Synthesis of Phospholipid-Protein Conjugates as New Antigens for Autoimmune Antibodies
Abstract
:1. Introduction
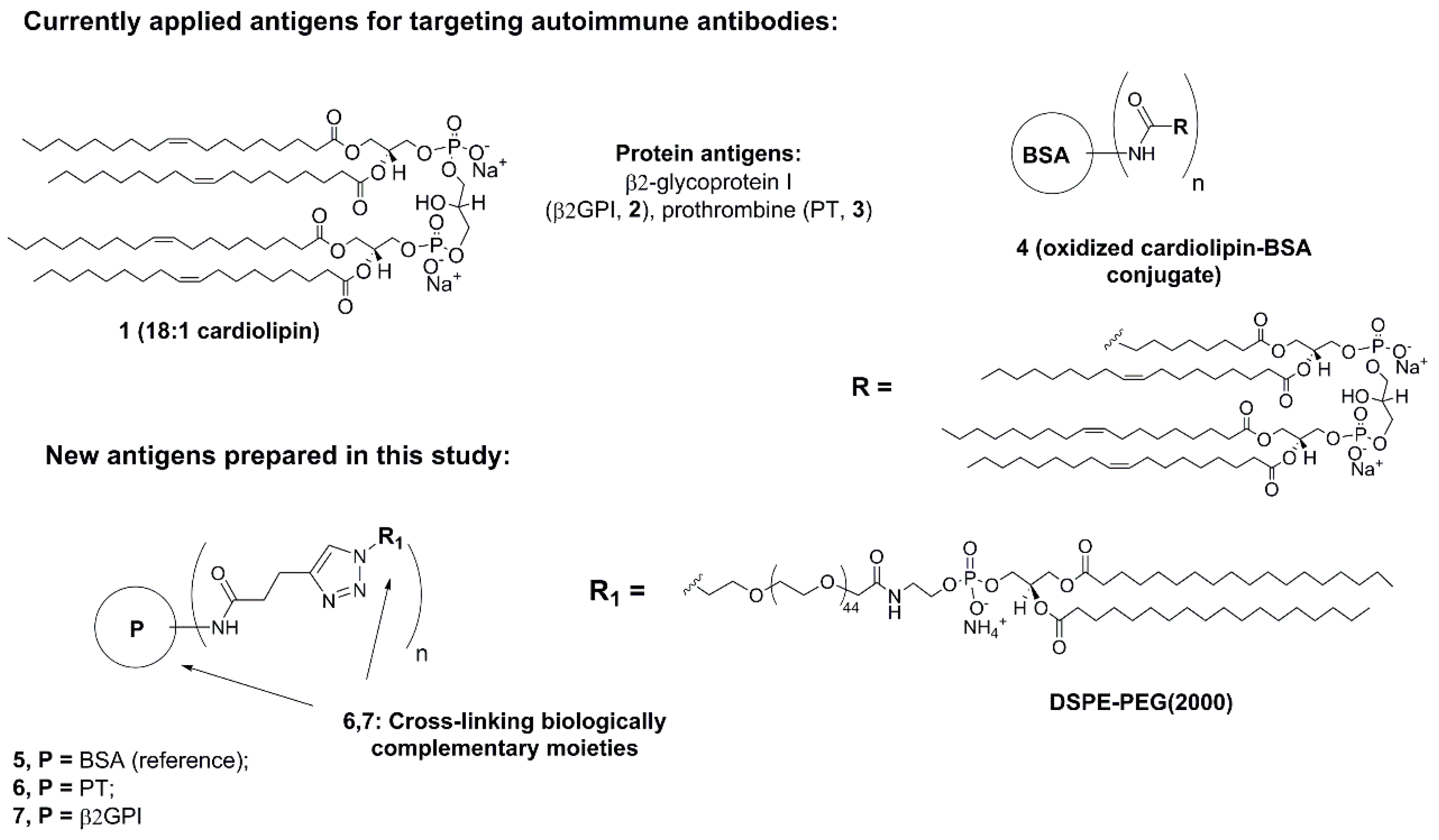
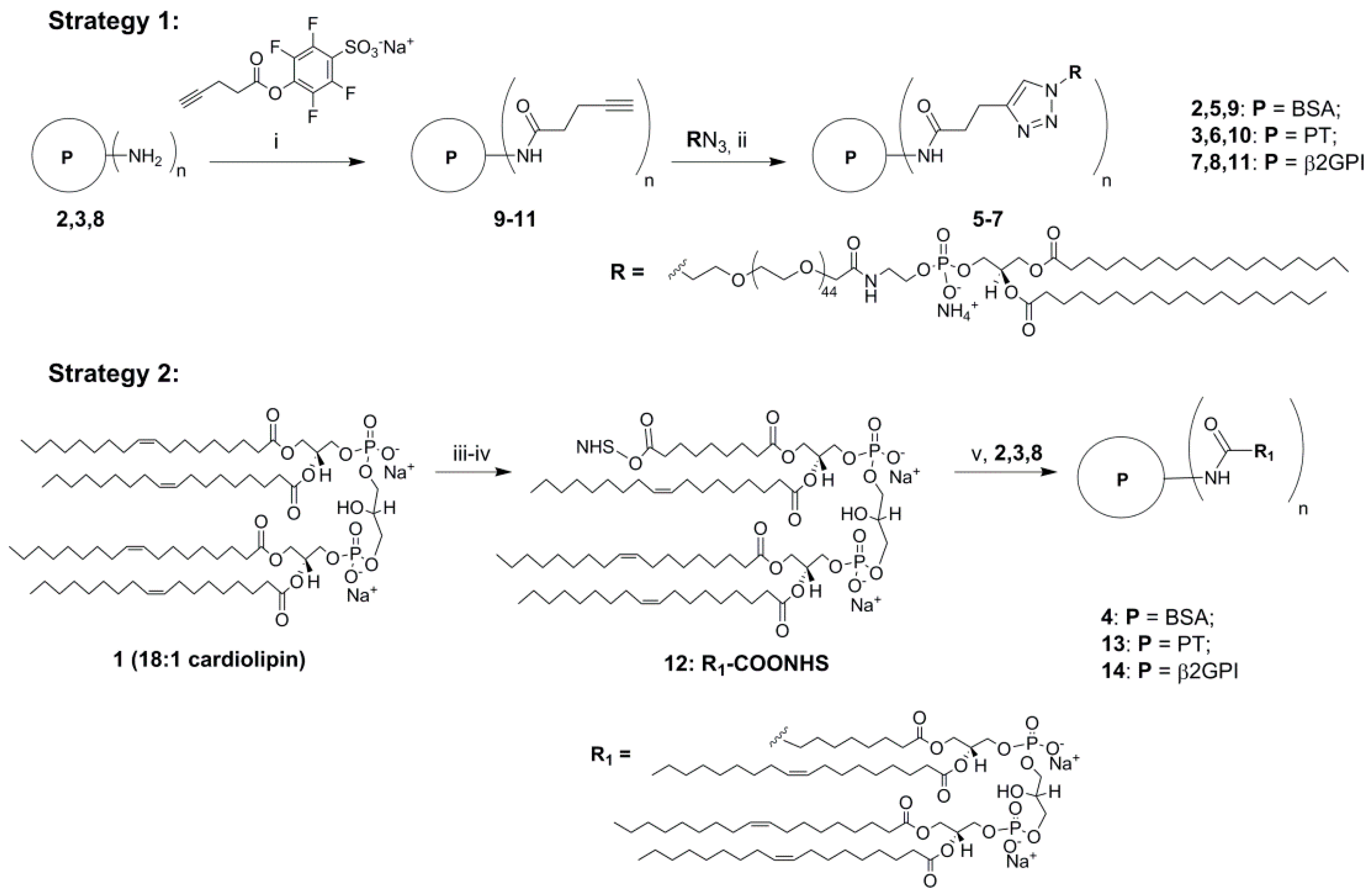
2. Results and Discussion

| Antigen | Absorbance at 450 nm: Analyte | ||||
|---|---|---|---|---|---|
| a-PL | a-β2GPI | a-ssDNA | a-dsDNA | HNP (n = 10) | |
| CL | 1.97 | 0.90 | 0.90 | 0.63 | 0.45 |
| β2GPI | 0.75 | 1.01 | 0.60 | 0.32 | 0.22 |
| CL:β2GPI § | 0.98 | 0.80 | 0.55 | 0.61 | 0.52 |
| PT | 0.45 | 0.31 | 0.43 | 0.45 | 0.23 |
| BSA | 0.31 | 0.25 | 0.34 | 0.28 | 0.24 |
| PE azide | 1.03 | 0.91 | 0.80 | 0.54 | 0.33 |
| 5 (BSA-PE) | 1.44 | 0.56 | 1.44 | 1.21 | 0.43 |
| 6 (PT-PE) | 1.05 | 0.44 | 1.01 | 0.72 | 0.37 |
| 7 (β2GPI-PE) | 1.02 | 1.15 | 0.45 | 0.34 | 0.19 |
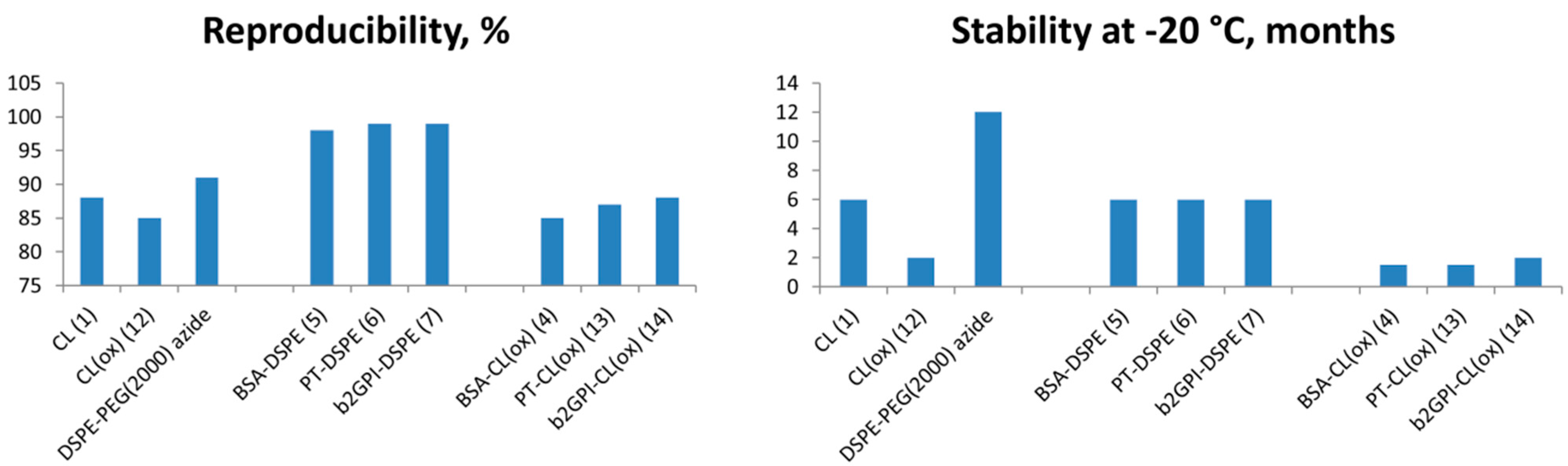
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hughes, G.R. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br. Med. J. (Clin. Res. Ed.) 1983, 287, 1088–1089. [Google Scholar]
- Roddick, J. Autoimmune Disease. Available online: http://www.healthline.com/health/autoimmune-disorders#Overview1,Retrieved (accessed on 13 March 2015).
- Giannakopoulos, B.; Passam, F.; Rahgozar, S.; Krilis, S.A. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood 2007, 109, 422–429. [Google Scholar]
- Rand, J.H. The antiphospholipid syndrome. Ann. Rev. Med. 2003, 54, 409–424. [Google Scholar]
- Pierangeli, S.S.; Chen, P.P.; Raschi, E.; Scurati, S.; Grossi, C.; Borghi, M.O. Antiphospholipid antibodies and the antiphospholipid syndrome: Pathogenic mechanisms. Semin. Thromb. Hemost. 2008, 34, 236–250. [Google Scholar]
- Ruiz-Irastorza, G.; Crowther, M.; Branch, W.; Khamashta, M.A. Antiphospholipid syndrome. Lancet 2010, 376, 1498–1509. [Google Scholar]
- Matzner, W.; Chong, P.; Xu, G.; Ching, W. Characterization of antiphospholipid antibodies in women with recurrent spontaneous abortions. J. Reprod. Med. 1994, 39, 27–30. [Google Scholar]
- Meroni, P.L.; Borghi, M.O.; Raschi, E.; Tedesco, F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat. Rev. Rheumatol. 2011, 7, 330–339. [Google Scholar]
- Bertolaccini, M.L. Antibodies to prothrombin. Lupus 2012, 21, 729–731. [Google Scholar]
- Bertolaccini, M.L.; Gomez, S.; Pareja, J.F.; Theodoridou, A.; Sanna, G.; Hughes, G.R. Antiphospholipid antibody tests: Spreading the net. Ann. Rheum. Dis. 2005, 64, 1639–1643. [Google Scholar]
- Bertolaccini, M.L.; Amengual, O.; Atsumi, T.; Binder, W.L.; de Laat, B.; Forastiero, R. ‘Non-criteria’ aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011, 20, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Sali, A.; Herzog, H.; Lahnstein, J.; Krilis, S.A. Site-directed mutagenesis of recombinant human beta 2-glycoprotein I identifies a cluster of lysine residues that are critical for phospholipid binding and anti-cardiolipin antibody activity. J. Immunol. 1996, 157, 3744–3751. [Google Scholar] [PubMed]
- Himoto, T.; Yoneyama, H.; Kurokohchi, K.; Mori, H.; Inukai, M.; Masugata, H.; Goda, F.; Haba, R.; Watanabe, S.; Senda, S.; et al. Clinical relevance of antibodies to cardiolipin in patients with chronic hepatitis C. J. Clin. Lab. Anal. 2012, 26, 342–348. [Google Scholar]
- Pierangeli, S.S.; Harris, E.N. A protocol for determination of anticardiolipin antibodies by ELISA. Nat. Protoc. 2008, 3, 840–848. [Google Scholar]
- Mehdi, H.; Naqvi, A.; Kamboh, M.I. Recombinant hepatitis B surface antigen and anionic phospholipids share a binding region in the fifth domain of beta2-glycoprotein I (apolipoprotein H). Biochim. Biophys. Acta 2008, 1782, 163–168. [Google Scholar]
- Delgado, A.J.; Kumar, S.; Isenberg, D.A. Cross-reactivity between anti-cardiolipin, anti-high-density lipoprotein and anti-apolipoprotein A-I IgG antibodies in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Rheumatology 2003, 42, 893–899. [Google Scholar]
- Mehdi, H.; Naqvi, A.; Kamboh, M.I. A hydrophobic sequence at position 313–316 (Leu-Ala-Phe-Trp) in the fifth domain of apolipoprotein H (beta2-glycoprotein I) is crucial for cardiolipin binding. Eur. J. Biochem. 2000, 267, 1770–1776. [Google Scholar]
- Castro, A.R.; Wang, H. Modified Cardiolipin and Uses There for. WO 2007061793 A2, 31 May 2007. [Google Scholar]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar]
- Hein, C.; Liu, X.-M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I) catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar]
- Yu, H.; Nie, Y.; Dohmen, C.; Li, Y.; Wagner, E. Epidermal growth factor-PEG functionalized PAMAM-pentaethylene hexamine dendron for targeted gene delivery produced by click chemistry. Biomacromolecules 2011, 12, 2039–2047. [Google Scholar]
- Bratcher, P.E.; Gaggar, A. Factors Influencing the Measurement of Plasma/Serum Surfactant Protein D Levels by ELISA. PLoS ONE 2014, 9, e111466. [Google Scholar]
- Cheung, Y.B.; Xu, Y.; Remarque, E.J.; Milligan, P. Statistical estimation of antibody concentration using multiple dilutions. J. Immunol. Methods 2015, 417, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Jin, H.; Suenaga, T.; Morikami, S.; Arase, N.; Kishida, K.; Hirayasu, K.; Kohyama, M.; Ebina, Y.; Yasuda, S.; et al. β2-glycoprotein I/HLA class II complexes are novel autoantigens in antiphospholipid syndrome. Blood 2015, 125, 2835–2844. [Google Scholar]
- Korematsu, S.; Miyahara, H.; Kakita, A.; Izumi, T. Elevated serum anti-phosphatidylcholine IgG antibodies in patients with influenza vaccination-associated optic neuritis. Vaccine 2014, 32, 6345–6348. [Google Scholar]
- Pengo, V.; Bison, E.; Denas, G.; Jose, S.P.; Bracco, A.; Banzato, A. The paradox of the lupus anticoagulant: History and perspectives. Semin. Thromb. Hemost. 2014, 40, 860–865. [Google Scholar]
- Sikara, M.P.; Routsias, J.G.; Samiotaki, M.; Panayotou, G.; Moutsopoulos, H.M.; Vlachoyiannopoulos, P.G. {beta}2 Glycoprotein I ({beta}2GPI) binds platelet factor 4 (PF4): implications for the pathogenesis of antiphospholipid syndrome. Blood 2010, 115, 713–723. [Google Scholar]
- Kumar, S.; Nagl, S.; Kalsi, J.K.; Ravirajan, C.T.; Athwal, D.; Latchman, D.S.; Pearl, L.H.; Isenberg, D.A. Anti-cardiolipin/beta-2 glycoprotein activities co-exist on human anti-DNA antibody light chains. Mol. Immunol. 2003, 40, 517–530. [Google Scholar]
- Abe, H.; Tsuboi, N.; Suzuki, S.; Sakuraba, H.; Takanashi, H.; Tahara, K.; Tonozuka, N.; Hayashi, T.; Umeda, M. Anti-apolipoprotein A-I autoantibody: Characterization of monoclonal autoantibodies from patients with systemic lupus erythematosus. J. Rheumatol. 2001, 28, 990–995. [Google Scholar]
- Ravirajan, C.T.; Harmer, I.; McNally, T.; Hohmann, A.; Mackworth-Young, C.G.; Isenberg, D.A. Phospholipid binding specificities and idiotype expression of hybridoma derived monoclonal autoantibodies from splenic cells of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 1995, 54, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, L.; Ruffatti, A.; Gavasso, S.; Tonello, M.; Mattia, E.; Spiezia, L.; Campello, E.; Hoxha, A.; Fedrigo, M.; Punzi, L.; et al. The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-β2 glycoprotein I antibodies. A comparison with a homemade ELISA method. Clin. Chem. Lab. Med. 2015. [Google Scholar] [CrossRef]
- Emmi, G.; Silvestri, E.; Squatrito, D.; Ciucciarelli, L.; Cameli, A.M.; Denas, G.; D’Elios, M.M.; Pengo, V.; Emmi, L.; Prisco, D. An Approach to Differential Diagnosis of Antiphospholipid Antibody Syndrome and Related Conditions. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Koike, T. Antiphospholipid syndrome: 30 years and our contribution. Int. J. Rheum. Dis. 2015, 18, 233–241. [Google Scholar]
- Wilson, R.F.; Rinne, R.W. Effect of Freezing and Cold Storage on Phospholipids in Developing Soybean Cotyledons. Plant Physiol. 1976, 57, 270–273. [Google Scholar]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. [Google Scholar]
- Sample Availability: Samples of the phospholipid-protein conjugates 4–7,13,14 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maity, A.; Macaubas, C.; Mellins, E.; Astakhova, K. Synthesis of Phospholipid-Protein Conjugates as New Antigens for Autoimmune Antibodies. Molecules 2015, 20, 10253-10263. https://doi.org/10.3390/molecules200610253
Maity A, Macaubas C, Mellins E, Astakhova K. Synthesis of Phospholipid-Protein Conjugates as New Antigens for Autoimmune Antibodies. Molecules. 2015; 20(6):10253-10263. https://doi.org/10.3390/molecules200610253
Chicago/Turabian StyleMaity, Arindam, Claudia Macaubas, Elizabeth Mellins, and Kira Astakhova. 2015. "Synthesis of Phospholipid-Protein Conjugates as New Antigens for Autoimmune Antibodies" Molecules 20, no. 6: 10253-10263. https://doi.org/10.3390/molecules200610253






