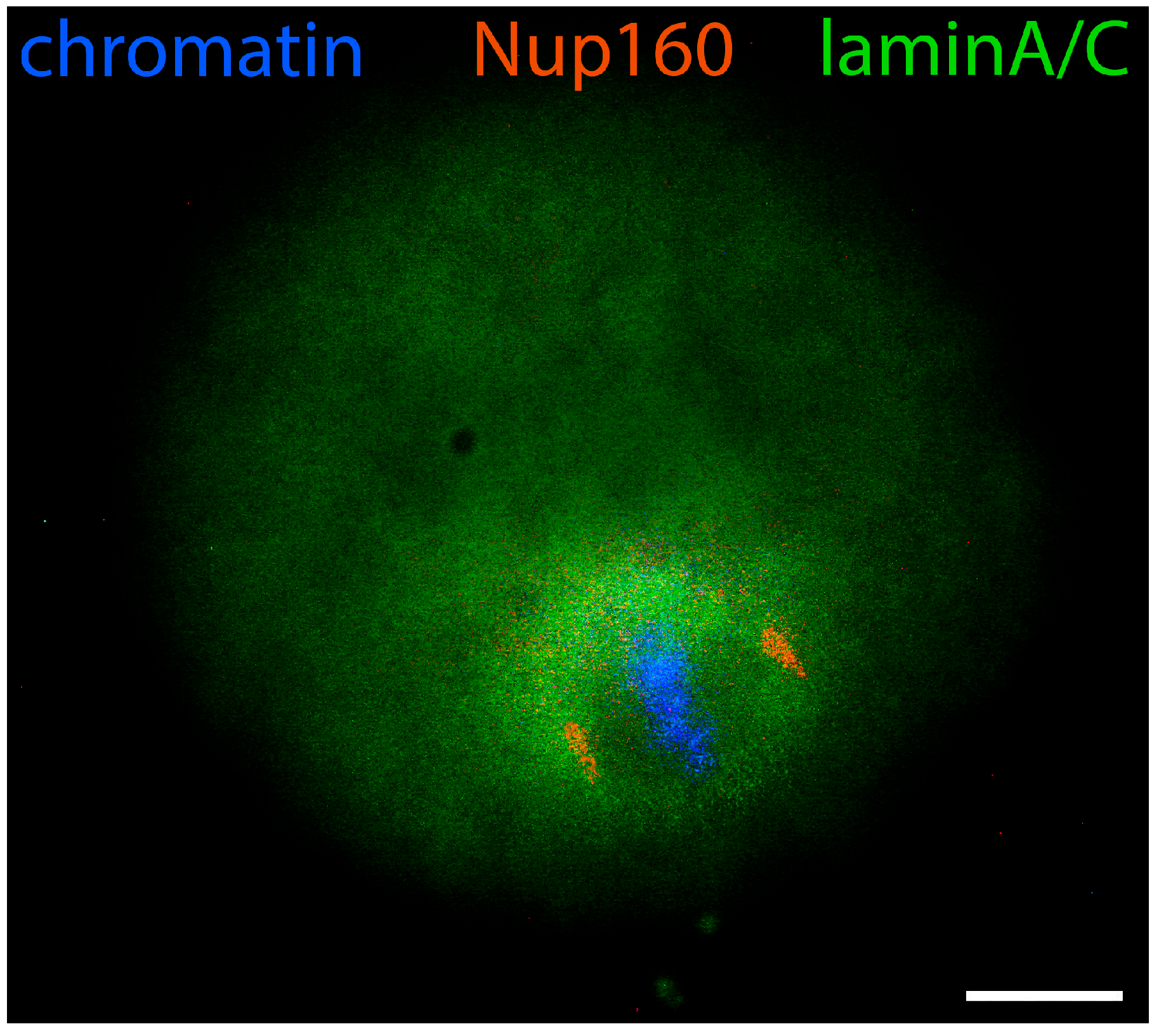
- Nikolova, V.; Delimitreva, S.; Chakarova, I.; Zhivkova, R.; Hadzhinesheva, V.; Markova, M. Dynamics of Lamins B and A/C and Nucleoporin Nup160 during Meiotic Maturation in Mouse Oocytes. Folia Biol. 2017, 63, 6–12. [Google Scholar] [CrossRef]
- Koncicka, M.; Tetkova, A.; Jansova, D.; Del Llano, E.; Gahurova, L.; Kracmarova, J.; Prokesova, S.; Masek, T.; Pospisek, M.; Bruce, A.W.; et al. Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females. Int. J. Mol. Sci. 2018, 19, 2841. [Google Scholar] [CrossRef]
- Koncicka, M.; Cervenka, J.; Jahn, D.; Sucha, R.; Vodicka, P.; Gad, A.; Alsheimer, M.; Susor, A. Expression of lamin C2 in mammalian oocytes. PLoS ONE 2020, 15, e0229781. [Google Scholar] [CrossRef]
- Prentice-Biensch, J.R.; Singh, J.; Alfoteisy, B.; Anzar, M. A simple and high-throughput method to assess maturation status of bovine oocytes: Comparison of anti-lamin A/C-DAPI with an aceto-orcein staining technique. Theriogenology 2012, 78, 1633–1638. [Google Scholar] [CrossRef]
- Meng, X.Q.; Fan, H.Y.; Zhong, Z.S.; Zhang, G.; Li, Y.L.; Chen, D.Y.; Sun, Q.Y. Localization of gamma-tubulin in mouse eggs during meiotic maturation, fertilization, and early embryonic development. J. Reprod. Dev. 2004, 50, 97–105. [Google Scholar] [CrossRef][Green Version]
- Markova, M.D.; Nikolova, V.P.; Chakarova, I.V.; Zhivkova, R.S.; Dimitrov, R.K.; Delimitreva, S.M. Intermediate filament distribution patterns in maturing mouse oocytes and cumulus cells. Biocell 2015, 39, 1–7. [Google Scholar]
- Bogolyubova, I.; Salimov, D.; Bogolyubov, D. Chromatin Configuration in Diplotene Mouse and Human Oocytes during the Period of Transcriptional Activity Extinction. Int. J. Mol. Sci. 2023, 24, 11517. [Google Scholar] [CrossRef]
- Pochukalina, G.N.; Ilicheva, N.V.; Podgornaya, O.I.; Voronin, A.P. Nucleolus-like body of mouse oocytes contains lamin A and B and TRF2 but not actin and topo II. Mol. Cytogenet. 2016, 9, 50. [Google Scholar] [CrossRef]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, A.; Plancha, C.E.; Overstrom, E.W.; Albertini, D.F. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: Insights into the relationship between nuclear and cytoplasmic quality. Hum. Reprod. 2004, 19, 2889–2899. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dong, J.; Fu, J.; Kuang, Y.; Chen, B.; Gu, H.; Luo, Y.; Gu, R.; Zhang, M.; Li, W.; et al. The mechanism of acentrosomal spindle assembly in human oocytes. Science 2022, 378, eabq7361. [Google Scholar] [CrossRef] [PubMed]
- Mchedlishvili, N.; Matthews, H.K.; Corrigan, A.; Baum, B. Two-step interphase microtubule disassembly aids spindle morphogenesis. BMC Biol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Mühlhäusser, P.; Kutay, U. An in vitro nuclear disassembly system reveals a role for the RanGTPase system and microtubule-dependent steps in nuclear envelope breakdown. J. Cell Biol. 2007, 178, 595–610. [Google Scholar] [CrossRef]
- Harasimov, K.; Uraji, J.; Mönnich, E.U.; Holubcová, Z.; Elder, K.; Blayney, M.; Schuh, M. Actin-driven chromosome clustering facilitates fast and complete chromosome capture in mammalian oocytes. Nat. Cell Biol. 2023, 25, 439–452. [Google Scholar] [CrossRef]
- Kitajima, T.S.; Ohsugi, M.; Ellenberg, J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell 2011, 146, 568–581. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Wang, S.; Heidinger, J.M.; Shumaker, D.K.; Adam, S.A.; Goldman, R.D.; Zheng, Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 2006, 311, 1887–1893. [Google Scholar] [CrossRef]
- Shi, C.; Channels, W.E.; Zheng, Y.; Iglesias, P.A. A computational model for the formation of lamin-B mitotic spindle envelope and matrix. Interface Focus 2014, 4, 20130063. [Google Scholar] [CrossRef]
- Bury, L.; Coelho, P.A.; Simeone, A.; Ferries, S.; Eyers, C.E.; Eyers, P.A.; Zernicka-Goetz, M.; Glover, D.M. Plk4 and Aurora A cooperate in the initiation of acentriolar spindle assembly in mammalian oocytes. J. Cell Biol. 2017, 216, 3571–3590. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, V.; Markova, M.; Zhivkova, R.; Chakarova, I.; Hadzhinesheva, V.; Delimitreva, S. How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins. J. Dev. Biol. 2024, 12, 28. https://doi.org/10.3390/jdb12040028
Nikolova V, Markova M, Zhivkova R, Chakarova I, Hadzhinesheva V, Delimitreva S. How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins. Journal of Developmental Biology. 2024; 12(4):28. https://doi.org/10.3390/jdb12040028
Chicago/Turabian StyleNikolova, Venera, Maya Markova, Ralitsa Zhivkova, Irina Chakarova, Valentina Hadzhinesheva, and Stefka Delimitreva. 2024. "How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins" Journal of Developmental Biology 12, no. 4: 28. https://doi.org/10.3390/jdb12040028
APA StyleNikolova, V., Markova, M., Zhivkova, R., Chakarova, I., Hadzhinesheva, V., & Delimitreva, S. (2024). How the Oocyte Nucleolus Is Turned into a Karyosphere: The Role of Heterochromatin and Structural Proteins. Journal of Developmental Biology, 12(4), 28. https://doi.org/10.3390/jdb12040028






