Impact of Canopy Gap Ecology on the Diversity and Dynamics of Natural Regeneration in a Tropical Moist Semi-Deciduous Forest, Ghana †
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Impact of Gap Ecology on Species Composition and Diversity
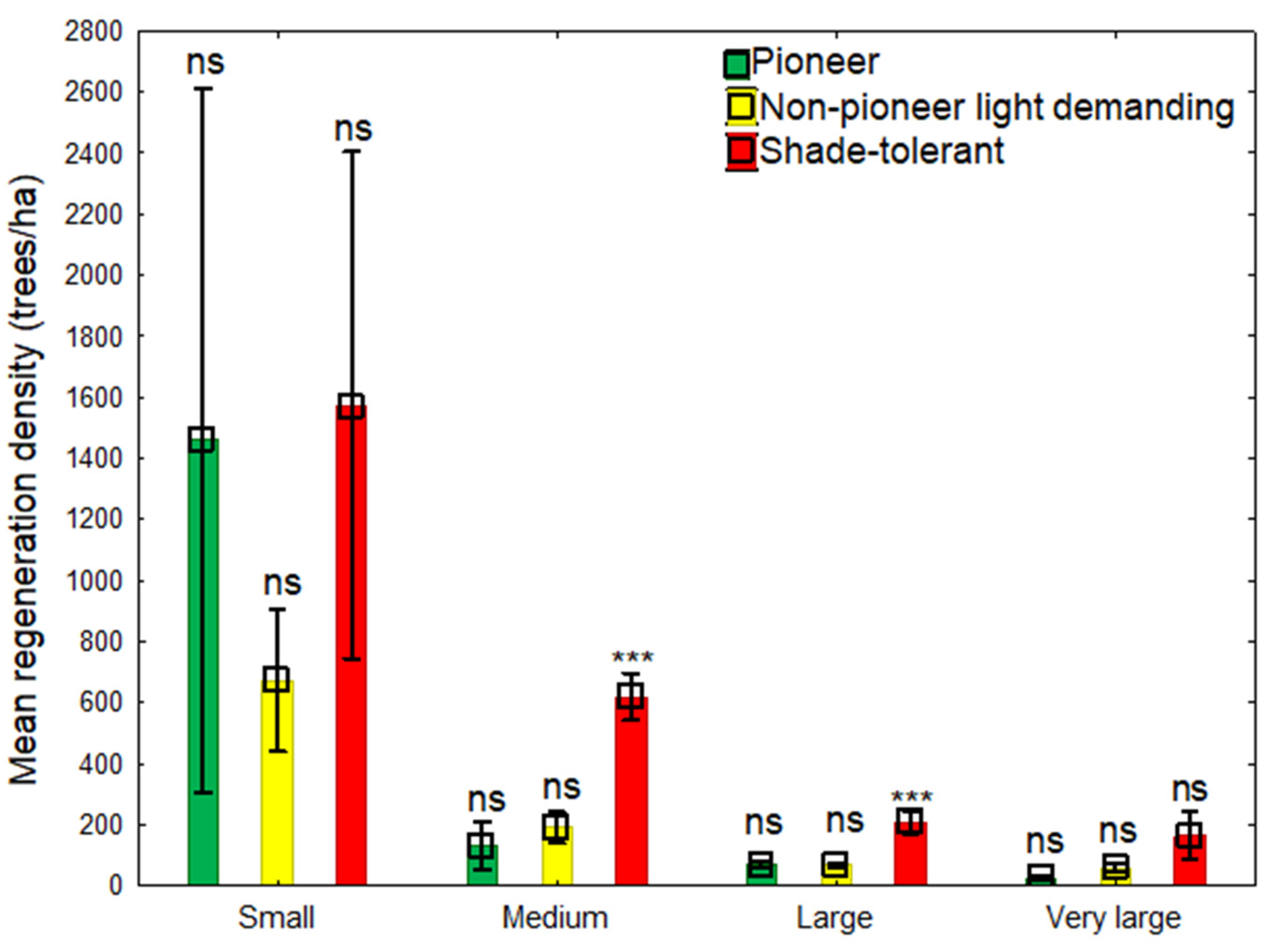
3.2. Impact of Gap Ecology on Regeneration Dynamics of Species with Different Shade Tolerance Mechanisms
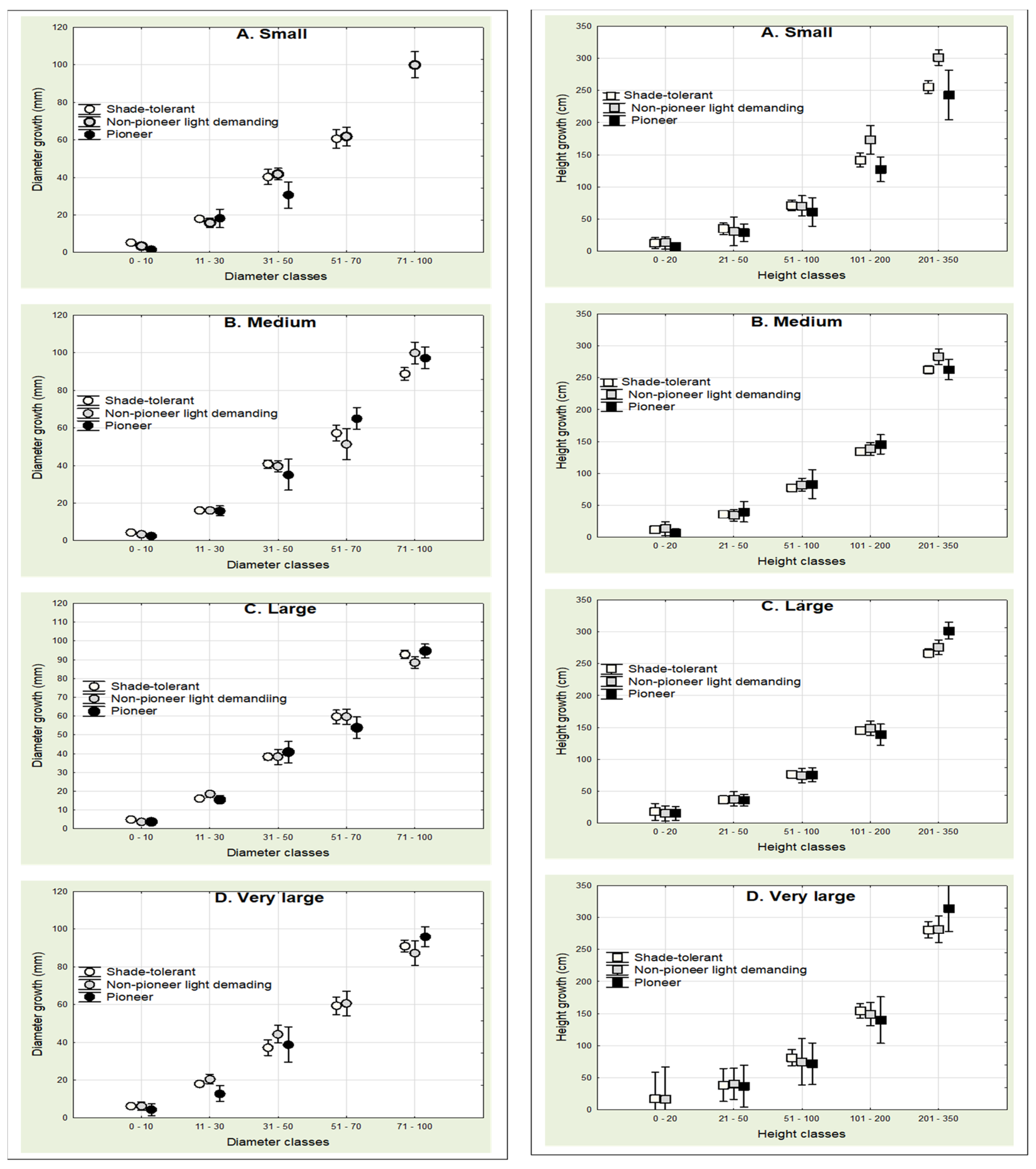
3.3. Impact of Gap Ecology on Growth Dynamics of Different Naturally Regenerated Tree Species
4. Conclusions
Supplementary Materials
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Sherman, R.E.; Fahey, T.J.; Battles, J.J. Small-scale disturbance and regeneration dynamics in a neotropical mangrove forest. J. Ecol. 2000, 88, 165–178. [Google Scholar] [CrossRef]
- Feldmann, E.; Glatthorn, J.; Ammer, C.; Leuschner, C. Regeneration Dynamics Following the Formation of Understory Gaps in a Slovakian Beech Virgin Forest. Forests 2020, 11, 585. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorny, R.; Dobrovolný, L.; Friedl, M.; Hiitola, N. Effect of gap size on tree species diversity of natural regeneration–case study from Masaryk Training Forest Enterprise Křtiny. J. For. Sci. 2020, 66, 407–419. [Google Scholar] [CrossRef]
- Hammond, M.E.; Pokorný, R. Diversity of Tree Species in Gap Regeneration under Tropical Moist Semi-Deciduous Forest: An Example from Bia Tano Forest Reserve. Diversity 2020, 12, 301. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology 2001, 82, 913–919. [Google Scholar] [CrossRef]
- Duah-Gyamfi, A.; Kyereh, B.; Adam, K.A.; Agyeman, V.K.; Swaine, M.D. Natural regeneration dynamics of tree seedlings on skid trails and tree gaps following selective logging in a tropical moist semi-deciduous forest in Ghana. Open J. For. 2014, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, A.L.; D’Oliveira, M.V.N.; Putz, F.E.; De Oliveira, L.C. Natural regeneration of trees in selectively logged forest in western Amazonia. For. Ecol. Manag. 2017, 392, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Harper, D.A. Numerical Palaeobiology: Computer-Based Modelling and Analysis of Fossils and Their Distributions; John Wiley and Sons Inc.: Chichester, UK, 1999. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of Planktonic Foraminifera in Deep-Sea Sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Rozendaal, D.M.A.; Bongers, F.; Aide, T.M.; Alvarez-Dávila, E.; Ascarrunz, N.; Balvanera, P.; Becknell, J.M.; Bentos, T.V.; Brancalion, P.H.S.; Cabral, G.A.L.; et al. Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 2019, 5, eaau3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozendaal, D.M.; During, H.J.; Sterck, F.J.; Asscheman, D.; Wiegeraad, J.; Zuidema, P.A. Long-term growth patterns of juvenile trees from a Bolivian tropical moist forest: Shifting investments in diameter growth and height growth. J. Trop. Ecol. 2015, 31, 519–529. [Google Scholar] [CrossRef]
- Coopersmith, D.J.; Hall, E. Experimental Project 1077: The Siphon Creek Mixedwood Trial: The Use of a Simple Height-to-Diameter Ratio to Predict the Growth Success of Planted White Spruce Seedlings Beneath Aspen Canopies; Prince George Forest Region, Forest Resources and Practices Team: Prince George, BC, Canada, 1999. [Google Scholar]
- Noyer, E.; Ningre, F.; Dlouhá, J.; Fournier, M.; Collet, C. Time shifts in height and diameter growth allocation in understory European beech (Fagus sylvatica L.) following canopy release. Trees 2018, 33, 333–344. [Google Scholar] [CrossRef]

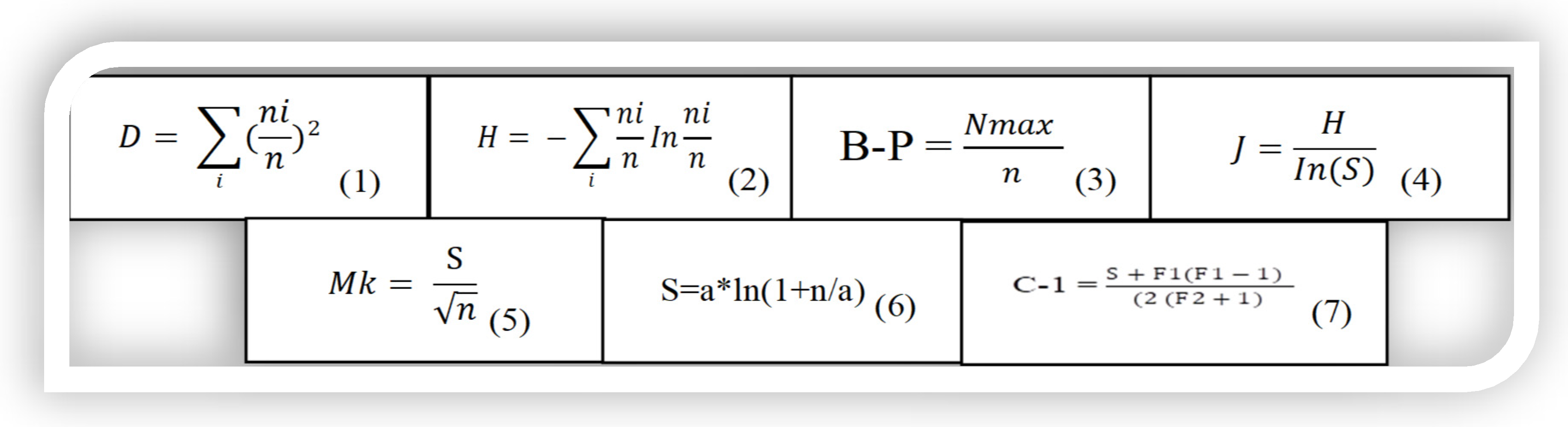
| Gap ID (Size/Area m2) | Estimated Biodiversity Indices | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | S | 1-D | H | B-P | J | Mk | α | C-1 | ||
| Gap1(Large/1896) | 123 | 29 | 0.87 | 2.60 | 0.29 | 0.78 | 2.49 | 11.14 | 29.41 | |
| Gap2(Medium/782) | 90 | 16 | 0.56 | 1.49 | 0.65 | 0.54 | 1.62 | 5.32 | 24.78 | |
| Gap3(Medium/782) | 57 | 11 | 0.65 | 1.56 | 0.55 | 0.65 | 1.46 | 4.06 | 17.72 | |
| Gap4(Large/1252) | 35 | 13 | 0.85 | 2.18 | 0.26 | 0.87 | 2.08 | 6.83 | 28.08 | |
| Gap5(Large/1098) | 46 | 17 | 0.87 | 2.39 | 0.25 | 0.86 | 2.36 | 8.78 | 34.62 | |
| Gap6(Very large/2557) | 102 | 14 | 0.77 | 1.86 | 0.36 | 0.72 | 1.32 | 4.11 | 20.57 | |
| Gap7(Large/1244) | 48 | 15 | 0.85 | 2.27 | 0.28 | 0.84 | 2.12 | 7.21 | 22.32 | |
| Gap8(Large/1457) | 12 | 9 | 0.84 | 1.96 | 0.25 | 0.94 | 2.31 | 12.50 | 14.03 | |
| Gap9(Medium/692) | 25 | 13 | 0.84 | 2.18 | 0.29 | 0.88 | 2.40 | 9.34 | 25.67 | |
| Gap10(Large/1249) | 52 | 11 | 0.81 | 1.95 | 0.31 | 0.81 | 1.48 | 4.07 | 17.67 | |
| Gap11(Large/1425) | 73 | 15 | 0.79 | 2.03 | 0.37 | 0.76 | 1.64 | 5.18 | 22.67 | |
| Gap12(Large/1420) | 37 | 9 | 0.83 | 1.94 | 0.27 | 0.89 | 1.48 | 3.79 | 12.00 | |
| Gap13(Large/1647) | 33 | 9 | 0.73 | 1.63 | 0.39 | 0.77 | 1.45 | 3.62 | 13.50 | |
| Gap14(Large/1058) | 39 | 15 | 0.86 | 2.29 | 0.25 | 0.87 | 2.24 | 7.92 | 23.31 | |
| Gap15(Very large/2728) | 20 | 9 | 0.83 | 1.95 | 0.27 | 0.90 | 1.94 | 5.84 | 14.42 | |
| Gap16(Large/1664) | 50 | 19 | 0.88 | 2.48 | 0.23 | 0.86 | 2.55 | 10.17 | 34.70 | |
| Gap17(Very large/2005) | 57 | 19 | 0.90 | 2.60 | 0.18 | 0.89 | 2.43 | 9.39 | 27.78 | |
| Gap18(Small/296) | 44 | 18 | 0.91 | 2.62 | 0.17 | 0.91 | 2.61 | 10.60 | 24.67 | |
| Gap19(Small/287) | 170 | 19 | 0.79 | 2.05 | 0.37 | 0.70 | 1.46 | 5.48 | 23.38 | |
| Gap20(Medium/607) | 108 | 29 | 0.91 | 2.81 | 0.18 | 0.84 | 2.76 | 12.75 | 42.05 | |
| Gap21(Medium/884) | 88 | 15 | 0.77 | 1.89 | 0.37 | 0.71 | 1.49 | 4.72 | 21.17 | |
| Gap22(Medium/971) | 100 | 20 | 0.71 | 1.98 | 0.51 | 0.66 | 2.00 | 7.52 | 37.00 | |
| Gap23(Medium/837) | 59 | 17 | 0.80 | 2.13 | 0.37 | 0.76 | 2.08 | 7.27 | 31.94 | |
| df | 22 | 22 | 22 | 22 | 22 | 22 | 22 | |||
| F-ratio | 5.94 | 7.52 | 4.21 | 6.04 | 15.37 | 7.72 | 2.15 | |||
| p-value | *** | *** | *** | *** | *** | *** | * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammond, M.E.; Pokorný, R. Impact of Canopy Gap Ecology on the Diversity and Dynamics of Natural Regeneration in a Tropical Moist Semi-Deciduous Forest, Ghana. Biol. Life Sci. Forum 2021, 2, 10. https://doi.org/10.3390/BDEE2021-09455
Hammond ME, Pokorný R. Impact of Canopy Gap Ecology on the Diversity and Dynamics of Natural Regeneration in a Tropical Moist Semi-Deciduous Forest, Ghana. Biology and Life Sciences Forum. 2021; 2(1):10. https://doi.org/10.3390/BDEE2021-09455
Chicago/Turabian StyleHammond, Maame Esi, and Radek Pokorný. 2021. "Impact of Canopy Gap Ecology on the Diversity and Dynamics of Natural Regeneration in a Tropical Moist Semi-Deciduous Forest, Ghana" Biology and Life Sciences Forum 2, no. 1: 10. https://doi.org/10.3390/BDEE2021-09455




