Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature
Abstract
:1. Introduction
2. Pathophysiology of Gastric Cancer
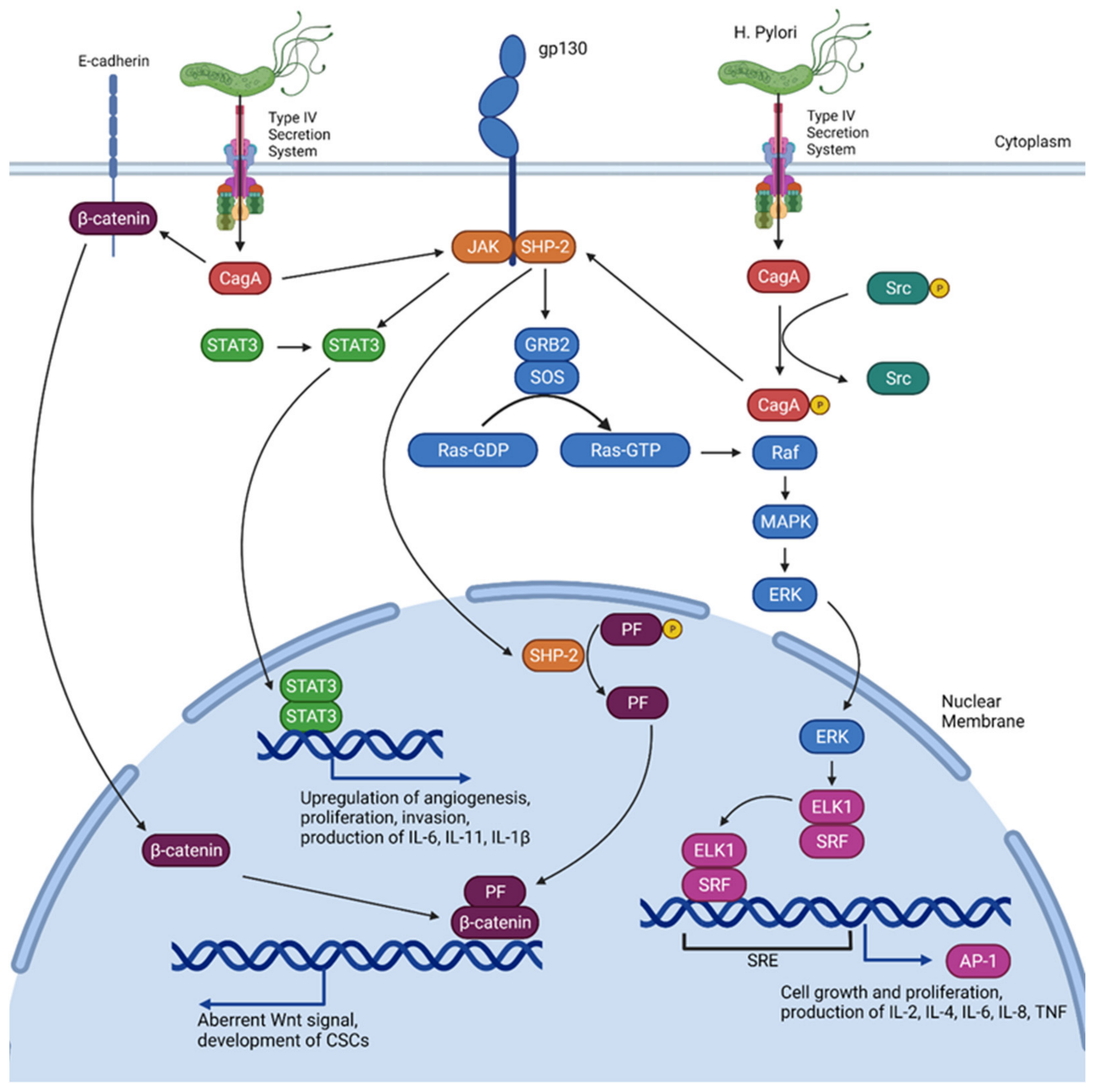
3. Implications of H. Pylori
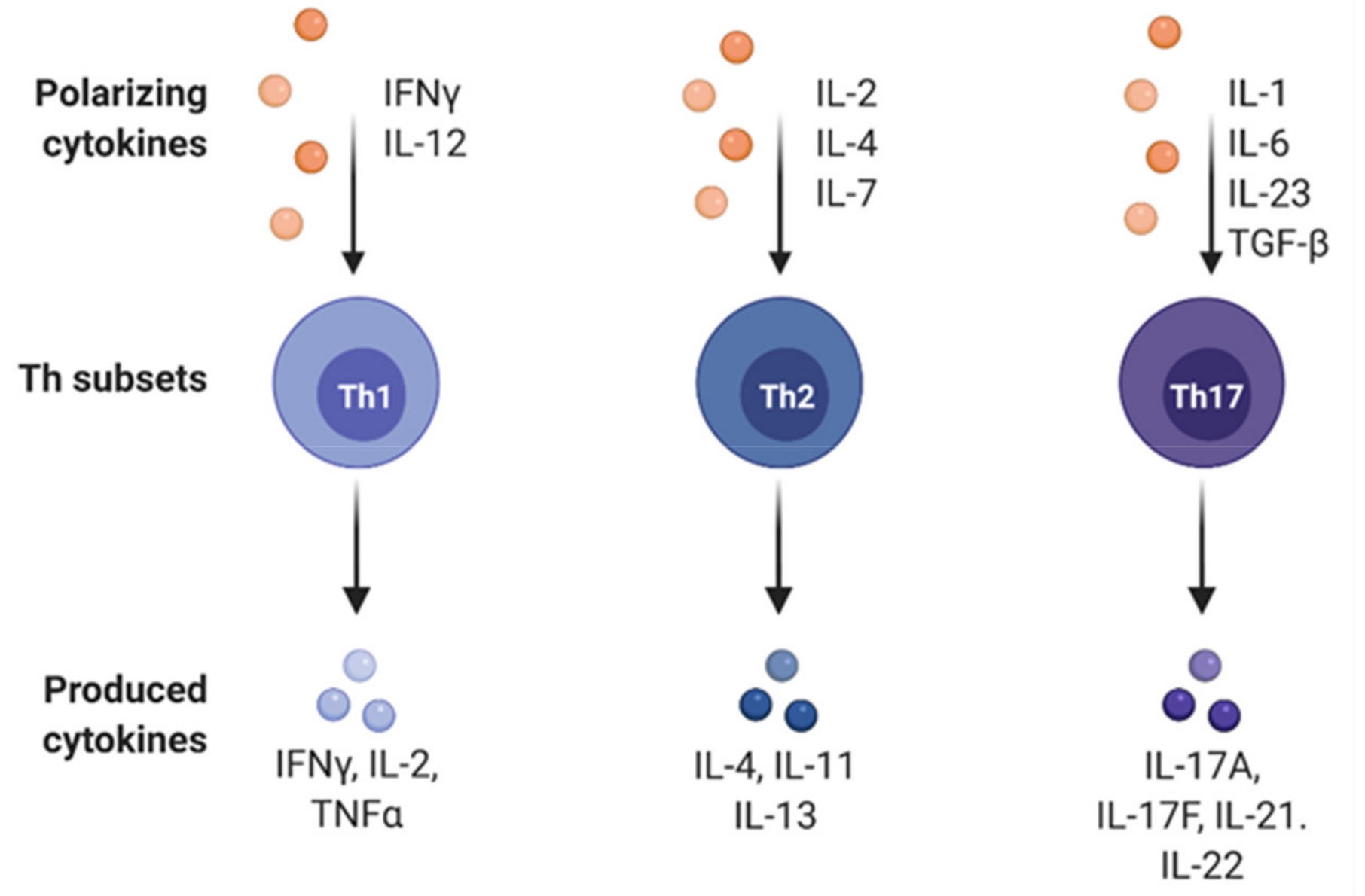
4. Effects of Immune Cell Differentiation in Gastric Cancer
5. Effects of Cytokines on Gastric Cancer Prognosis
5.1. IL-6
5.2. TNF
5.3. IFN-γ
5.4. IL-17A
5.5. IL-10
5.6. IL-8
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, J.; Maciejewski, R.; Polkowski, W. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Res. 2018, 10, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsueda, S.; Graham, D. Immunotherapy in gastric cancer. World J. Gastroenterol. 2014, 20, 1657. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Hayakawa, Y.; Koike, K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020, 111, 2696–2707. [Google Scholar] [CrossRef]
- Chung, H.W. Role of the tumor microenvironment in the pathogenesis of gastric carcinoma. World J. Gastroenterol. 2014, 20, 1667. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori CagA and Gastric Cancer: A Paradigm for Hit-and-Run Carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Tsujimoto, H.; Ono, S.; Ichikura, T.; Matsumoto, Y.; Yamamoto, J.; Hase, K. Roles of inflammatory cytokines in the progression of gastric cancer: Friends or foes? Gastric Cancer 2010, 13, 212–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gu, W.; Sun, B. Th1/Th2 Cell Differentiation and Molecular Signals; Springer: Dordrecht, The Netherlands, 2014; pp. 15–44. [Google Scholar]
- Bockerstett, K.A.; Dipaolo, R.J. Regulation of gastric carcinogenesis by inflammatory cytokines. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Madej-Michniewicz, A.; Budkowska, M.; Sałata, D.; Dołęgowska, B.; Starzyńska, T.; Błogowski, W. Evaluation of selected interleukins in patients with different gastric neoplasms: A preliminary report. Sci. Rep. 2015, 5, 14382. [Google Scholar] [CrossRef] [Green Version]
- Amedei, A.; Della Bella, C.; Silvestri, E.; Prisco, D.; D’Elios, M.M. T Cells in gastric cancer: Friends or foes. Clin. Dev. Immunol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Berlth, F. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World J. Gastroenterol. 2014, 20, 5679. [Google Scholar] [CrossRef]
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef]
- Kaneko, S.; Yoshimura, T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br. J. Cancer 2001, 84, 400–405. [Google Scholar] [CrossRef]
- Qiu, M.-Z.; Cai, M.-Y.; Zhang, D.-S.; Wang, Z.-Q.; Wang, D.-S.; Li, Y.-H.; Xu, R.-H. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J. Transl. Med. 2013, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Espinel, J. Treatment modalities for early gastric cancer. World J. Gastrointest. Endosc. 2015, 7, 1062. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van De Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Keam, B.; Im, S.-A.; Han, S.-W.; Ham, H.S.; Kim, M.A.; Oh, D.-Y.; Lee, S.-H.; Kim, J.H.; Kim, D.-W.; Kim, T.-Y.; et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer 2008, 8, 148. [Google Scholar] [CrossRef] [Green Version]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Brar, G.; Shah, M.A. The role of pembrolizumab in the treatment of PD-L1 expressing gastric and gastroesophageal junction adenocarcinoma. Ther. Adv. Gastroenterol. 2019, 12, 175628481986976. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Gastric Cancer (Version 5.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 11 November 2021).
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, Y.; Kato, M.; Asaka, M. Geographic Differences in Gastric Cancer Incidence Can be Explained by Differences between Helicobacter pylori Strains. Intern. Med. 2008, 47, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.O.; Kim, J.H.; Choi, Y.J.; Pillinger, M.H.; Kim, S.-Y.; Blaser, M.J.; Lee, Y.C. Helicobacter pylori CagA Phosphorylation Status Determines the gp130-activated SHP2/ERK and JAK/STAT Signal Transduction Pathways in Gastric Epithelial Cells. J. Biol. Chem. 2010, 285, 16042–16050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, M. Molecular Mechanism of Helicobacter pylori-induced gastric cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Lee, K.S.; Kalantzis, A.; Jackson, C.B.; O’Connor, L.; Murata-Kamiya, N.; Hatakeyama, M.; Judd, L.M.; Giraud, A.S.; Menheniott, T.R. Helicobacter pylori CagA Triggers Expression of the Bactericidal Lectin REG3γ via Gastric STAT3 Activation. PLoS ONE 2012, 7, e30786. [Google Scholar] [CrossRef] [Green Version]
- Bessède, E.; Staedel, C.; Acuña Amador, L.A.; Nguyen, P.H.; Chambonnier, L.; Hatakeyama, M.; Belleannée, G.; Mégraud, F.; Varon, C. Helicobacter pylori generates cells with cancer stem cell properties via epithelial–mesenchymal transition-like changes. Oncogene 2014, 33, 4123–4131. [Google Scholar] [CrossRef] [Green Version]
- Mitsuno, Y. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 2001, 49, 18–22. [Google Scholar] [CrossRef]
- Backert, S.; Naumann, M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010, 18, 479–486. [Google Scholar] [CrossRef]
- Judd, L.M.; Menheniott, T.R.; Ling, H.; Jackson, C.B.; Howlett, M.; Kalantzis, A.; Priebe, W.; Giraud, A.S. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS ONE 2014, 9, e95993. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Judd, L.; Menheniott, T.; Kronborg, I.; Dow, C.; Yeomans, N.; Boussioutas, A.; Robb, L.; Giraud, A. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J. Pathol. 2007, 213, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Howlett, M.; Menheniott, T.R.; Judd, L.M.; Giraud, A.S. Cytokine signalling via gp130 in gastric cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1623–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGeachy, M.J.; Cua, D.J. Th17 Cell Differentiation: The Long and Winding Road. Immunity 2008, 28, 445–453. [Google Scholar] [CrossRef] [Green Version]
- Maeda, S.; Yoshida, H.; Ogura, K.; Mitsuno, Y.; Hirata, Y.; Yamaji, Y.; Akanuma, M.; Shiratori, Y.; Omata, M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 2000, 119, 97–108. [Google Scholar] [CrossRef]
- Suzuki, N.; Murata-Kamiya, N.; Yanagiya, K.; Suda, W.; Hattori, M.; Kanda, H.; Bingo, A.; Fujii, Y.; Maeda, S.; Koike, K.; et al. Mutual reinforcement of inflammation and carcinogenesis by the Helicobacter pylori CagA oncoprotein. Sci. Rep. 2015, 5, 10024. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Lamb, A.; Chen, L.-F. The many roads traveled by Helicobacter pylori to NF-κB activation. Gut Microbes 2010, 1, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Wilson, K.T.; James, S.P. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect. Immun. 2000, 68, 6265–6272. [Google Scholar] [CrossRef]
- Ubukata, H.; Motohashi, G.; Tabuchi, T.; Nagata, H.; Konishi, S.; Tabuchi, T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J. Surg. Oncol. 2010, 102, 742–747. [Google Scholar] [CrossRef]
- Kryczek, I.; Wei, S.; Zou, L.; Altuwaijri, S.; Szeliga, W.; Kolls, J.; Chang, A.; Zou, W. Cutting Edge: Th17 and Regulatory T Cell Dynamics and the Regulation by IL-2 in the Tumor Microenvironment. J. Immunol. 2007, 178, 6730–6733. [Google Scholar] [CrossRef]
- Yamada, Y.; Saito, H.; Ikeguchi, M. Prevalence and clinical relevance of Th17 cells in patients with gastric cancer. J. Surg. Res. 2012, 178, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Li, H.; Zhang, H.; Chen, Y.F.; Cao, Y.F.; Li, R.C.; Lin, C.; Wei, Y.C.; Xiang, X.N.; Fang, H.J.; et al. Intratumoral IL17-producing cells infiltration correlate with antitumor immune contexture and improved response to adjuvant chemotherapy in gastric cancer. Ann. Oncol. 2019, 30, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Peng, L.; Yu, P.; Zhao, Y.; Shi, Y.; Mao, X.; Chen, W.; Cheng, P.; Wang, T.; Chen, N.; et al. Increased Circulating Th22 and Th17 Cells are Associated with Tumor Progression and Patient Survival in Human Gastric Cancer. J. Clin. Immunol. 2012, 32, 1332–1339. [Google Scholar] [CrossRef]
- Zhang, B.; Rong, G.; Wei, H.; Zhang, M.; Bi, J.; Ma, L.; Xue, X.; Wei, G.; Liu, X.; Fang, G. The prevalence of Th17 cells in patients with gastric cancer. Biochem. Biophys. Res. Commun. 2008, 374, 533–537. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Ham, I.-H.; Oh, H.J.; Jin, H.; Bae, C.A.; Jeon, S.-M.; Choi, K.S.; Son, S.-Y.; Han, S.-U.; Brekken, R.A.; Lee, D.; et al. Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer. Mol. Cancer 2019, 18, 68. [Google Scholar] [CrossRef]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Oka, M.; Murase, K.; Soda, H.; Isomoto, H.; Takeshima, F.; Mizuta, Y.; Murata, I.; Kohno, S. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J. Int. Med Res. 2003, 31, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-P.; Wu, M.-S.; Shun, C.-T.; Wang, H.-P.; Lin, M.-T.; Kuo, M.-L.; Lin, J.-T. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J. Biomed. Sci. 2004, 11, 517–527. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.; Zhang, L.; Li, W.; Wu, H.; Yuan, X.; Mao, F.; Wang, M.; Zhu, W.; Qian, H.; et al. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014, 5, e1295. [Google Scholar] [CrossRef]
- Ham, I.-H.; Lee, D.; Hur, H. Role of Cancer-Associated Fibroblast in Gastric Cancer Progression and Resistance to Treatments. J. Oncol. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Simondurairaj, C.; Krishnakumar, R.; Sundaram, S.; Venkatraman, G. Interleukin-6 Receptor (IL-6R) expression in human gastric carcinoma and its clinical significance. Cancer Investig. 2019, 37, 293–298. [Google Scholar] [CrossRef]
- Ashizawa, T.; Okada, R.; Suzuki, Y.; Takagi, M.; Yamazaki, T.; Sumi, T.; Aoki, T.; Ohnuma, S.; Aoki, T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: Role of IL-6 as a prognostic factor. Gastric Cancer 2005, 8, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Zauco, N.; Torres, J.; Gómez, A.; Camorlinga-Ponce, M.; Muñoz-Pérez, L.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Giono-Cerezo, S.; Maldonado-Bernal, C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ikeguchi, M.; Hatada, T.; Yamamoto, M.; Miyake, T.; Matsunaga, T.; Fukumoto, Y.; Yamada, Y.; Fukuda, K.; Saito, H.; Tatebe, S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009, 12, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Łukaszewicz-Zając, M.; Mroczko, B.; Gryko, M.; Kędra, B.; Szmitkowski, M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin. Exp. Med. 2011, 11, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.C.; Lin, J.T.; Wu, C.Y.; Huang, S.P.; Lin, M.T.; Wu, A.S.H.; Huang, Y.J.; Wu, M.S. Serum Interleukin-6 Level but not Genotype Predicts Survival after Resection in Stages II and III Gastric Carcinoma. Clin. Cancer Res. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ching, L.-M.; Goldsmith, D.; Joseph, W.R.; Körner, H.; Sedgwick, J.D.; Baguley, B.C. Induction of Intratumoral Tumor Necrosis Factor (TNF) Synthesis and Hemorrhagic Necrosis by 5,6-Dimethylxanthenone-4-Acetic Acid (DMXAA) in TNF Knockout Mice. Cancer Res. 1999, 59, 3304–3307. [Google Scholar] [PubMed]
- Zhao, C.; Lu, X.; Bu, X.; Zhang, N.; Wang, W. Involvement of tumor necrosis factor-α in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer 2010, 10, 419. [Google Scholar] [CrossRef] [Green Version]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Oshima, H.; Ishikawa, T.; Yoshida, G.J.; Naoi, K.; Maeda, Y.; Naka, K.; Ju, X.; Yamada, Y.; Minamoto, T.; Mukaida, N.; et al. TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene 2014, 33, 3820–3829. [Google Scholar] [CrossRef]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, M.; Watanabe, T.; Sueoka, E.; Lim, I.K.; Fujiki, H. Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies. Toxins 2021, 13, 181. [Google Scholar] [CrossRef]
- Yang, J.-P.; Hyun, M.-H.; Yoon, J.-M.; Park, M.-J.; Kim, D.; Park, S. Association between TNF-α-308 G/A gene polymorphism and gastric cancer risk: A systematic review and meta-analysis. Cytokine 2014, 70, 104–114. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, S.; Zhang, S.; Min, L.; Wang, Y.; Xie, J.; Hou, Y.; Tian, X.; Cheng, J.; Liu, K.; et al. The relationship between tumor necrosis factor-α polymorphisms and gastric cancer risk: An updated meta-analysis. Biomed. Rep. 2017, 7, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, M.R.; Merlino, G. The Two Faces of Interferon-γ in Cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef] [Green Version]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 469. [Google Scholar] [CrossRef]
- Tu, S.P.; Quante, M.; Bhagat, G.; Takaishi, S.; Cui, G.; Yang, X.D.; Muthuplani, S.; Shibata, W.; Fox, J.G.; Pritchard, D.M.; et al. IFN-γ Inhibits Gastric Carcinogenesis by Inducing Epithelial Cell Autophagy and T-Cell Apoptosis. Cancer Res. 2011, 71, 4247–4259. [Google Scholar] [CrossRef] [Green Version]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.-F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Allison, C.C.; Ferrand, J.; McLeod, L.; Hassan, M.; Kaparakis-Liaskos, M.; Grubman, A.; Bhathal, P.S.; Dev, A.; Sievert, W.; Jenkins, B.J.; et al. Nucleotide Oligomerization Domain 1 Enhances IFN-γ Signaling in Gastric Epithelial Cells during Helicobacter pylori Infection and Exacerbates Disease Severity. J. Immunol. 2013, 190, 3706–3715. [Google Scholar] [CrossRef] [Green Version]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J.; et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef]
- Gu, C.; Wu, L.; Li, X. IL-17 family: Cytokines, receptors and signaling. Cytokine 2013, 64, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhou, C.H.; Ma, J.; Jiang, C.; Ji, P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med. Oncol. 2012, 29, 3024–3028. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Zhao, Y.; Ma, P.; Cao, Y.; Liu, C.; Zhang, X.; Wang, W.; Chen, L.; Li, Y. Cancer-associated fibroblasts promote the migration and invasion of gastric cancer cells via activating IL-17a/JAK2/STAT3 signaling. Ann. Transl. Med. 2020, 8, 877. [Google Scholar] [CrossRef]
- Karabulut, M.; Usul Afsar, C.; Serimez, M.; Karabulut, S. Serum IL-17 levels can be diagnostic for gastric cancer. Off. J. Balk. Union Oncol. 2019, 24, 1601–1609. [Google Scholar]
- Cheng, G.; Wei, L.; Xiurong, W.; Xiangzhen, L.; Shiguang, Z.; Songbin, F. IL-17 stimulates migration of carotid artery vascular smooth muscle cells in an MMP-9 dependent manner via p38 MAPK and ERK1/2-dependent NF-kappaB and AP-1 activation. Cell. Mol. Neurobiol. 2009, 29, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Wu, X.; Bian, Z.; Gao, Q. Interleukin 17A Promotes Gastric Cancer Invasiveness via NF-κB Mediated Matrix Metalloproteinases 2 and 9 Expression. PLoS ONE 2014, 9, e96678. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, A.R.; Pendas, A.M.; Sanchez, L.M.; Lopez-Otin, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Yang, T.; Liu, X.; Guo, J.N.; Xie, T.; Ding, Y.; Lin, M.; Yang, H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumor Biol. 2016, 37, 5493–5501. [Google Scholar] [CrossRef] [PubMed]
- Lazăr, D.; Tăban, S.; Raica, M.; Sporea, I.; Cornianu, M.; Goldiş, A.; Vernic, C. Immunohistochemical evaluation of the tumor neoangiogenesis as a prognostic factor for gastric cancers. Rom. J. Morphol. Embryol. 2008, 49, 137–148. [Google Scholar]
- Jiang, Y.X.; Yang, S.W.; Li, P.A.; Luo, X.; Li, Z.Y.; Hao, Y.X.; Yu, P.W. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene 2017, 36, 1256–1264. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, T.-J.; Jiang, Y.-M.; Hu, Y.-F.; Huang, L.; Yu, J.; Zhao, L.-Y.; Deng, H.-J.; Mou, T.-Y.; Liu, H.; Yang, Y.; et al. Interleukin-17–Producing Neutrophils Link Inflammatory Stimuli to Disease Progression by Promoting Angiogenesis in Gastric Cancer. Clin. Cancer Res. 2017, 23, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Zhang, Q.; Wang, H.; Lu, M.; Kong, H.; Zhang, Y.; Shi, H. Prognostic significance of interleukin-17 in solid tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10515–10536. [Google Scholar]
- Chen, J.-G.; Xia, J.-C.; Liang, X.-T.; Pan, K.; Wang, W.; Lv, L.; Zhao, J.-J.; Wang, Q.-J.; Li, Y.-Q.; Chen, S.-P.; et al. Intratumoral Expression of IL-17 and Its Prognostic Role in Gastric Adenocarcinoma Patients. Int. J. Biol. Sci. 2011, 7, 53–60. [Google Scholar] [CrossRef]
- Iida, T.; Iwahashi, M.; Katsuda, M.; Ishida, K.; Nakamori, M.; Nakamura, M.; Naka, T.; Ojima, T.; Ueda, K.; Hayata, K.; et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol. Rep. 2011, 25, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev.™ Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Shi, Y.; Zhu, X.; Guo, W.; Zhang, M.; Che, Y.; Tang, L.; Yang, X.; You, Q.; Liu, Z. IL-10 secreted by cancer-associated macrophages regulates proliferation and invasion in gastric cancer cells via c-Met/STAT3 signaling. Oncol. Rep. 2019, 2, 595–604. [Google Scholar] [CrossRef]
- Kindlund, B.; Sjöling, Å.; Yakkala, C.; Adamsson, J.; Janzon, A.; Hansson, L.-E.; Hermansson, M.; Janson, P.; Winqvist, O.; Lundin, S.B. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer 2017, 20, 116–125. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Terai, M.; Tamura, Y.; Alexeev, V.; Mastrangelo, M.J.; Selvan, S.R. Interleukin 10 in the tumor microenvironment: A target for anticancer immunotherapy. Immunol. Res. 2011, 51, 170–182. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, D.; Wu, P.; Wang, Z.; Huang, J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS ONE 2015, 10, e0139598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Pan, R.; Xu, L.; Ma, Q.; Ying, X.; Zhao, J.; Zhao, H.; Miao, L.; Xu, Y.; Duan, S.; et al. IL10 hypomethylation is associated with the risk of gastric cancer. Oncol. Lett. 2021, 21, 241. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.E. Helicobacter pylori and interleukin-8 in gastric cancer. World J. Gastroenterol. 2013, 19, 8192. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pinto-Ribeiro, I.; Wen, X.; Marcos-Pinto, R.; Dinis-Ribeiro, M.; Carneiro, F.; Figueiredo, C. Helicobacter pylori cagA Promoter Region Sequences Influence CagA Expression and Interleukin 8 Secretion. J. Infect. Dis. 2016, 213, 669–673. [Google Scholar] [CrossRef]
- Chen, X.; Jin, R.; Chen, R.; Huang, Z. Complementary action of CXCL1 and CXCL8 in pathogenesis of gastric carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1036–1045. [Google Scholar]
- Gobert, A.P.; Wilson, K.T. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. In Molecular Pathogenesis and Signal Transduction by Helicobacter pylori; Springer International Publishing: New York, NY, USA, 2017; Volume 400, pp. 27–52. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef] [Green Version]
- Kitadai, Y.; Haruma, K.; Sumii, K.; Yamamoto, S.; Ue, T.; Yokozaki, H.; Yasui, W.; Ohmoto, Y.; Kajiyama, G.; Fidler, I.J.; et al. Expression of interleukin-8 correlates with vascularity in human gastric carcinomas. Am. J. Pathol. 1998, 152, 93. [Google Scholar]
- Martin, D.; Galisteo, R.; Gutkind, J.S. CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFκB through the CBM (Carma3/Bcl10/Malt1) Complex. J. Biol. Chem. 2009, 284, 6038–6042. [Google Scholar] [CrossRef] [Green Version]
- Kido, S.; Kitadai, Y.; Hattori, N.; Haruma, K.; Kido, T.; Ohta, M.; Tanaka, S.; Yoshihara, M.; Sumii, K.; Ohmoto, Y.; et al. Interleukin 8 and vascular endothelial growth factor— prognostic factors in human gastric carcinomas? Eur. J. Cancer 2001, 37, 1482–1487. [Google Scholar] [CrossRef]
- Yeni, M.; Korkut, E.; Aksungur, N.; Kara, S.; Askin, S.; Kartal, M. Determination of Pentraxin-3, Interleukin-8 and Vascular Endothelial Growth Factor Levels in Patients with Gastric Adenocarcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 1507–1512. [Google Scholar] [CrossRef]
- Lin, C.S.; He, P.J.; Hsu, W.T.; Wu, M.S.; Wu, C.J.; Shen, H.W.; Hwang, C.H.; Lai, Y.K.; Tsai, N.M.; Liao, K.W. Helicobacter pylori-derived Heat shock protein 60 enhances angiogenesis via a CXCR2-mediated signaling pathway. Biochem. Biophys. Res. Commun. 2010, 397, 283–289. [Google Scholar] [CrossRef]
- Ju, D.; Sun, D.; Xiu, L.; Meng, X.; Zhang, C.; Wei, P. Interleukin-8 is associated with adhesion, migration and invasion in human gastric cancer SCG-7901 cells. Med. Oncol. 2012, 29, 91–99. [Google Scholar] [CrossRef]
- Chen, L.; Min, L.; Wang, X.; Zhao, J.; Chen, H.; Qin, J.; Chen, W.; Shen, Z.; Tang, Z.; Gan, Q.; et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR-302c/IL8 Signaling Loop. Cancer Res. 2015, 75, 3832–3841. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; He, H.; Liu, H.; Li, R.; Chen, Y.; Qi, Y.; Jiang, Q.; Chen, L.; Zhang, P.; Zhang, H.; et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 2019, 68, 1764. [Google Scholar] [CrossRef]
- Hu, W.; Wang, J.; Luo, G.; Luo, B.; Wu, C.; Wang, W.; Xiao, Y.; Li, J. Proteomics-based analysis of differentially expressed proteins in the CXCR1-knockdown gastric carcinoma MKN45 cell line and its parental cell. Acta Biochim. Biophys. Sin. 2013, 45, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, L.; Rajarathnam, K. Ligand Selectivity and Affinity of Chemokine Receptor CXCR1. J. Biol. Chem. 2004, 279, 30000–30008. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cytokine | Overall Effect | Sources within GC TME | Mechanisms | Effect on Prognosis |
|---|---|---|---|---|
| IL-6 | Pro-tumour | Tumour-associated fibroblasts, tumour cells [51,52,54] | IL-6 serum levels and IL-6R levels in GC tissue have been linked to GC severity and poorer OS. A systematic review by Vainer et al. [64] Found that all six papers which assessed clinical characteristics of GC and IL-6 levels linked decreased OS to increased IL-6. Ashizawa et al. [60] Demonstrated GC survival rates of 43% and 87% in patients with high and low serum levels of IL-6, respectively. | |
| OS | Pro- and anti-tumour | Epithelial Cells, stromal cells [67] |
| No studies which investigated the relationship between TNF levels and GC patient prognosis were found. |
| IFN-γ | Pro- and anti-tumour | CD4+ Th1 and CD8+ cytotoxic T-cells, NK cells [77] | N Sánchez-Zauco. et al. [61] showed that high plasma/serum levels of IFN-γ are associated with GC incidence rate. No studies were found to investigate the relationship between IFN-γ levels and GC patient prognosis. | |
| IL-17A | Pro-tumour | CD4+ Th17 and CD8+ cytotoxic T-cells, NK cells, neutrophils [86,87,88] |
| Mixed evidence: Iida et al. [99] (n = 82) linked increased IL-17 mRNA to increased tumour depth and lymph node involvement, but a meta-analysis by Zeng et al. [97] concluded that there was no statistical significance between IL-17 and worsened prognosis in GC. |
| IL-10 | Pro- and anti-tumour | CD4+ Th1 and Th2 cells, Treg cells, TAMs [101,102,103] | No studies which investigated the relationship between levels of IL-10 and GC prognosis/outcome were found. | |
| IL-8 | Pro-tumour | Macrophages, neutrophils [113], tumour cells [109,114], epithelial cells [111] | Limited evidence available. Kido et al. showed decreased OS in GC patients with high levels of IL-8 vs. low IL-8 (n = 56), but results were not statistically significant [117]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.; Chua, W.; Ng, W.; Roberts, T.L. Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines 2021, 9, 1916. https://doi.org/10.3390/biomedicines9121916
Yang E, Chua W, Ng W, Roberts TL. Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines. 2021; 9(12):1916. https://doi.org/10.3390/biomedicines9121916
Chicago/Turabian StyleYang, Elton, Wei Chua, Weng Ng, and Tara Laurine Roberts. 2021. "Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature" Biomedicines 9, no. 12: 1916. https://doi.org/10.3390/biomedicines9121916
APA StyleYang, E., Chua, W., Ng, W., & Roberts, T. L. (2021). Peripheral Cytokine Levels as a Prognostic Indicator in Gastric Cancer: A Review of Existing Literature. Biomedicines, 9(12), 1916. https://doi.org/10.3390/biomedicines9121916






