Solvent-Free Copper-Catalyzed Azide-Alkyne Cycloaddition under Mechanochemical Activation
Abstract
:1. Introduction
2. Results and Discussion
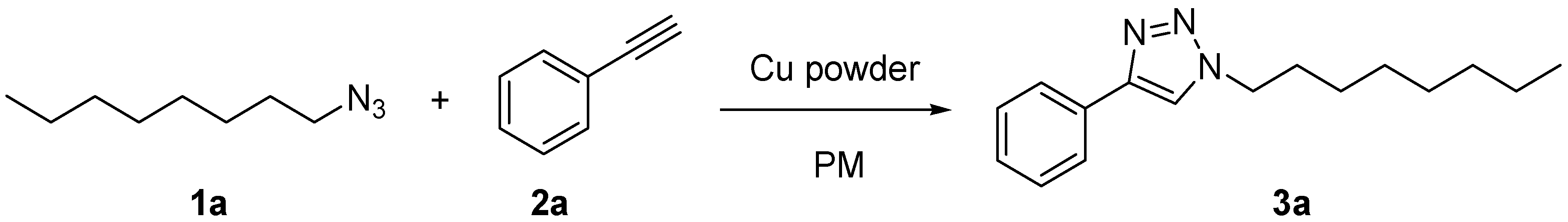
| Entry | Ball Number | Active Surface Area b (mm2) | Yield (%) a | ||
|---|---|---|---|---|---|
| Small (Ø = 2 mm) | Medium (Ø = 5 mm) | Big (Ø = 10 mm) | |||
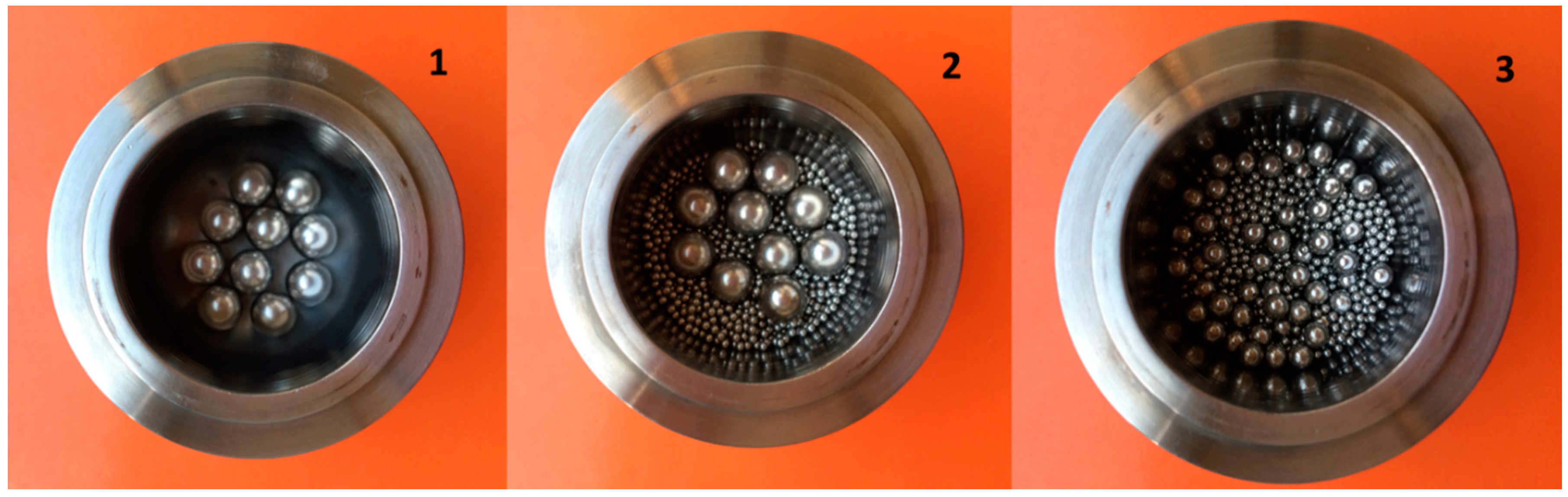
| 1 | none | none | 10 | 10,666 | 67 |
| 2 | 625 | none | 10 | 18,520 | 80 |
| 3 | 1500 | 48 | none | 30,144 | 99 |
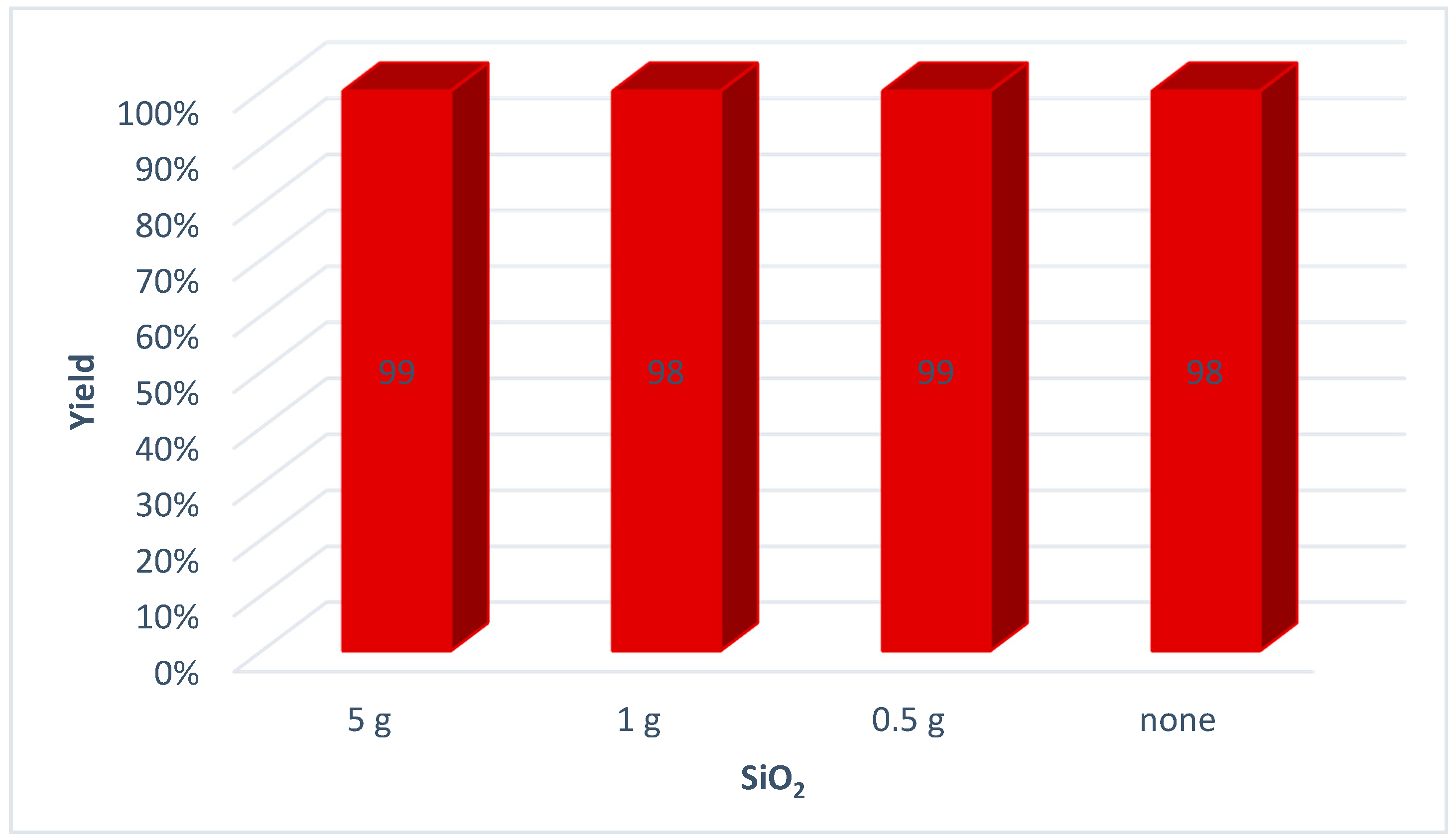

| Entry | Octyl Azide (1a) | Yield a | ||
|---|---|---|---|---|
| mmol | g | % | g | |
| 1 b | 1 | 0.1552 | 99 | 0.254 |
| 2 b | 10 | 1.5524 | 98 | 2.519 |
| 3 c | 50 | 7.7620 | 95 | 12.208 |
| Entry | Alkyne | Azide | Product | Yield b % (Conv. c) |
|---|---|---|---|---|
| 1 d | 2a |  1b |  3b | 98 (>99) |
| 2 d | 2a |  1c |  3c | 97 (>99) |
| 3 | 2a |  1d |  3d | 95 (>99) |
| 4 d | 2a |  1e |  3e | 91 (95) |
| 4 d | 2a |  1f |  3f | 94 (97) |
| 5 d | 2a |  1g |  3g | 97 (>99) |
| 6 | 2a |  1h |  3h | 90 (92) |
| 7 d |  2b |  1i |  3i | 98 (>99) |
| 8 |  2c | 1a |  3j | 92 (96) |
| 9 |  2d | 1a |  3k | 88 (91) |
| 10 e |  2e | 1a |  3l | 98 (>99) |
| 11 f | 2a |  1j |  3m | 81 |
3. Experimental Section
3.1. General Information
3.2. General Reaction Protocols
3.2.1. General Procedure A for Alkyne-Azide Click Reaction (Preparation of 3a, 3d, 3h)
3.2.2. General Procedure B for Alkyne-Azide Click Reaction (Preparation of 3b, 3c, 3e, 3f, 3g, 3i)
3.2.3. General Procedure C for Alkyne-Azide Click Reaction (Preparation of 3l)
3.2.4. General Procedure D for Alkyne-Azide Click Reaction (Preparation of 3m)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huisgen, R. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, NY, USA, 1984; Volume 1, pp. 1–176. [Google Scholar]
- Padwa, A. Intermolecular 1,3-dipolar cycloaddition. In Comprehensive Organic Synthesis; Trost, B.M., Ed.; Pergamon: Oxford, UK, 1991; Volume 4, pp. 1069–1109. [Google Scholar]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide-Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Whiting, M.; Tripp, J.C.; Lin, Y.C.; Lindstrom, W.; Olson, A.J.; Elder, J.H.; Sharpless, K.B.; Fokin, V.V. Rapid discovery and structure-activity profiling of novel inhibitors of human immunodeficiency virus type 1 protease enabled by the copper(I)-catalyzed synthesis of 1,2,3-triazoles and their further functionalization. J. Med. Chem. 2006, 49, 7697–7710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper(I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Fokin, V.V.; Finn, M.G.; Sharpless, K.B. Bringing Efficiency to Materials Synthesis: The Philosophy of Click Chemistry. Aust. J. Chem. 2007, 60, 381–383. [Google Scholar] [CrossRef]
- Lévêque, J.-M.; Cravotto, G. Microwaves, power ultrasound, and ionic liquids. A new synergy in green organic synthesis. Chimia 2006, 60, 313–320. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The Combined Use of Microwaves and Ultrasound. In Methods and Practice, Microwaves in Organic Synthesis, 3rd ed.; de La Hoz, A., Loupy, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 1, pp. 541–562. [Google Scholar]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131–1141. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Barge, A.; Tagliapietra, S.; Binello, A.; Cravotto, G. Click chemistry under microwave or ultrasound irradiation. Curr. Org. Chem. 2011, 15, 189–203. [Google Scholar] [CrossRef]
- Kappe, C.O.; van der Eycken, E. Click chemistry under non-classical reaction conditions. Chem. Soc. Rev. 2010, 39, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Strack, M.; Langklotz, S.; Bandow, J.E.; Metzler-Nolte, N.; Albada, H.B. Silyl-Based Alkyne-Modifying Linker for the Preparation of C-Terminal Acetylene-Derivatized Protected Peptides. J. Org. Chem. 2012, 77, 9954–9958. [Google Scholar] [CrossRef] [PubMed]
- Capicciotti, C.J.; Trant, J.F.; Leclere, M.; Ben, R.N. Synthesis of C-Linked Triazole-Containing AFGP Analogues and their Ability to Inhibit Ice Recrystallization. Bioconjugate Chem. 2011, 22, 605–616. [Google Scholar] [CrossRef]
- Glowacka, I.E.; Balzarini, J.; Wroblewski, A.J.E. Synthesis and biological evaluation of novel 1,2,3-triazolonucleotides. Arch. Pharm. 2013, 346, 278–291. [Google Scholar] [CrossRef]
- Isobe, H.; Fujino, T.; Yamazaki, N.; Guillot-Nieckowski, M.; Nakamura, E. Triazole-linked analogue of deoxyribonucleic acid (TLDNA): Design, synthesis, and double-strand formation with natural DNA. Org. Lett. 2008, 10, 3729–3732. [Google Scholar] [CrossRef] [PubMed]
- Barge, A.; Caporaso, M.; Cravotto, G.; Martina, K.; Tosco, P.; Aime, S.; Carrera, C.; Gianolio, E.; Pariani, G.; Corpillo, D. Design and Synthesis of a γ(1)β(8)-Cyclodextrin Oligomer: A New Platform with Potential Application as a Dendrimeric Multicarrier. Chemistry 2013, 19, 12086–12092. [Google Scholar] [CrossRef]
- Carmona, T.; Marcelo, G.; Rinaldi, L.; Martina, K.; Cravotto, G.; Mendicuti, F. Soluble cyanine dye/β-cyclodextrin derivatives: Potential carriers for drug delivery and optical imaging. Dyes Pigments 2015, 114, 204–214. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Cai, C.-Z. Rapid Grafting of Azido-Labeled Oligo(ethylene glycol)s onto an Alkynyl-Terminated Monolayer on Nonoxidized Silicon via Microwave-Assisted “Click” Reaction. Langmuir 2011, 27, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, A.E.; Hansen, T.S.; Larsen, N.B.; Hvilsted, S. Microwave assisted click chemistry on a conductive polymer film. Synth. Met. 2011, 161, 812–816. [Google Scholar] [CrossRef]
- Martina, K.; Baricco, F.; Berlier, G.; Caporaso, M.; Cravotto, G. Efficient green protocols for the preparation of highly functionalized β-cyclodextrin grafted silica. ACS Sustain. Chem. Eng. 2014, 2, 2595–2603. [Google Scholar] [CrossRef]
- Tagliapietra, S.; Cravotto, G.; Gaudino, E.C.; Visentin, S.; Mussi, V. Functionalization of Single-Walled Carbon Nanotubes through 1,3-Cycloaddition of Carbonyl Ylides under Microwave Irradiation. Synlett 2012, 23, 1459–1462. [Google Scholar] [CrossRef]
- Nagao, Y.; Takasu, A. “Click polyester”: Synthesis of polyesters containing triazole units in the main chain via safe and rapid “click” chemistry and their properties. J. Polym. Sci. Pol. Chem. 2010, 48, 4207–4218. [Google Scholar] [CrossRef]
- Marullo, S.; D’Anna, F.; Rizzo, C.; Noto, R. The ultrasound-ionic liquids synergy on the copper catalyzed azide–alkyne cycloaddition between phenylacetylene and 4-azidoquinoline. Ultrason. Sonochem. 2015, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Cintas, P.; Palmisano, G.; Cravotto, G. Power ultrasound in metal-assisted synthesis: From classical Barbier-like reactions to click chemistry. Ultrason. Sonochem. 2011, 18, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Cintas, P.; Carnaroglio, D.; Rinaldi, L.; Cravotto, G. Complementary and synergic effects of microwaves and ultrasound in metal-assisted synthesis. Chem. Today 2012, 30, 33–35. [Google Scholar]
- Cravotto, G.; Calcio Gaudino, E.; Cintas, P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 2013, 42, 7521–7534. [Google Scholar] [CrossRef] [PubMed]
- Antilla, J.C.; Klapars, A.; Buchwald, S.L. The Copper-Catalyzed N-Arylation of Indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef] [PubMed]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gommermann, N.; Gehrig, A.; Knochel, P. Enantioselective Synthesis of Chiral α-Aminoalkyl-1,2,3-triazoles Using a Three-Component Reaction. Synlett 2005, 2796–2798. [Google Scholar] [CrossRef]
- Cintas, P.; Barge, A.; Tagliapietra, S.; Boffa, L.; Cravotto, G. Alkyne-azide click reaction catalyzed by metallic copper under ultrasound. Nat. Prot. 2010, 5, 607–616. [Google Scholar] [CrossRef]
- Cravotto, G.; Fokin, V.V.; Garella, D.; Binello, A.; Boffa, L.; Barge, A. Ultrasound-Promoted Copper-Catalyzed Azide-Alkyne Cycloaddition. J. Comb. Chem. 2010, 12, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Harnessing mechanochemical effects with ultrasound-induced reactions. Chem. Sci. 2012, 3, 295–307. [Google Scholar] [CrossRef]
- Cintas, P.; Martina, K.; Robaldo, B.; Garella, D.; Boffa, L.; Cravotto, G. Improved protocols for microwave-assisted Cu(I)-catalyzed Huisgen 1,3-dipolar cycloadditions. Collect. Czech. Chem. C 2007, 72, 1014–1024. [Google Scholar] [CrossRef]
- Balaz, P.; Achimovicova, M.; Balaz, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutkova, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Schmidt, R.; Jacob, K. Scale-up of organic reactions in ball mills: Process intensification with regard to energy efficiency and economy of scale. Faraday Discuss. 2014, 170, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Addario, D.D.; Polito, M.; Grepioni, F. Mechanically induced expeditious and selective preparation of disubstituted pyridine/pyrimidine ferrocenyl complexes. Organometallics 2004, 23, 2810–2812. [Google Scholar] [CrossRef]
- Ranu, B.C.; Stolle, A. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Cravotto, G.; Garella, D.; Carnaroglio, D.; Calcio Gaudino, E.; Rosati, O. Solvent-free chemoselective oxidation of thioethers and thiophenes by mechanical milling. Chem. Commun. 2012, 48, 11632–11634. [Google Scholar] [CrossRef]
- Trotzki, R.; Hoffmann, M.M.; Ondruschka, B. Studies on the solvent-free and waste-free Knoevenagel condensation. Green Chem. 2008, 10, 767–772. [Google Scholar] [CrossRef]
- Tan, Y.-J.; Zhang, Z.; Wang, F.-J.; Wu, H.-H.; Li, Q.-H. Mechanochemical milling promoted solvent-free imino Diels-Alder reaction catalyzed by FeCl3: Diastereoselective synthesis of cis-2,4-diphenyl-1,2,3,4-tetrahydroquinolines. RSC Adv. 2014, 4, 35635–35638. [Google Scholar] [CrossRef]
- Groote, R.; Szyja, B.M.; Leibfarth, F.A.; Hawker, C.J.; Doltsinis, N.L.; Sijbesma, R.P. Strain-Induced Strengthening of the Weakest Link: The Importance of Intermediate Geometry for the Outcome of Mechanochemical Reactions. Macromolecules 2014, 47, 1187–1192. [Google Scholar] [CrossRef]
- Zhu, S.-E.; Li, F.; Wang, G.-W. Mechanochemistry of fullerene and related materials. Chem. Soc. Rev. 2013, 42, 7535–7570. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Energetic assessment of the Suzuki-Miyaura reaction: A curtate life cycle assessment as an easily understandable and applicable tool for reaction optimization. Green Chem. 2009, 11, 1894–1899. [Google Scholar] [CrossRef]
- Thorwirth, R.; Stolle, A.; Ondruschka, B.; Wild, A.; Schubert, U.S. Fast, ligand- and solvent-free copper-catalyzed click reactions in a ball mill. Chem. Commun. 2011, 47, 4370–4372. [Google Scholar] [CrossRef]
- Tan, D.; Mottillo, C.; Katsenis, A.D.; Štrukil, V.; Friščić, T. Development of C-N coupling using mechanochemistry: Catalytic coupling of arylsulfonamides and carbodiimides. Angew. Chem. Int. Ed. 2014, 53, 9321–9324. [Google Scholar] [CrossRef]
- Boldyrev, V.V. ; Mechanochemistry and sonochemistry. Ultrason. Sonochem. 1995, 2, S143–S145. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Raston, C.L.; Scotta, J.L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility. Chem. Commun. 2001, 2159–2169. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef] [PubMed]
- Klufers, P.; Piotrowski, H.; Uhlendorf, J. Homoleptic Cuprates(II) with Multiply Deprotonated α-Cyclodextrin Ligands. Chem. Eur. J. 1997, 3, 601–608. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3a–3m are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, L.; Martina, K.; Baricco, F.; Rotolo, L.; Cravotto, G. Solvent-Free Copper-Catalyzed Azide-Alkyne Cycloaddition under Mechanochemical Activation. Molecules 2015, 20, 2837-2849. https://doi.org/10.3390/molecules20022837
Rinaldi L, Martina K, Baricco F, Rotolo L, Cravotto G. Solvent-Free Copper-Catalyzed Azide-Alkyne Cycloaddition under Mechanochemical Activation. Molecules. 2015; 20(2):2837-2849. https://doi.org/10.3390/molecules20022837
Chicago/Turabian StyleRinaldi, Laura, Katia Martina, Francesca Baricco, Laura Rotolo, and Giancarlo Cravotto. 2015. "Solvent-Free Copper-Catalyzed Azide-Alkyne Cycloaddition under Mechanochemical Activation" Molecules 20, no. 2: 2837-2849. https://doi.org/10.3390/molecules20022837