Biorecognition Assays for Mycotoxins
A topical collection in Toxins (ISSN 2072-6651). This collection belongs to the section "Mycotoxins".
Viewed by 159137Editors
Interests: development and validation of analytical methods for food contaminants, sample preparation; chromatographic methods; rapid methods
Special Issues, Collections and Topics in MDPI journals
Interests: nucleic acid aptamers; targeted delivery; biorecognition; biosensing, DNA origami
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
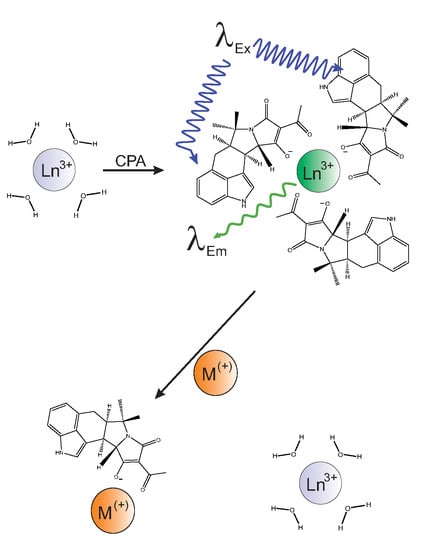
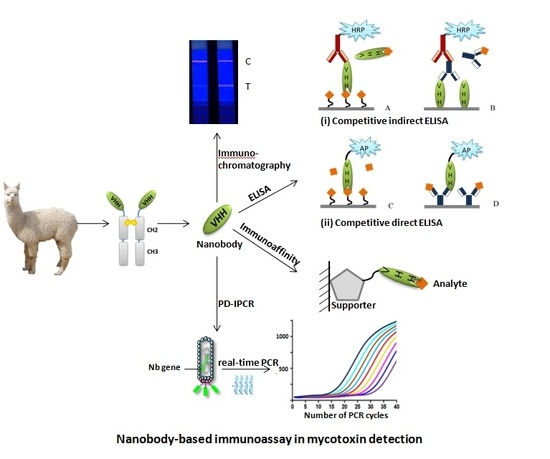
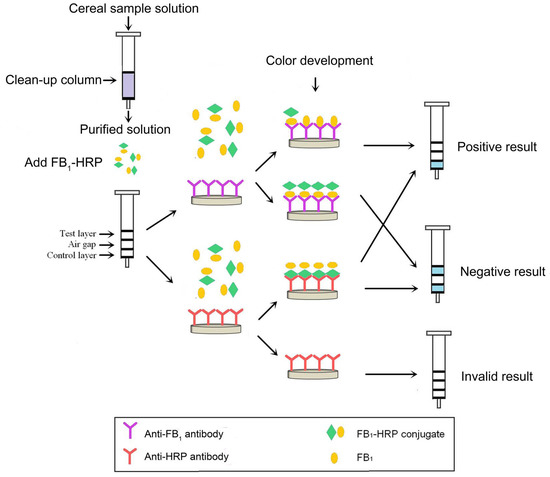
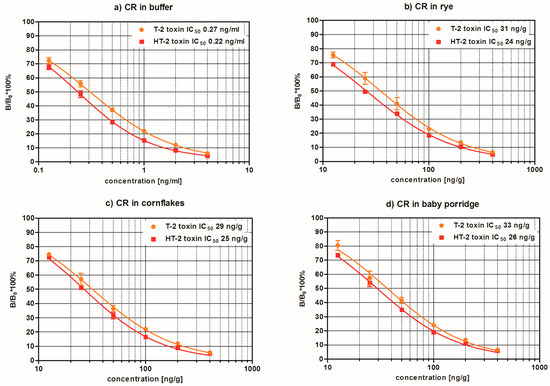
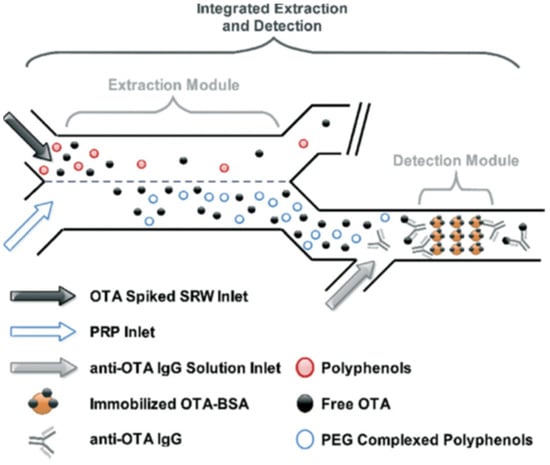
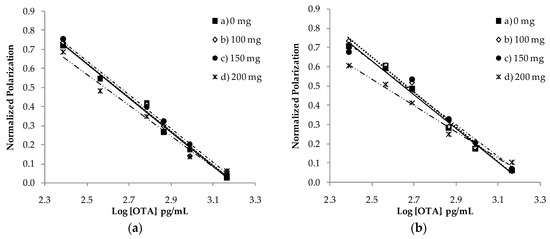
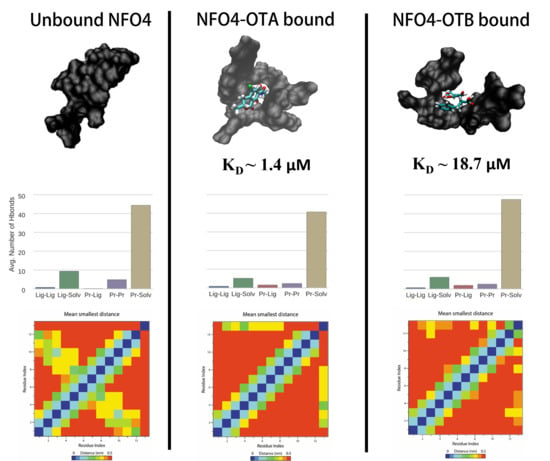
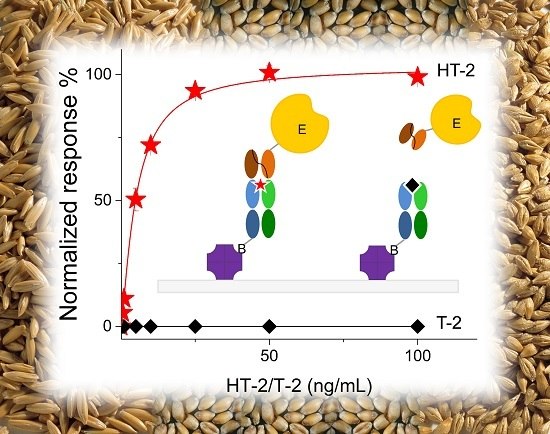
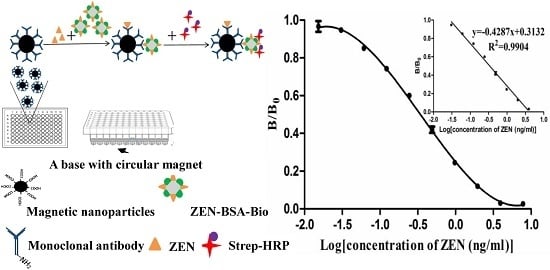
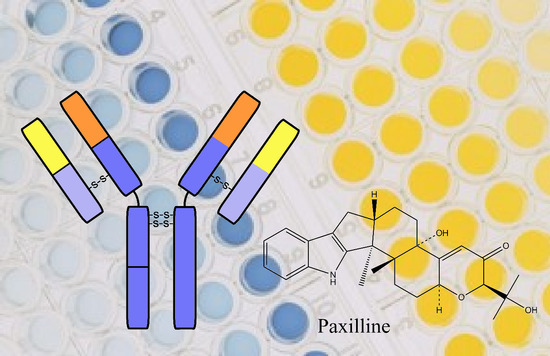
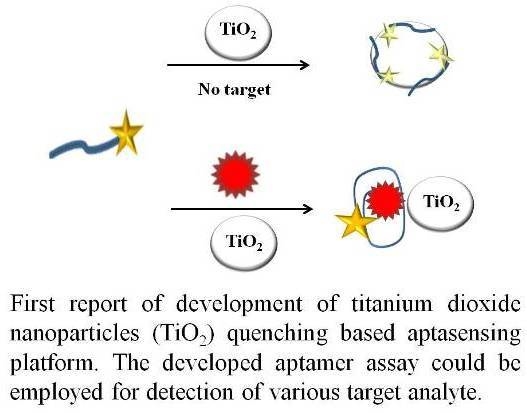
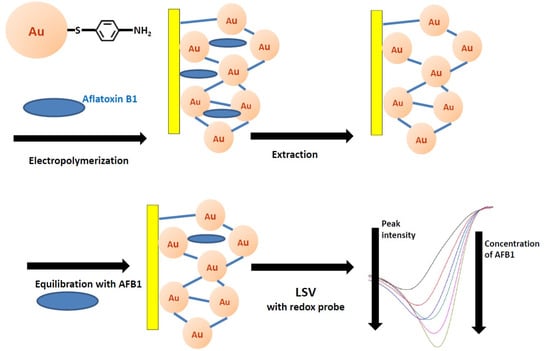
Mycotoxins are toxic fungal metabolites that contaminate several crops worldwide and could represent a significant hazard to human and animal health. Maximum levels for the major mycotoxins have been established in several commodities worldwide. Therefore, a continuous monitoring of these natural contaminants in food and feed is essential to prevent the risk of exposure. Liquid chromatographic methods, coupled with ultraviolet, fluorescence, or mass spectrometry detectors are commonly used for the determination of a large number of mycotoxins. However they are expensive, time-consuming, and require sample clean-up and trained personnel. There is a growing need for rapid and high-throughput screening methods to be used in the food/feed chains and this is reflected in the continued growth of global markets for such assays. Screening methods, based on bio-sensing technologies, are fast, inexpensive, and require visual evaluation or simple instruments. Enzyme immunosorbent assays, lateral flow devices, strip tests, flow-through immunoassays, fluorescence polarization immunoassays based on antibodies, aptamers, peptides or bio-mimetic recognition elements, i.e., molecularly imprinted polymers, are some examples of biorecognition assays for mycotoxin detection. This Special Issue aims to highlight recent developments in this important field.
Dr. Michelangelo Pascale
Asso. Prof. Maria C. DeRosa
Collection Editors
Submission
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Toxins is an international peer-reviewed Open Access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1200 CHF (Swiss Francs).
Keywords
- Mycotoxin
- Immunoassay
- Biorecognition
- Antibody
- Aptamer
- Biosensor
- Biosensing Technique
- Lab-on-a-chip