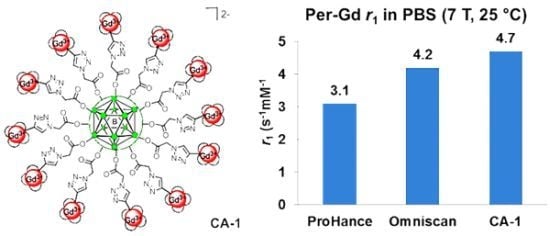
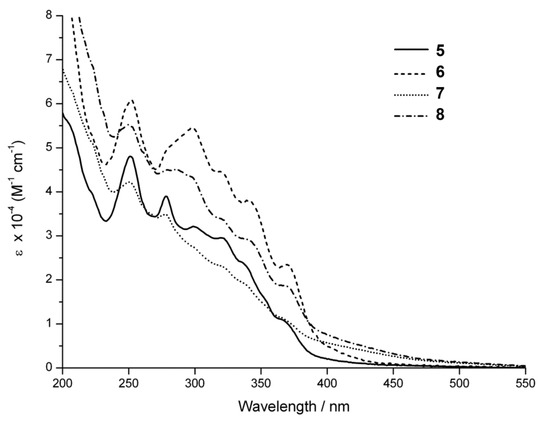
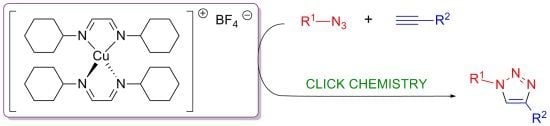
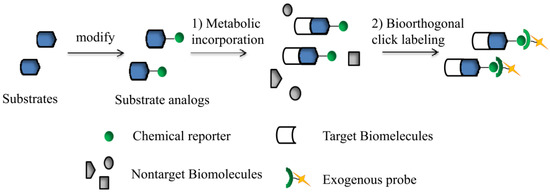
Advances in Click Chemistry
A topical collection in Molecules (ISSN 1420-3049). This collection belongs to the section "Organic Chemistry".
Viewed by 247571Editor
Topical Collection Information
Dear Colleagues,
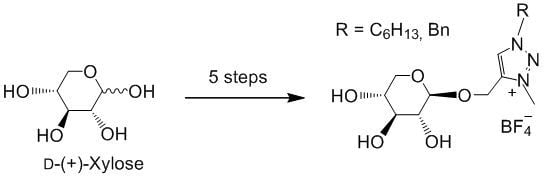
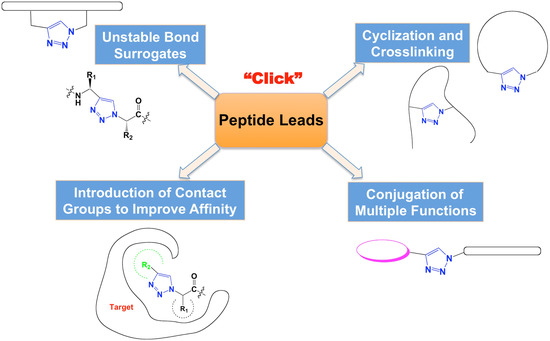
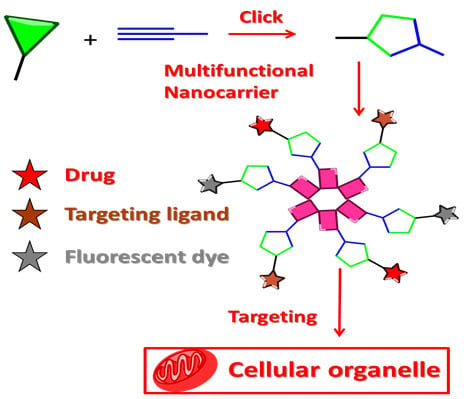
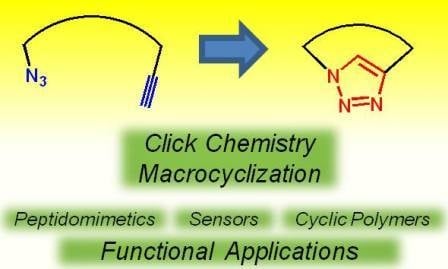
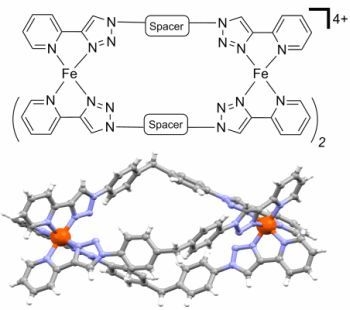
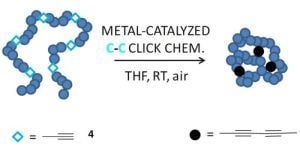
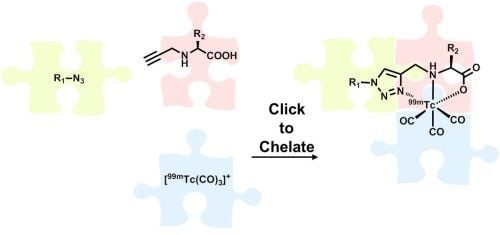
The rise of "click chemistry" as a toolbox gathering only simple, high yielding and easily workable transformations has facilitated an extraordinary increase in the number of molecules available for catalysis, medicinal chemistry, biology, material science and nanotechnologies. Among the synthetic "click tools", the regioselective copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC), is considered as the historical breakthrough in the domain, and has received unrivalled attention. For this reaction, major improvements in the reaction rate and, consequently on the application scopes have arisen from ligand design. However, click chemistry is not restricted to the CuAAC reaction and actually the interest for chemical ligation using strain-promoted alkyne-azide cycloaddition (SPAAC), a copper-free click reaction, is constantly increasing. This allows now pletorious applications at the frontiers of chemistry and biology.
This Special Issue of Molecules will highlight important facets of these milestone reactions, covering all click flavours ranging from methodology to applications. I strongly encourage colleagues to submit their manuscript for this Special Issue to promote and celebrate this exceptional synthetic toolbox.
Dr. Arnaud Gautier
Guest Editor
Submission
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. Papers will be published continuously (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Molecules is an international peer-reviewed Open Access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs).
Keywords
- click chemistry
- CuAAC
- RuAAC
- SPAAC
- chemical ligation
- catalysis
- medicinal chemistry
- material science
- nanosciences